Abstract
Background and aims: Interstitial cells of Cajal (ICC) are pacemakers and mediators of motor neurotransmission in gastrointestinal smooth muscles. ICC require cellular signalling via Kit, a receptor tyrosine kinase, for development and maintenance of phenotype. Much of the evidence demonstrating the functions of ICC comes from studies of W/WV mice, which have reduced Kit function and reductions in specific populations of ICC. The aim of the present study was to differentially examine gene expression in the small intestines of wild-type and W/WV mutant mice.
Methods and results: RNA from the jejunums of wild-type and W/WV mutants was analysed using a differential gene display method. Eighteen queries were identified as novel genes that were differentially displayed in wild-type and W/WV mice. One candidate gene, encoding a novel acid phosphatase-like protein, was significantly suppressed in fed and starved W/WV mice. The full length clone of the murine gene and its human counterpart were designated acid phosphatase-like protein 1 (ACPL1). Human ACPL1 cDNA encodes a protein of 428 amino acids with homology to human prostatic acid phosphatase protein. This gene is located at 1q21. ACPL1 was abundantly expressed in the human small intestine and colon. Gene products were found to be cytoplasmic in transfected COS-7 cells. Reverse transcription-polymerase chain reaction analysis revealed expression of ACPL1 mRNA within single isolated ICCs.
Conclusions: Gene analysis showed that ACPL1 was differentially expressed in the small intestines of normal and W/WV mice. ICC within the small intestine expressed mRNA for ACPL1. Specific downregulation of ACPL1 in the jejunums of W/WV mice and high expression in human intestinal tissue suggest that the ACPL1 gene could be associated with ICC function in mice and humans.
Keywords: gastrointestinal motility, interstitial cells of Cajal, pacemaker deficient mice, acid phosphatase family, acid phosphatase-like protein 1, chromosome 1q21 differential gene display method
Interstitial cells of Cajal (ICC) are gastrointestinal pacemakers that generate and propagate electrical slow waves.1–4 These cells also mediate excitatory and inhibitory motor neurotransmission.5,6 The important physiological functions of ICC have been deduced primarily from studies on the murine small intestine and stomach. ICC express Kit, a receptor tyrosine kinase, which is essential for the development and maintenance of the ICC phenotype in the gastrointestinal tract.7,8 Mutations in the W locus of mice (for example, W/WV) result in animals with reduced Kit function. These mutants develop few ICC at the level of the myenteric plexus (IC-MY) in the small intestine,2,3 and loose intramuscular ICC (IC-IM) in the stomach and sphincters.5 Associated with the loss of IC-MY is the absence of electrical slow waves in the small intestines of W/WV animals. IC-IM, found within the circular and longitudinal muscle layers of the stomach (IC-IM) and at the level of the deep muscular plexus in the small intestine (termed IC-DMP), play a critical role in enteric motor transmission.5,6
Reduced numbers of ICC have been reported in several gastrointestinal motility disorders, such as chronic intestinal pseudo-obstruction,9,10 infantile hypertrophic pyloric stenosis,11–13 Hirschsprung's disease,14–16 chronic constipation, and certain forms of paresis.17,18 Loss of IC-MY in the small intestine in W/WV mice leads to a distended abdomen and prolonged transit time of intestinal contents.19,20 These pseudo-obstruction-like symptoms are pathologies frequently associated with motility disorders in humans.21 The association between motility disorders and loss of specific populations of ICC suggests that a more complete understanding of the molecular and cell biology of ICC networks within the gastrointestinal tract may help in understanding the aetiology of gastrointestinal motor pathologies. At the present time, impairment in Kit function in W/WV mice is the only molecular defect that has been characterised. Loss of specific populations of ICC in W/WV mutant mice provides a unique opportunity to study the molecular changes that occur in these mutants.
The aim of the present study was to characterise genetic sequences that are expressed in the gastrointestinal tract and may encode important functional elements of the gastrointestinal pacemaker system. We pursued the hypothesis that such genes might show differential expression in the small intestines of wild-type mice and W/WV mice. To determine differential expression, an extensive analysis was performed to examine gene expression in the gastrointestinal tracts of wild-type and W/WV mice. We identified numerous bands from the small intestines that were differentially expressed, and we also identified 18 novel candidate genes. Among these we found a novel cDNA clone, expression of which was greatly suppressed in the small intestines of W/WV mice. This gene encodes a protein which shows significant similarity to acid phosphatases (designated as acid phosphatase-like protein 1 (ACPL1)). In the present study, we report for the first time the isolation and characterisation of human and mouse ACPL1, a novel gene, downregulated in jejunal tissues of W/WV mice.
MATERIALS AND METHODS
Animals and tissue preparation
The use and treatment of experimental animals was approved by the Guideline Governing Animal Experiment Committee at the Yamanashi Medical University School of Medicine and University of Nevada School of Medicine. Six adult male WBB6F1 +/+ mice and six adult male WBB6F1 W/WV mice, weighing 20–30 g, were purchased from Japan SLC Inc (Shizuoka, Japan). They were anaesthetised with ether or carbon dioxide inhalation and sacrificed by cervical dislocation followed by exsanguination.
