Abstract
Background: Activation of the gastrin-cholecystokininB (CCKB) receptor stimulates cell proliferation and increases production of ligands for the epidermal growth factor receptor (EGF-R).
Aims: To determine the role of gastrin-CCKB activation in stimulation of cell proliferation via paracrine activation of EGF-R.
Methods: AGS cells were transfected with the gastrin-CCKB receptor (AGS-GR cells) or with green fluorescent protein (AGS-GFP cells). Proliferation was determined by [3H] thymidine incorporation, flow cytometry, and cell counting.
Results: Gastrin inhibited proliferation of AGS-GR cells by delaying entry into S phase. However, when AGS-GR cells were cocultured with AGS-GFP cells, gastrin stimulated proliferation of the latter. Immunoneutralisation and pharmacological studies using metalloproteinase and kinase inhibitors indicated that the proliferative response was mediated by paracrine stimulation of EGF-R and activation of the mitogen activated protein kinase pathway through release of heparin binding EGF.
Conclusions: Gastrin can directly inhibit, and indirectly stimulate, proliferation of gastric AGS cells.
Keywords: gastrin, proliferation, gastric epithelium, epidermal growth factor, cholecystokinin, AGS cells
It is well recognised that the gastric hormone gastrin stimulates gastric epithelial cell proliferation as well as acutely regulating acid secretion.1 Elevated plasma gastrin concentrations are associated with increased parietal cell mass,2 hyperplasia of histamine producing enterochromaffin-like (ECL) cells,3 and increased labelling of proliferating cells by bromodeoxyuridine.4–7 Gastrin precursor peptides (progastrin and Gly-gastrin) are thought to stimulate proliferation of normal and neoplastic colon cells8–12 although the receptor at which they act is still uncertain. In contrast, proliferation of gastric epithelial cells is thought to be primarily regulated by the amidated gastrins acting at the gastrin-cholecystokininB (CCKB) receptor.5,13The picture is complicated however by reports that in some cell lines expression of the gastrin-CCKB receptor is associated with inhibition of proliferation.14,15 Moreover, in the normal gastric epithelium, it is not clear that the gastrin-CCKB receptor is expressed by proliferating cells, aside from ECL cells.16 It is however recognised that gastrin increases production of ligands of the epidermal growth factor receptor (EGF-R).17–19 The extent to which the proliferative effects of gastrin might be indirect and secondary to release of EGF-R ligands remains uncertain. In the present study we have developed a coculture system which allows study of both the direct effects of gastrin (mediated by cells expressing the receptor) and indirect effects mediated through paracrine release of secondary mediators. We report here that expression of the gastrin-CCKB receptor is linked to direct inhibition of proliferation of cells expressing the receptor (AGS-GR cells), and to indirect stimulation of proliferation of cocultured cells that do not express the receptor (AGS-GFP cells).
METHODS
Tissue culture and transfection
AGS cells were cultured in HAMS/F12 medium supplemented with 10% fetal bovine serum (FBS) and 1% w/v penicillin/streptomycin (Life Technologies, Paisley, UK). Cells stably transfected with full length cDNA encoding the human gastrin-CCKB receptor, or with the empty vector, were generated as previously described.20 In addition, AGS cells stably expressing green fluorescent protein (AGS-GFP cells) were generated by transfection with pEGFP-C1 (Clontech, Basingstoke, UK) using TransFast reagent (Promega, Southampton, UK), and clones resistant to G418 (Life Technologies) selected by fluorescence microscopy. Cells were counted by a haemocytometer and viability determine by trypan blue exclusions (over 95% in all experiments).
Drugs, growth factors, and antibodies
Heptadecapeptide amidated gastrin (that is, G17) was obtained from Peninsula (St Helens, Merseyside, UK); L-740,093 was obtained from Merck (Harlow, UK). Ro-32-0432, GM6001, AG1478, and BB2516 were obtained from Calbiochem (Nottingham, UK). Phorbol-12-myristate-13-acetate (PMA), CRM197, transforming growth factor α (TGF-α), and heparin binding (HB)-EGF were obtained from Sigma (Poole, Dorset, UK); neutralising monoclonal antibodies to TGF-α and EGF-R were obtained from Oncogene (Nottingham, UK), and neutralising goat HB-EGF antibody from RDI (Flanders, New Jersey, USA). Mouse monoclonal antibody to phospho-mitogen activated protein (MAP) kinase was obtained from NEB (Hitchin, Herts, UK), and phycoerythrin conjugated goat antimouse gamma globulin was obtained from Sigma.