For morphological studies, the gross anatomy of the gastrointestinal tract was photographed immediately following the opening of the abdomen and prior to any fixation. For immunohistochemistry and conventional electron microscopy, tissues were prepared and observed in the same manner as described previously.6,22 For gene analyses, fresh specimens of the proximal jejunum were rapidly resected from mice and immediately frozen in liquid nitrogen (−196°C). Following freezing tissues were stored at −80°C.
Differential gene display and RT-PCR
For differential gene display, poly(A) RNA was isolated from each tissue sample by Trizol (Life Technologies, Inc., Gaithersburg, Maryland, USA). The differential display procedure was performed as described elsewhere.23,24 The blots were autoradiographed and analysed with a fluorescent image analyser (FMBIO; Takara Co., Kyoto, Japan). For reverse transcription-polymerase chain reaction (RT-PCR), total RNA was isolated from resected specimens by Trizol. cDNA was prepared from 2 μg of total RNA by dT15 priming and synthesised in the same manner as in the differential gene display experiments. Levels of mouse ACPL1 mRNA expression were analysed by comparative RT-PCR from proximal jejunums of fed/starved wild-type mice and W/WV mice.
Isolation and sequencing of ACPL1
Isolation and DNA sequencing of cDNA mouse and human ACPL1 were performed as previously described.25,26 Briefly, by using the differential gene display method we found that one novel clone, termed 54M, was highly homologous to proteins belonging to the family of an acid phosphatase, especially to human prostatic acid phosphatase protein (PAP, GenBank accession No X53605).27 Using the 0.5 kb cDNA insert of this clone as a probe, we screened a mouse stomach cDNA library (approximately 1×106 plaques). The nucleotide sequences of the cDNA clones were determined with an ABI Prism 377 DNA sequencer (Applied Biosystems, Foster City, California, USA), using T3 (5`-TAACCCTCACTAAAGGGA-3`), T7 (5`-TACGACTCACTATAGGGC-3`), or synthetic oligonucleotide primers according to the manufacturer's instructions. The expressed sequence tags (ESTs) of the human counterpart were identified by screening the FASTA database (the Genome Net Database Service, Japan; http://www.genome.ad.jp),28 and these sequences were assembled by means of the ABI “Assembler” computer software (Applied Biosystems). We then subjected the connected cDNA fragment, termed 54H as the human counterpart of 54M, to 5` and 3` rapid amplification of cDNA ends (RACE) using human prostate mRNA as a template and the Marathon cDNA amplification kit (Clontech, Palo Alto, California, USA), according to the manufacturer's instructions.
Northern blot, FISH, and assay for subcellular localisation
For northern blot analysis, human multiple tissue blots (Clontech) were hybridised with a cDNA fragment of 54H, human ACPL1, labelled by the random oligonucleotide priming method. Prehybridisation, hybridisation, and washing were performed according to the supplier's recommendations. The blots were autoradiographed with intensifying screens at −80°C for 12 hours. For fluorescence in situ hybridisation (FISH), a human PAC DNA pool (Genome Systems Inc., St Louis, Missouri, USA) was screened in accordance with the manufacturer's instruction. To determine the chromosomal location of the gene, we performed direct R-banding FISH as previously described.29
To observe subcellular localisation of ACPL1 by immunohistochemistry, plasmids designed to express ACPL1 were constructed by cloning the coding region of ACPL1 that is conserved between human and mouse (codons 52–426) cDNA into the pcDNA3.1(+) vector (Invitrogen, Carlsbad, California, USA). To achieve c-myc tagged ACPL1, we constructed pcDNA3.1(+)/ACPL1 that contained c-myc-epitope sequences (LDEESILKQE) at the C terminal of the ACPL1 protein and transfected them into COS-7 cells. Transiently transfected COS-7 cells were incubated with a mouse anti-c-myc antibody as reported elsewhere.30 Antibodies were stained with a goat antimouse secondary antibody conjugated to fluorescein, and viewed under a fluorescence microscope. To confirm expression of ACPL1/c-myc tagged protein in transfected cells, we also performed western blot analysis, in a manner described previously.31
Confirmation of expression of ACPL1 mRNA within isolated pacemaker interstitial cells
To determine whether ICC express mRNA for ACPL1, enzymatically dispersed and Kit labelled single ICC were collected with modified patch clamp glass pipettes. Total RNA was isolated from single ICC and cDNA prepared as previously described.32 Expression of mRNA for ACPL1 within ICC from the proximal jejunum of wild-type mice was analysed using RT-PCR.
RESULTS
Morphological studies
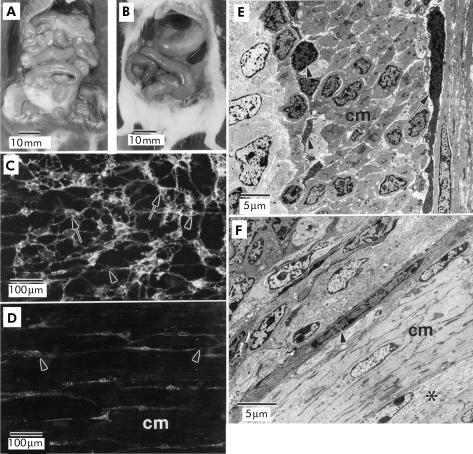
The gross morphology of the gastrointestinal tracts of age matched wild-type and W/WV mice were compared under a dissecting microscope. Gross anatomical observations revealed normal gastrointestinal tracts in wild-type mice (fig 1A ▶). In contrast, W/WV mice presented with distended stomachs and slightly distended small intestines (fig 1B ▶). Kit-like immunoreactivity, used to identify ICC, was found in cells at the level of the myenteric plexus (IC-MY) and deep muscular plexus (IC-DMP) in the jejunums of wild-type animals (fig 1C ▶). In contrast, only IC-DMP were detected with Kit-like immunoreactivity in W/WV mutants; there was almost complete absence of IC-MY in these animals (fig 1D ▶). Tissues were also examined with transmission electron microscopy to confirm that loss of immunoreactivity reflected loss of IC-MY. In wild-type animals, IC-MY and IC-DMP populations were both readily observed (fig 1E ▶) but in W/WV mutant animals, only IC-DMP were observed (fig 1F ▶).
Figure 1.
(A, B) Comparison of the gross morphology of the gastrointestinal tracts from wild-type and W/WV mutant animals, respectively. Note the distended appearance of the stomach and intestines of W/WV mutants (B). Kit-like immunoreactivity revealed the presence of interstitial cells of Cajal (ICC) at the level of the myenteric plexus (IC-MY) (C; arrows) and ICC at the level of the deep muscular plexus (IC-DMP) (arrowheads) in wild-type animals. In W/WV mutants, only IC-DMP were observed (D; arrowheads). The absence of IC-MY in W/WV mutants was also confirmed by electron microscopy (E, F). IC-MY (arrow) and IC-DMP (arrowheads) were readily observed within the circular muscle layer (cm) of jejunums from wild-type animals (E) but only IC-DMP were observed within the cm of W/WV muscles. IC-MY were never observed at the level of the myenteric plexus (*) of W/WV mutant animals. Scale bars are as indicated on each panel.
Differential gene display and RT-PCR
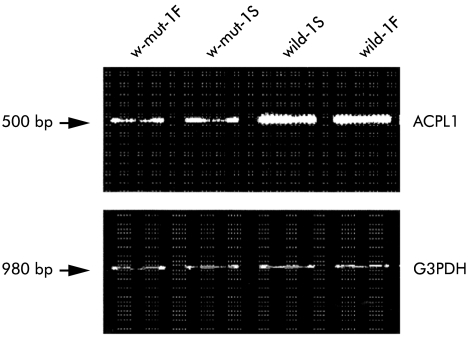
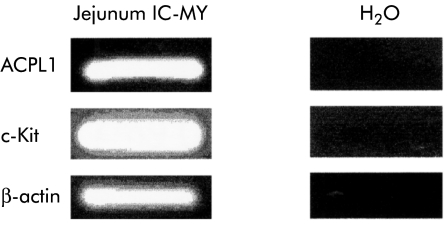
To identify novel genes that may be selectively associated with ICC-MY, we compared gene expression patterns among jejunal tissues derived from wild-type and W/WV mice by a differential gene display method.23,24 Using various primer sets, we identified dozens of cDNA bands that were likely to be expressed at different levels in these tissues. cDNA bands for 54M showed intense labelling in the jejunum of wild-type animals but very weak labelling in W/WV jejunums (data not shown). To confirm the differential display profiles, we further examined expression levels by RT-PCR analysis using murine jejunal mRNA derived from two wild-type and two W/WV mice maintained under fed/starved conditions as templates. In both starved and fed states, expression of the 54M clone was considerably reduced in the jejunums of W/WV mice compared with age matched wild-type mice (fig 2 ▶).
Figure 2.
Expression of acid phosphatase-like protein 1 (ACPL1) using reverse transcription-polymerase chain reaction analysis in jejunum tissues from two wild-type and two W/WV mutant mice maintained under fed and starved conditions. F and S indicate samples of fed and staved mice, respectively. Glyceraldehyde-3- phosphate dehydrogenase (G3PDH) levels were measured as a control. Expression of the 54M clone was considerably reduced in the jejunums of W/WV mice compared with age matched wild-type mice.
Cloning and sequencing of human and murine ACPL1
We subsequently tried to clone and sequence the murine and human cDNAs identified above. To obtain a full length clone of the murine gene, we screened a mouse stomach cDNA library (nearly one million independent plaques) using the 0.5 kb cDNA fragment originally isolated by differential display as a probe and isolated seven cDNA clones. Assembling DNA sequences of these clones, we determined the entire coding nucleotide sequence of the mouse cDNA. It consisted of 0.5 kb that revealed significant homology to acid phosphatase genes, human PAP,25–27 and lysosomal acid phosphatase (ACP2).33 Subsequently, we screened the FASTA database using the coding sequence of mouse cDNA, identified several ESTs, and also determined the human homologue.