[3H] Thymidine incorporation
Synthesis of DNA was assessed by incorporation of [3H] thymidine (Amersham, Little Chalfont, Bucks, UK). Cells (5×104) were cultured in six well plates in 2 ml of medium containing 10% FBS with or without G17 (10 pM to 10 nM) for up to 96 hours. Alternatively, cells were cultured in 2 ml of medium containing 10% FBS for 24 hours and were then synchronised in G0/G1 by incubation in serum free medium for 48 hours. Progression through S phase was stimulated by addition of medium containing 10% FBS with or without G17 (10 pM to 10 nM), PMA (10–100 nM), or TGF-α (10 ng/ml). Cells were then incubated for one hour with 2 μCi [3H] thymidine and washed three times in 2 ml of ice cold phosphate buffered saline (PBS). Trichloroacetic acid (2 ml, 5%) was added and dishes were incubated at 4°C for 20 minutes; cells were washed twice with 2 ml of ice cold ethanol, DNA solubilised in 1 ml of 0.1 M NaOH (60 minutes, 60°C), and an aliquot taken for scintillation counting.
Flow cytometry
In initial experiments on subconfluent AGS-GR cells, cultures were washed in PBS, cells harvested using trypsin, fixed in 70% ethanol, and treated with 100 μg/ml RNAase (Sigma, Poole, UK). Cellular DNA was stained with 20 μg/ml propidium iodide (Sigma) and quantified by cytometry using a Becton Dickinson FacScan (Becton Dickinson, Cowley, Oxford, UK). Cell cycle analysis was performed using Modifit Software (Verity Software House Inc.). In experiments on cocultures of AGS-GFP and AGS-GR, cells were recovered with trypsin, washed once in PBS, resuspended in PBS containing 2 μg/ml Hoechst 33342 (Molecular Probes, Leiden the Netherlands), and incubated for 30 minutes at 37°C prior to analysis by flow cytometery using a Becton Dickinson FACS Vantage. Signals were gated for GFP fluorescence using excitation at 488 nm and emission at 510 nm. Hoechst 33342 fluorescence was detected by excitation at 355 nm and emission at 424 nm. Cell cycle data were analysed using Modfit Software running on an Apple powermac G4 computer.
Proliferation of AGS-GFP cells
Cocultures of 105 AGS-GFP and AGS-GR cells were plated in full media in 12 well plates. After 24 hours, cells were washed and incubated in serum free media with and without gastrin for up to 72 hours. Media was replaced with 0.5 ml of PBS, and fluorescence counted in a FluoroCount Plate Reader (Packard, Brook House, Pangbourne, Berks, UK) with an excitation wavelength of 485 nM and an emission wavelength of 510 nM.
Phospho-MAP kinase detection
Phospho-MAP kinase was detected by the method recently described by Chow and colleagues.21 Briefly, cocultures of AGS-GFP and ASG-GR cells were washed with PBS, recovered with trypsin, pelleted, resuspended in 2% paraformaldehyde, incubated for 10 minutes at 37°C, and then transferred onto ice for two minutes. Ice cold 100% methanol was added to the cells to give a final concentration of 90% and the cells were kept on ice for a further 30 minutes after which they were either labelled immediately or stored at −20°C in 90% methanol. For labelling, cells were washed in PBS containing 4% v/v FBS and then incubated with antibody to phospho-MAP kinase (1:200) for 15 minutes at room temperature, washed once with PBS in 4% FBS, and then labelled with secondary antibody (phycoerythrin conjugated goat antimouse gamma globulin (1:100)) for 15 minutes at room temperature. Cells were then washed once with PBS containing 4% FBS and resuspended in PBS prior to FACS analysis. GFP fluorescence was detected as described above, and phospho MAP kinase detected from the phycoerythrin conjugated secondary antibody using excitation at 488 nm and emission at 575 nm.
Antisense treatment
Cells were preincubated for 24 hours with either random or EGF-R antisense oligonucleotides (Biognostik, Gottingen, Germany) before addition of gastrin. Uptake indicated by FITC labelled oligonucleotides was more than 80% after 24 hours.
Western blotting
Cells were stimulated with gastrin (1 nM, up to 24 hours), extracted in lysis buffer and western blotting was performed as previously described20 using a goat anti-HB-EGF antibody (Santa Cruz, California, USA).
Statistics
Results are presented as means (SEM); comparisons were made using a t test and were considered significant at p<0.05.
RESULTS
Expression of the gastrin-CCKB receptor is linked to inhibition of proliferation
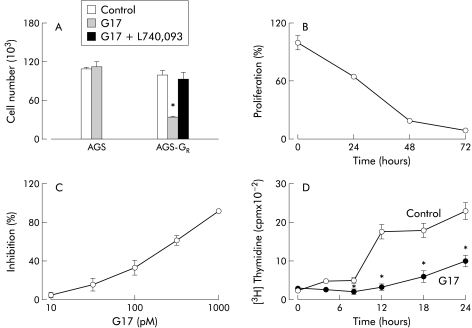
To examine the effect of expression of the gastrin-CCKB receptor on proliferation of AGS cells, we determined cell numbers and incorporation of [3H] thymidine in cells stably expressing the receptor. In the presence of 1 nM G17 for 72 hours, cell numbers did not increase compared with controls but suppression of proliferation was reversed by the gastrin-CCKB receptor antagonist L-740,093 100 nM (fig 1A ▶). Gastrin had no effect on either the parental cell line (fig 1A ▶) or AGS cells stably transfected with the empty vector (not shown). When AGS-GR cells were incubated in media containing 10% FBS, G17 produced a time dependent inhibition of [3H] thymidine incorporation (fig 1B ▶). This response was related to the concentration of gastrin and was detectable with gastrin concentrations in the physiological range (that is, <100 pM) (fig 1C ▶). When AGS-GR cells were synchronised in the G1 phase of the cell cycle by incubation in serum free medium for 48 hours, [3H] thymidine incorporation was reduced by over 90%. Introduction of 10% FBS increased [3H] thymidine incorporation after 12–15 hours (corresponding to cells entering S phase) and this was inhibited by G17 (fig 1D ▶).
Figure 1.
Inhibition of proliferation of cells expressing the gastrin-cholecystokininB receptor. (A) The number of wild-type AGS cells was not changed by incubation in 1 nM heptadecapeptide gastrin (G17) for 72 hours but G17 inhibited the increase in AGS-GR cell numbers and this was reversed by L-740,093 (100 nM). (B) Incorporation of [3H] thymidine into AGS-GR cells was decreased by incubation with G17 for up to 72 hours, and (C) by G17 in concentrations of 300 pM to 10 nM. (D) In cells synchronised in the G1 phase of the cell cycle by incubation in serum free medium for 48 hours, addition of 10% fetal calf serum increased [3H] thymidine incorporation after 12–15 hours, and this was inhibited by G17. Means (SEM), n=4 – 8. *p<0.05 compared with controls.
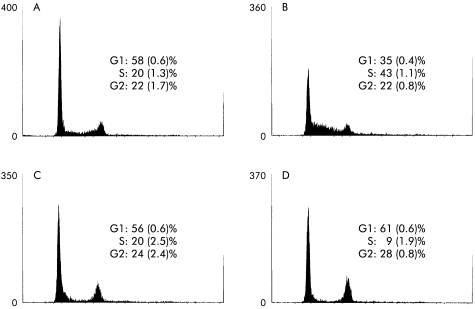
Expression of the gastrin-CCKB receptor is linked to arrest in G1 phase of the cell cycle
To characterise the effects of gastrin-CCKB receptor expression on progression through the cell cycle, we used flow cytometry. Addition of 10% serum to cells incubated in serum free medium for 24 hours significantly increased the proportion of cells in S phase (20 (1.3) to 43 (1.1)%; p<0.05) (fig 2A ▶, B). Addition of gastrin completely inhibited the effect of serum (fig 2C ▶). Similarly, the protein kinase C stimulant PMA arrested AGS cells in the G1 phase of the cell cycle (fig 2D ▶), and similar to gastrin inhibited [3H] thymidine incorporation (19.9 (6.2)% compared with controls; p<0.05).
Figure 2.
FACS analysis of AGS-GR cells. (A) Control incubation in serum free medium. (B) Cells cultured in serum free medium for 48 hours followed by 12 hours in 10% fetal calf serum showing progression into S phase. (C) Incubation with heptadecapeptide gastrin (G17 10 nM) blocked progression into S phase in synchronised cells, and (D) phorbol-12-myristate-13-acetate (100 nM) also inhibited progression into S phase. Means (SEM), n=4.