Mouse cDNA consists of 1687 nucleotides, including an open reading frame of 1122 nucleotides that encodes a 374 amino acid peptide, while the 1610 bp of the human counterpart contains an open reading frame of 1284 nucleotides encoding 428 amino acids (fig 3 ▶; DNA sequences are available from GenBank: see Nos AB030038 and AB030039, respectively). The human homologue conserved 77% identity in amino acids with the murine products. A homology search using the FASTA program revealed that these predicted proteins were similar to proteins belonging to the family of acid phosphatases, especially human PAP and ACP2 (fig 3 ▶; 27–28% identity in amino acids, respectively; see the section “ACPL1 and acid phosphatase family members” in the discussion). Hence we designated this gene ACPL1. A comparison of the deduced amino acid sequences of human ACPL1 and two other human acid phosphatases indicated a similarity in the overall structures that share the conserved histidine acid phosphatases phosphohistidine signature
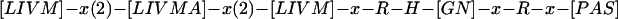 |
in the N terminal region, although the amino acid sequence of mouse ACPL1 does not seem to contain the complete phosphohistidine signature.
Figure 3.
Alignment of the predicted amino acid sequences of the acid phosphatase (ACP) family. Included is human acid phosphatase-like protein 1 (ACPL1), mouse ACPL1, human prostatic acid phosphatase (PAP), and human lysosomal acid phosphatase (ACP2) (GenBank accession Nos AB030038, AB0300039, X53605, and P11117, respectively). Shading indicates homologues residues among ACPL1 (human and mouse) and two known human acid phosphatases. ACPL1 is highly conserved between the two species and is also similar to proteins belonging to the family of acid phosphatases (PAP and ACP2).
Northern blot and chromosomal assignment of human ACPL1
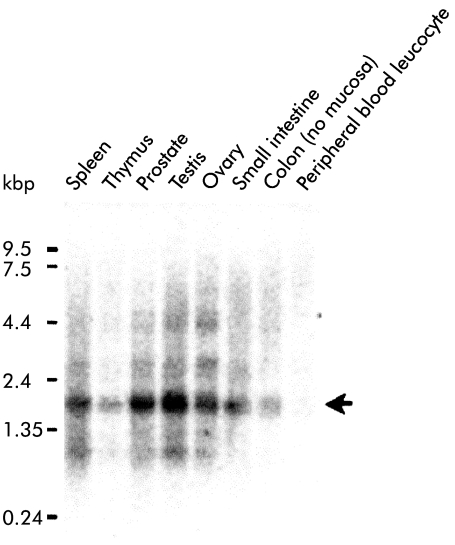
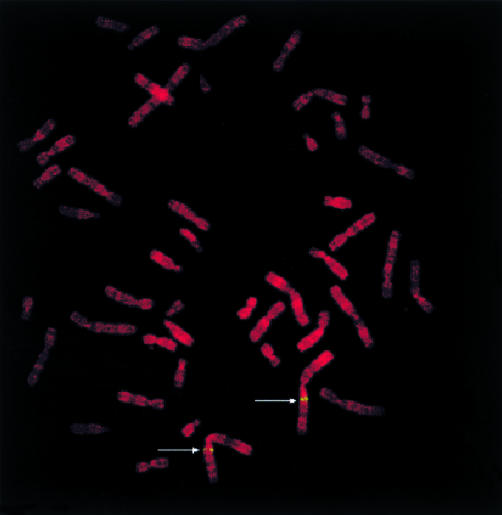
Northern blot analysis using human cDNA as a probe detected a transcript of 1.7 kb in all human tissues examined (fig 4 ▶); the prostate, testis, small intestine, and colon seemed to express the gene more abundantly than other tissues. FISH using a PAC clone including the human ACPL1 gene as a probe showed clear twin signals specifically on chromosomal band 1q21; no signals were detected on any other chromosomes in 100 metaphase cells examined (fig 5 ▶).
Figure 4.
Northern blot analysis of acid phosphatase-like protein 1 (ACPL1) in various human tissues including the spleen, thymus, prostate, testis, ovary, small intestine, colon without mucosa, and peripheral blood leucocytes. Each lane contained approximately 2 μg of poly A+ RNA. Molecular sizes are indicated as kbp on the left of the column. Arrow donates the position of ACPL1.
Figure 5.
Chromosomal mapping of the acid phosphatase-like protein 1 (ACPL1) gene. Prometaphase chromosomes stained with propidium iodide show twin spot signals (green) on chromosome 1q21 (indicated by arrows) mapping the location of the ACPL1 gene.
Subcellular localisation of ACPL1 protein in transfected COS cells
To investigate the cellular localisation of ACPL1 protein in mammalian cells, we transfected COS-7 cells with pcDNA3.1(+)/ACPL1, a plasmid that contained c-myc-epitope sequences (LDEESILKQE) at the C terminal of the ACPL1 protein. First we confirmed expression of ACPL1 in transfected cells by immunoblotting. After transient expression of the ACPL1/c-myc protein in COS-7 cells, proteins were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis. The 49 kDa ACPL1/c-myc protein was detected by western blot analysis using anti-c-myc antibodies (data not shown). Using the same antibodies, we detected the ACPL1/c-myc protein mainly in cytoplasm when the COS-7 cells were transfected with the ACPL1 expression plasmid (fig 6 ▶).
Figure 6.