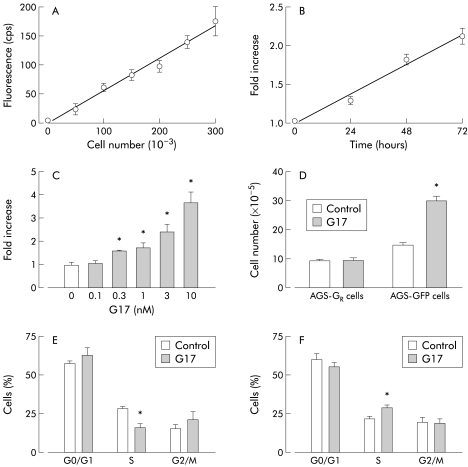
Gastrin-CCKB receptor expression is linked to paracrine stimulation of proliferation
Gastrin stimulates the production of multiple paracrine mediators in vivo, including EGF-R ligands, histamine, Reg, and somatostatin.22 To determine the effects of paracrine mediators on proliferative responses to gastrin, we cocultured cells expressing the gastrin-CCKB receptor (AGS-GR cells) with cells expressing GFP but not the gastrin receptor (AGS-GFP cells). There was a linear relationship between fluorescence and AGS-GFP cell number (fig 3A ▶). Interestingly, in cocultures of AGS-GFP and AGS-GR cells under serum free conditions, gastrin increased the numbers of AGS-GFP cells while the numbers of AGS-GR cells remained constant. The increase in AGS-GFP cell number was dependent on time and the concentration of gastrin, and was detectable with concentrations in the physiological range (fig 3B ▶–D). To determine the effect of gastrin on progression of cocultured cells through the cell cycle, we used flow cytometry to quantify cells labelled with Hoechst 33342. In the case of AGS-GR cells, gastrin increased the proportion of cells in G0/G1 and decreased cells in S phase (p<0.05). In contrast, in the case of AGS-GFP cells in the same cultures, there was a decrease in the proportion of cells in G0/G1 and an increase in S phase (fig 3E, F ▶).
Figure 3.
Proliferation of AGS-GFP cells in coculture with AGS-GR cells. (A) Fluorescence (cps) of AGS-GFP cells was linearly related to cell number (coefficient of correlation 0.985; p<0.05). (B) In cocultures, heptadecapeptide gastrin (G17 1 nM) stimulated a time dependent increase in the numbers of AGS-GFP cells, and (C) this effect of G17 was concentration dependent. (D) In cocultures, G17 (1 nM) increased the numbers of AGS-GFP cells but not AGS-GR cells, determined by cytometry. Flow cytometry of cocultured AGS-GR and AGS-GFP cells incubated with gastrin showed (E) increased abundance of AGS-GR cells in G0/G1 and decreased numbers in S phase, while (F) there was a decrease in AGS-GFP cells in G0/G1 and an increase in S phase. Means (SEM), n=4–8. *p<0.05 versus control.
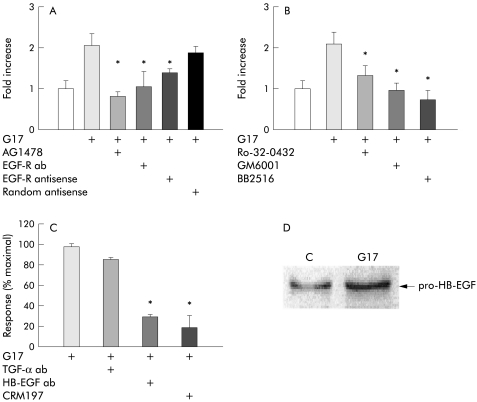
Expression of the gastrin-CCKB receptor is linked to release of an EGF-R ligand
To determine whether stimulation of EGF-R might mediate the effect of gastrin-CCKB receptor activation, we first confirmed that the EGF-R ligand TGF-α stimulated [3H] thymidine incorporation into cells synchronised in G0/G1 phase by incubation in serum free medium (control 100 (3.4); TGF-α 10 ng/ml for 16 hours 245.0 (5.5)%; p<0.01). Similarly, TGF-α stimulated proliferation of AGS-GFP cells in the system described above (control 100 (4.2); TGF-α 221 (10.2)%; p<0.05). We then studied how the proliferative response to gastrin of AGS-GFP cells in coculture with ASG-GR cells was influenced by (A) AG-1478 which inhibits EGF-R tyrosine kinase activity, (B) neutralising antibody to EGF-R, and (C) antisense inhibition of EGF-R expression. All three treatments significantly inhibited the effect of gastrin on ASG-GFP cell proliferation indicating a role for the EGF-R in this response (fig 4A ▶).