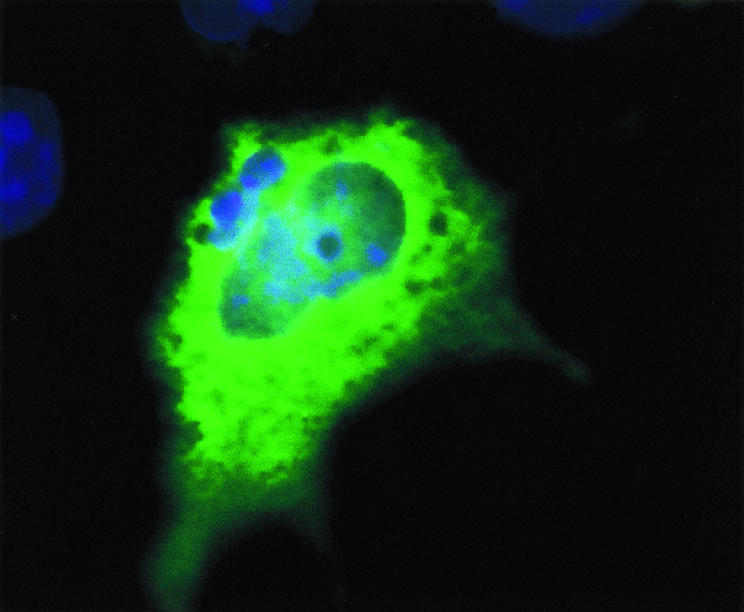
Cellular localisation of the acid phosphatase-like protein 1 (ACPL1) in mammalian cells after transfection of COS-7 cells using immunohistochemistry. Cells were labelled with antibodies against ACPL1/c-myc protein and DAPI. ACPL1/c-myc protein was located within the cytoplasm of CPS-7 cells but was not specifically associated with any cellular organelle.
Confirmation of expression of ACPL1 mRNA within isolated pacemaker interstitial cells
Enzymatic dispersion of murine small intestine revealed numerous spindle shaped smooth muscle cells and a second population of cells that were identified using immunohistochemistry as being Kit immunopositive.32 RT-PCR analysis on single Kit-like immunopositive ICC, isolated from the jejunums of wild-type animals, revealed coexpression of mRNA for Kit and ACPL1 (fig 7 ▶).
Figure 7.
Using reverse transcription-polymerase chain reaction, coexpression of mRNA for acid phosphatase-like protein 1 (ACPL1) and Kit was identified within a single interstitial cell isolated from the jejunum of a wild-type animal by enzymatic dispersion.; IC-DMP, ICC at the level of the deep muscular plexus; IC-MY, interstitial cells of Cajal ICC at the level of the myenteric plexus.
DISCUSSION
Morphological studies for wild-type and W/WV mice
Morphological observations in the present study revealed: (1) distension of the stomach and slight distension of the small intestine of W/WV mice, and (2) disappearance of IC-MY, but an essentially normal distribution of IC-DMP, in the jejunums of W/WV mice. We suggest the former finding is a result of loss of intramuscular IC-IM and motor neurotransmission in the stomach and impairment of the electrical slow wave pacemaker system in the jejunum of these animals, as previously described.2,7,8,22 Our study confirms previous reports that IC-DMP are present in W/WV mutants,34 and these cells have been reported in the deep muscular plexus of Steel mutants and in Ws/Ws mutant rats.35,36 Other than a slight distension of the small intestinal wall and the absence of IC-MY, we failed to identify any other pathological findings in the jejunums of W/WV mice.2,7,8,22 The loss of IC-MY in W/WV and Steel mutants is associated with loss of spontaneous electrical activity (slow waves), and IC-DMP are recognised as an independent population of ICC that are apparently not capable of generating electrical rhythmicity in the murine small intestine.37 Consequently, the present morphological and molecular observations suggest that differential expression of ACPL1 in the jejunum of W/WV mice results from loss of IC-MY. At the present time we do not know whether ACPL1 is important to the function of the pacemaker apparatus or was downregulated as a result of loss of ICC and impairment of normal gastrointestinal pacemaker activity.
Expression and characteristics of ACPL1
Differential gene display indicated that expression of ACPL1 was significantly suppressed in W/WV mice. We confirmed this result with RT-PCR of tissues from four additional mice (two each of wild-type and W/WV mice), and these data suggest that expression of ACPL1 is specifically reduced in W/WV mice. We also described the characterisation of novel human and mouse genes: ACPL1, which are candidate members of the acid phosphatase family. Northern blot analysis showed that human ACPL1 was expressed prominently in the prostate, testis, small intestine, and colon. Comparison of the predicted peptide sequence of human ACPL1 with that of the mouse homologue revealed 77% homology. The N terminal region of the human ACPL1 is highly conserved and is thought to include a putative histidine acid phosphatases phosphohistidine signature (see fig 3 ▶).25–27 This motif is also highly conserved in other acid phosphatase members of several species, such as Caenorhabditis elegans, Saccharomyces cerevisiae, and Escherichia coli. This domain includes not only residues (Arg and His) that have been identified previously as active site residues but also ones (Gly and Pro) that are frequently conserved for structural reasons.38
Human ACPL1 was mapped to chromosome 1q21. It is also found in the human genome project database in chromosome 1. Chromosome band 1q21 is reported to be the site of several genetic diseases caused by multigenetic variables, including an axonal form of autosomal recessive Charcot-Marie-Tooth disease,39 autosomal dominant medullary cystic kidney disease,40 pycnodysostosis,41 and hereditary hyperparathyroidism-jaw tumour syndrome.42 Some types of human chronic intestinal pseudo-obstruction, which could be related to gastrointestinal pacemaker defects, are also hereditary, although the chromosomal position of these defects has not been clarified.9,10 Further investigation is required to elucidate whether ACPL1 is involved in human hereditary diseases.