Figure 4.
Stimulation of AGS-GFP cell proliferation by gastrin in cocultures with AGS-GR cells was mediated by epidermal growth factor receptor (EGF-R) ligands. (A) Proliferative responses of AGS-GFP cells to heptadecapeptide gastrin (G17 1 nM) were inhibited by AG1478 (1 μg/ml), monoclonal antibody (ab) to EGF-R (8 μg/ml), and antisense inhibition of EGF-R synthesis (oligonucleotides 2 μM). (B) G17 stimulation of AGS-GFP cell proliferation was reversed by the protein kinase C inhibitor Ro-32-0432 (1 μM), and the metalloproteinase inhibitors BB2516 (150 μM) and GM6001 (25 μM). (C) G17 responses were inhibited by antibody to heparin binding (HB)-EGF (5 μg/ml) but not transforming growth factor α (TGF-α 4 μg/ml), and by the diphtheria toxin mutant CRM197 (10 μg/ml). (D) Western blot shows that G17 (1nM) for four hours increased the abundance of the precursor of HB-EGF. Means (SEM), n=4–6. *p<0.05 versus controls.
Proliferative responses following expression of the gastrin-CCKB receptor are linked to metalloproteinase activity
Shedding of EGF-R ligands from the cell surface is thought to depend on metalloproteinase activity that can be stimulated by protein kinase C (PKC).23 We found that the PKC inhibitor Ro-32-0432 reduced the proliferative response of AGS-GFP cells to gastrin when cocultured with AGS-GR cells. Moreover, two metalloproteinase inhibitors, BB2516 and GM6001, also reversed gastrin stimulated proliferative responses (fig 4B ▶), compatible with PKC dependent release of an EGF-R ligand.
We then examined the effect of neutralising antibodies to two EGF-R ligands, TGF-α and HB-EGF. Antibody to the latter, but not the former, substantially inhibited the proliferative response of AGS-GFP cells when cocultured with AGS-GR cells. The proliferative response of AGS-GFP cells was also inhibited by the diphtheria toxin mutant CRM197 which is known to act as an HB-EGF receptor and to block proliferative responses to HB-EGF (fig 4C ▶).24 Direct evidence that gastrin increased HB-EGF production was provided by the finding that the abundance of the HB-EGF precursor detected by western blot was increased in gastrin stimulated cultures by four hours (fig 4D ▶).
Gastrin stimulates MAP kinase both directly and indirectly
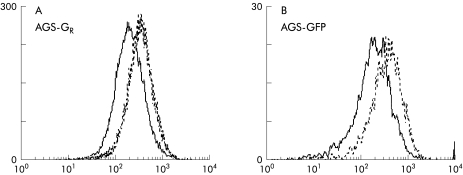
As stimulation of EGF-R is linked to increased MAP kinase, we assessed if gastrin increased p42/44 MAP kinase phosphorylation in AGS-GFP cells in coculture with AGS-GR. Using flow cytometry of fixed, permeabilised, cocultured cells incubated with gastrin, and detection of phosphorylated p42/44MAP kinase by specific antibody,21 we found that in both cell lines there was gastrin dependent stimulation of MAP kinase that peaked at 30 minutes (fig 5 ▶).
Figure 5.
Effect of gastrin (1 nM, 30 minutes) on phospho-mitogen activated protein (MAP) kinase detected by flow cytometry. In cocultures, gastrin (broken line) increased phospho-MAP kinase compared with controls (solid line) in both AGS-GR cells (A) and AGS-GFP cells (B). Representative traces from four independent experiments.
DISCUSSION
Our results showed that expression of the gastrin-CCKB receptor is associated with both stimulation and inhibition of cell proliferation. In cells expressing the receptor, gastrin inhibited proliferation. However, when these cells were cocultured with cells not expressing the receptor, gastrin stimulated proliferation of the latter. We present evidence that the proliferative response was attributable to paracrine stimulation of EGF-R and that this stimulation required release of HB-EGF as a consequence of metalloproteinase activity.