ACPL1 and acid phosphatase family members
Acid phosphatases are a group of enzymes that can hydrolyse phosphomonoesters under acidic conditions and can be differentiated according to their immunological properties, tissue distribution, and subcellular location.43 Several human genes have been cloned that are members of the acid phosphatase family.44–48 The reactions in which these proteins participate in each tissue are not fully understood.
Members of the acid phosphatase have different roles in the various tissues in which they are expressed. For example, PAP is a cytosolic protein which has tyrosine kinase activity.47 On the other hand, ACP2 is a membrane associated protein found in lysosomes of almost all cells, and acts as a recognition marker for lysosomes.49 Using immunostaining, we determined that ACPL1 was localised within the cytoplasm. On the basis of its sequence similarities and a conserved motif with respect to other acid phosphatase proteins, we suspect that ACPL1 plays an important role in intracellular metabolism, such as hydrolysing phosphomonoesters under acidic conditions.50
Genetic approaches to investigate gastrointestinal pacemaker systems
In the present study, we isolated, sequenced, and analysed human and murine ACPL1. Murine ACPL1 gene encodes a functional protein that was differentially expressed in the W/WV mouse. Specific downregulation of ACPL1 in the jejunum of W/WV mouse, and high expression in human intestinal tissues, suggest that the ACPL1 gene could have a function in gastrointestinal pacemaker cells, and its downregulation could possibly be important in human gastrointestinal motility disorders. Expression of mRNA for ACPL1 within single isolated ICC, which was confirmed by expression of mRNA for c-kit, suggests the direct importance of ACPL1 in gastrointestinal pacemaker activity and motility.
It should also be noted that we isolated 18 additional novel candidate genes that were specifically down or upregulated in W/WV mice (data not shown). Considering that none of these genes has been implicated in any aspect of gastrointestinal motility, we suggest that many unknown genes are involved in normal motility and the cellular changes that lead to motility disorders. Differential gene display is an effective method for screening genes that vary in expression during the development of abnormal motility. This technique may provide important insights into the molecular mechanisms of normal gastrointestinal motor function and pacemaker activity.
Acknowledgments
The authors thank Dr Hiroaki Zai, Department of Gastroenterology, Tokai University School of Medicine, Isehara, Japan for critical reading of the present manuscript. Thanks are due to Dr Moritoshi Kinoshita and Dr Satoshi Takami, Gene Diagnosis Center, Otsuka Assay Laboratories, Tokushima, Japan, for their technical assistance. The present study was supported by a Grant-in-Aid (No B2–08457165) to Masayuki A Fujino from the Japanese Ministry of Education, Science, Sports and Culture for gene analysis, and by a Grant-in-Aid (No PO1 DK41315) to Sean M Ward, Kenton M Sanders, and Burton Horowitz from the National Institutes of Health, USA, for morphological studies and to Becky Walker and Burton Horowitz for molecular studies.
Abbreviations
ACPL1, acid phosphatase-like protein 1
ACP2, lysosomal acid phosphatase
ESTs, expressed sequence tags
FISH, fluorescence in situ hybridisation
ICC, interstitial cells of Cajal
IC-DMP, ICC at the level of the deep muscular plexus
IC-IM, intramuscular ICC
IC-MY, ICC at the level of the myenteric plexus
PAP, prostatic acid phosphatase
RT-PCR, reverse transcription-polymerase chain reaction
REFERENCES
- 1.Langton PD, Ward SM, Carl A, et al. Spontaneous electrical activity of interstitial cells of Cajal isolated from canine proximal colon. Proc Natl Acad Sci USA 1989;86:7280–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ward SM, Burns AJ, Torihashi S, et al. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol (Lond) 1994;480:91–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huizinga JD, Thuneberg L, Kluppel M, et al. W/Kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature (Lond) 1995;373:347–9. [DOI] [PubMed] [Google Scholar]
- 4.Ordog T, Ward SM, Sanders KM. Interstitial cells of Cajal generate electrical slow waves in the murine stomach. J Physiol (Lond) 1999;518:257–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burns AJ, Herbert TM, Ward SM, et al. Interstitial cells of Cajal inhibitory neurotransmission in the stomach. Proc Natl Acad Sci USA 1996;93:12008–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ward SM, Beckett EAH, Wang XY, et al. Interstitial cells of Cajal mediate enteric excitatory neurotransmission in the murine fundus. J Neurosci 2000;20:1393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishi K, Tokutomi N, Sato D, et al. Identification of gastrointestinal tract pacemaker cells and their functional significance. In: Tsuchiya M, Matsuo Y, Kasuga Y, et al, eds. Gastrointestinal function. Regulation and disturbance, vol 14. Tokyo: Excepta Medica, 1996:27–43.