There is abundant evidence to suggest that gastrin stimulates proliferation of gastric mucosal cells.5,13 In the case of the ECL cell, this may be a direct mitogenic effect.25 In general however it is not clear whether mitogenic responses to gastrin in the stomach are direct or reflect indirect effects mediated by other growth factors. Studies in cell lines indicate both stimulation and inhibition of proliferation in response to gastrin-CCKB receptor activation. Precisely how these different responses might be determined is unclear. Thus in several cells lines, including AR4-2J cells, small cell lung cancer cells, and Rat-1 cells expressing this receptor, there is increased proliferation in response to gastrin-CCKB receptor stimulation.26–28 However, in some pancreatic cell lines and CHO cells,14,15 as in AGS-GR cells, expression of the gastrin-CCKB receptor is linked to inhibition of proliferation. This receptor is coupled to Gαq/11, and other receptors signalling through Gαq/11 (for example, the muscarinic M3 receptor) have also been found to both stimulate and inhibit cell proliferation depending on cellular context.29 In addition to variation between cell lines, there may be differences between clones of the same cell line as in some AGS cell clones G17 is reported to stimulated AGS cell proliferation.30 The parental cell line used for the present study did not express gastrin-CCKB receptors and did not respond to gastrin. Some AGS cell clones may express the gastrin gene31 but as these cells cannot process the product to mature gastrin this does not result in the release of a ligand for the gastrin-CCKB receptor.
It is well established that stimulation of G protein coupled receptors, such as the gastrin-CCKB receptor, can lead to phosphorylation of EGF-Rs,32 which in turn leads to activation of the MAP kinase pathway and proliferation.33–35 In some systems this is attributable to an intracellular, or direct, signalling pathway possible due to PKC activation of Ras via recruitment of SoS-Grb2 complexes.35 In other cases however it now seems likely that G protein coupled receptor stimulation leads to increased intracellular calcium or activation of PKC which leads to release of growth factors by proteolysis of membrane associated precursors, and subsequent stimulation of EGF-R.23,36,37 However, the relative importance of this type of event in mediating proliferative responses to G protein coupled receptor stimulation remains uncertain. The combined results of using specific HB-EGF antibodies, a mutant diphtheria toxin that binds HB-EGF,24 and metalloproteinase inhibitors, clearly indicate that proliferative responses of AGS cells following gastrin-CCKB receptor activation require cleavage of HB-EGF. The data therefore support the idea that proliferative responses are due to activation of a paracrine stimulant of cells adjacent to those expressing the gastrin-CCKB receptor, and are not simply due to autocrine stimulation. The shedding HB-EGF is stimulated by PKC so that PKC inhibitors reverse proliferative responses. The system is complex however because in AGS cells and in other cell types, activation of PKC by phorbol esters leads to arrest in the G1 phase of the cell cycle. It therefore appears that activation of PKC leads to direct inhibition of proliferation and indirect (HB-EGF mediated) stimulation of proliferation. As our data show that there is activation of the MAP kinase pathway in both AGS-GR and AGS-GFP cells, it would appear that in the receptor expressing cells the pathway associated with inhibition of proliferation dominates.
There is growing interest in the role of gastrin and related peptides as growth factors in gastrointestinal cancer. The gastrin gene encodes multiple putative growth factors including progastrin itself, the Gly-gastrins, and the amidated gastrins.38,39 Only the latter are thought to act at gastrin-CCKB receptors. The combination of elevated gastrin and H pylori is reported to predispose to gastric cancer.40 The present studies provide clear evidence that proliferative responses to gastrin may be due to paracrine effects, mediated by increased production of growth factors that need not necessarily act on the cells expressing the receptor. Moreover, in cells which do express the gastrin-CCKB receptor, the major response may be inhibition of proliferation. It seems plausible to suppose that in other systems, responses to gastrin reflect a balance between direct inhibitory responses and indirect stimulatory responses. These findings therefore provide a basis for understanding physiological mechanisms in vivo where there is clear evidence that members of the EGF family (TGF-α, HB-EGF, amphiregulin) stimulate gastric epithelial cell proliferation, and that gastrin increases the synthesis of these growth factors.17–19 They also have implications for interpretation of responses to wound healing where gastrin-CCKB receptor expression is increased in mucus cells adjacent to the wound.41 Finally, they are relevant to an understanding of the role of gastrin in gastrointestinal neoplasia as it seems possible that gastrin stimulated proliferation and migration of tumour cells may be secondary to growth factor production.