- 8.Takayama I, Takeda M, Ohno S, et al. Experimental pacemaker disease model in mouse intestine: Impairment of interstitial cells of Cajal by an antagonistic antibody for c-kit. Yamanashi Med J 1998;13:53–63. [Google Scholar]
- 9.Takayama I, Kojima Y, Ohtsuka H, et al. Pathology of human idiopathic intestinal pseudo-obstruction and W/Wv mice in gut motility: A gut pacemaker disease? Gut 1995;37(suppl):A85. [Google Scholar]
- 10.Isozaki K, Hirota S, Miyagawa J, et al. Deficiency of c-kit+ cells in patients with a myopathic form of chronic idiopathic intestinal pseudo-obstruction. Am J Gastroenterol 1997;92:332–4. [PubMed] [Google Scholar]
- 11.Vanderwinden JM, Liu H, Menu R, et al. The pathology of infantile hypertrophic pyloric stenosis after healing. J Pediatr Surg 1996;31:1530–4. [DOI] [PubMed] [Google Scholar]
- 12.Vanderwinden JM, Liu H, DeLaet MH, et al. Study of the interstitial cells of Cajal in infantile hypertrophic pyloric stenosis. Gastroenterology 1996;111:279–88. [DOI] [PubMed] [Google Scholar]
- 13.Yamataka A, Fujiwara T, Kato Y, et al. Lack of intestinal pacemaker (C-KIT-positive) cells in infantile hypertrophic pyloric stenosis. J Pediatr Surg 1996;31:96–8. [DOI] [PubMed] [Google Scholar]
- 14.Yamataka A, Kato Y, Tibboel D. A lack of intestinal pacemaker (c-kit) in aganglionic bowel of patients with Hirschsprung's disease. J Pediatr Surg 1995;30:441–4. [DOI] [PubMed] [Google Scholar]
- 15.Yamataka A, Ohshiro K, Kobayashi H, et al. Interstitial C-KIT+ cells and synapse in allied Hirschsprung's disorders. J Pediatr Surg 1997;30:1069–74. [DOI] [PubMed] [Google Scholar]
- 16.Vanderwinden JM, Liu H, DeLaet NH, et al. Interstitial cells of Cajal in human colon and in Hirschsprung's disease. Gastroenterology 1996;111:901–10. [DOI] [PubMed] [Google Scholar]
- 17.He CL, Burgart L, Wang L, et al. Decreased interstitial cell of Cajal volume in patients with slow-transit constipation. Gastroenterology 2000;118:14–21. [DOI] [PubMed] [Google Scholar]
- 18.Ordog T, Takayama I, Cheung WKT, et al. Remodeling of networks of interstitial cells of Cajal in a murine model of diabetic gastroparesis. Diabetes 2000;49:1731–9. [DOI] [PubMed] [Google Scholar]
- 19.Der-Silaphet T, Malysz J, Hagel S, et al. Interstitial cells of Cajal direct normal propulsive contractile activity in the mouse small intestine. Gastroenterology 1998:114:724–36. [DOI] [PubMed] [Google Scholar]
- 20.Takayama I, Seto E, Zai H, et al. Changes of in vivo gastrointestinal motor pattern in pacemaker-deficient (WsRC-Ws/Ws) rats. Dig Dis Sci 2000;45:1901–6. [DOI] [PubMed] [Google Scholar]
- 21.Koch KL. Diabetic gastropathy. Gastric neuromuscular dysfunction in diabetes mellitus. A review of symptoms, pathophysiology, and treatment. Dig Dis Sci 1999;44:1061–75. [DOI] [PubMed] [Google Scholar]
- 22.Torihashi S, Ward SM, Nishikawa S, et al. c-kit-dependent development of interstitial cells and electrical activity in the murine gastrointestinal tract. Cell Tissue Res 1995;280:97–111. [DOI] [PubMed] [Google Scholar]
- 23.Liang P, Pardee AB. Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science 1992;257:967–71. [DOI] [PubMed] [Google Scholar]
- 24.Liang P, Pardee AB. Differential display. A general protocol. Mol Biotechnol 1998;10:261–7. [DOI] [PubMed] [Google Scholar]
- 25.Yeh LCC, Lee AJ, Lee NE, et al. Molecular cloning of cDNA for human prostatic acid phosphatase. Gene 1987;60:191–6. [DOI] [PubMed] [Google Scholar]
- 26.Vihko P, Virkkunen P, Henttu P, et al. Molecular cloning and sequence analysis of cDNA encoding human prostatic acid phosphatase. FEBS Lett 1988;236:275–81. [DOI] [PubMed] [Google Scholar]
- 27.Tailor PG, Govindan MV, Patel PC. Nucleotide sequence of human prostatic acid phosphatase determined from a full-length cDNA clone. Nucleic Acids Res 1990;18:4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Altschul SF, Gish W, Miller W, et al. Basic local alignment search tool. J Mol Biol 1990;215:403–10. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi E, Yamakawa K, Nakamura Y, et al. A high-resolution cytogenetic map of human chromosome 3: localization of 291 new cosmid markers by direct R-banding fluorescence in situ hybridization. Genomics 1992;13:1047–55. [DOI] [PubMed] [Google Scholar]
- 30.Algrain M, Turunen O, Vaheri A, et al. Ezrin contains cytoskeleton and membrane binding domains accounting for its proposed role as a membrane-cytoskeletal linker. J Cell Biol 1993;120:129–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Canfield VA, Levenson R. Transmembrane organization of the Na, K-ATPase determined by epitope addition. Biochemistry 1993;32:13782–6. [DOI] [PubMed] [Google Scholar]
- 32.Epperson A, Hatton WJ, Callaghan B, et al. Molecular markers expressed in cultured and freshly isolated interstitial cells of Cajal. Am J Physiol 2000;279:C529–39. [DOI] [PubMed] [Google Scholar]
- 33.Pohlmann R, Krentler C, Schmidt B, et al. Human lysosomal acid phosphatase: cloning, expression and chromosomal assignment. EMBO J 1988;7:2343–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malyz J, Thuneberg L, Mikkelsen HB, et al. Action potential generation in the small intestine of W mutant mice that lack interstitial cells of Cajal. Am J Physiol 1996;271: G387–99. [DOI] [PubMed] [Google Scholar]
- 35.Ward SM, Burns AJ, Torihashi S, et al. Impaired development of interstitial cells and intestinal electrical rhythmicity in steel mutants. Am J Physiol 1995;269:C1577–85. [DOI] [PubMed] [Google Scholar]
- 36.Ishikawa K, Komuro T, Hirota S, et al. Ultrastructural identification of the c-kit-expressing interstitial cells in the rat stomach: a comparison of control and Ws/Ws mutant rats. Cell Tissue Res 1997;289:137–43. [DOI] [PubMed] [Google Scholar]
- 37.Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology 1996;111:492–515. [DOI] [PubMed] [Google Scholar]
- 38.Van Etten RL, Davidson R, Stevis PE, et al. Covalent structure, disulfide bonding, and identification of reactive surface and active site residues of human prostatic acid phosphatase. J Biol Chem 1991;266:2313–19. [PubMed] [Google Scholar]
- 39.Bouhouche A, Benomar A, Birouk N, et al. A locus for an axonal form of autosomal recessive Charcot-Marie-Tooth disease maps to chromosome 1q21.2-q21.3. Am J Hum Genet 1999;65:722–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Christodoulou K, Tsingis M, Stavrou C, et al. Chromosome 1 localization of a gene for autosomal dominant medullary cystic kidney disease. Hum Mol Genet 1998;7:905–11. [DOI] [PubMed] [Google Scholar]
- 41.Gelb BD, Edelson JG, Desnick RJ. Linkage of pycnodysostosis to chromosome 1q21 by homozygosity mapping. Nat Genet 1995;10:235–7. [DOI] [PubMed] [Google Scholar]
- 42.Szabo J, Heath B, Hill VM, et al. Hereditary hyperparathyroidism-jaw tumor syndrome: the endocrine tumor gene HRPT2 maps to chromosome 1q21-q31. Am J Hum Genet 1995;56:944–50. [PMC free article] [PubMed] [Google Scholar]
- 43.Yam LT. Clinical significance of the human acid phosphatases: a review. Am J Med 1974;56:604–16. [DOI] [PubMed] [Google Scholar]
- 44.Wo YYP, McCormack AL, Shabanowitz J, et al. Sequencing, cloning, and expression of human red cell-type acid phosphatase, a cytoplasmic phosphotyrosyl protein phosphatase. J Biol Chem 1992;267:10856–65 [PubMed] [Google Scholar]
- 45.Sharief FS, Li SSL. Nucleotide sequence of human prostatic acid phosphatase ACPP gene, including 7 Alu repeats. Biochem Mol Biol Int 1994;33:561–5. [PubMed] [Google Scholar]
- 46.Ikemoto S, Hinohara H, Tsuchida S, et al. Phenotype and gene frequencies of acid phosphatase (s-AcP) in the human parotid saliva. Hum Genet 1985;71:30–2. [DOI] [PubMed] [Google Scholar]
- 47.Ketcham CM, Roberts RM, Simmen RC, et al. Molecular cloning of the type 5, iron-containing, tartrate-resistant acid phosphatase from human placenta. J Biol Chem 1988;264:557–563. [PubMed] [Google Scholar]
- 48.Lin MF, Li SS, Chu TM, et al. Comparison of prostate acid phosphatase with acid phosphatase isoenzymes from the lung and spleen. J Clin Lab Anal 1990;4:420–5. [DOI] [PubMed] [Google Scholar]
- 49.Hauser H, Geuze H, von Figura K. Human lysosomal acid phosphatase is transported as a transmembrane protein to lysosomes in transfected baby hamster kidney cells. EMBO J 1988;7:2351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Farquhar MG, Palade GE. The Golgi apparatus (complex)-(1954–1981)-from artifact to center stage. J Cell Biol 1981;91:77–103. [DOI] [PMC free article] [PubMed] [Google Scholar]