Acknowledgments
This work was supported by grants from the Medical Research Council. We are grateful to Drs C Thompson and M Jones for assistance with flow cytometry, and Cathy McLean for skilled technical assistance.
Abbreviations
CCK, cholecystokinin
ECL, enterochromaffin-like cell
EGF, epidermal growth factor
EGF-R, epidermal growth factor receptor
FBS, fetal bovine serum
GFP, green fluorescent protein
HB-EGF, heparin binding EGF
G17, heptadecapeptide gastrin
MAP, mitogen activated protein
PBS, phosphate buffered saline
PKC, protein kinase C
PMA, phorbol-12-myristate-13-acetate
TGF-α, transforming growth factor α
REFERENCES
- 1.Walsh JH. Gastrin. In: Walsh JH, Dockray GJ, eds. Gut peptides. New York: Raven Press, 1994:75–121.
- 2.Pisegna JR, Norton JA, Slimak GG, et al. Effects of curative gastrinoma resection on gastric secretory function and antisecretory drug requirement in the Zollinger-Ellison syndrome. Gastroenterology 1992;102:767–78. [DOI] [PubMed] [Google Scholar]
- 3.Lamberts R, Creutzfeldt W, Struber HG, et al. Long term omeprazole therapy in peptic ulcer disease: gastrin, endocrine cell growth and gastritis. Gastroenterology 1993;104:1356–70. [DOI] [PubMed] [Google Scholar]
- 4.Rozengurt E, Walsh JH. Gastrin, CCK, signaling, and cancer. Annu Rev Physiol 2001;63:49–76. [DOI] [PubMed] [Google Scholar]
- 5.Ohning GV, Wong HC, LLoyd KCK, et al. Gastrin mediates the gastric mucosal proliferative response to feeding. Am J Physiol 1996;271:G470–6. [DOI] [PubMed] [Google Scholar]
- 6.Larsson H, Carlsson E, Mattsson H, et al. Plasma gastrin and gastric enterochromaffinlike cell activation and proliferation: studies with omeprazole and randitidine in intact and antrectomized rats. Gastroenterology 1986;90:391–9. [DOI] [PubMed] [Google Scholar]
- 7.Bordi C, D'Adda T, Azzoni C, et al. Hypergastrinemia and gastric enterochromaffin-like cells. Am J Surg Pathol 1995;19:S8–19. [PubMed] [Google Scholar]
- 8.Wang TC, Koh TJ, Varro A, et al. Processing and proliferative effects of human progastrin in transgenic mice. J Clin Invest 1996;98:1918–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh P, Owlia A, Varro A, et al. Gastrin gene expression is required for the proliferation and tumorigenicity of human colon cancer cells. Cancer Res 1996;56:4111–15. [PubMed] [Google Scholar]
- 10.Koh TJ, Dockray GJ, Varro A, et al. Overexpression of glycine-extended gastrin in transgenic mice results in increased colonic proliferation. J Clin Invest 1999;103:1119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hollande F, Imdahl A, Mantamadiotis T, et al. Glycine-extended gastrin acts as an autocrine growth factor in a nontransformed colon cell line. Gastroenterology 1997;113:1576–88. [DOI] [PubMed] [Google Scholar]
- 12.Watson SA, Michaeli D, Grimes S, et al. Gastrimmune raises antibodies that neutralize amidated and glycine-exended gastrin-17 and inhibit the growth of colon cancer. Cancer Res 1996;56:880–5. [PubMed] [Google Scholar]
- 13.Johnson LR. Regulation of gastrointestinal mucosal growth. Physiol Rev 1988;68:456–502. [DOI] [PubMed] [Google Scholar]
- 14.Detjen K, Yule D, Tseng MJ, et al. CCK-B receptors produce similar signals but have opposite growth effects in CHO and Swiss 3T3 cells. Am J Physiol 1997;273:C1449–57. [DOI] [PubMed] [Google Scholar]
- 15.Detjen K, Fenrich MC, Logsdon CD. Transfected cholecystokinin receptors mediate growth inhibitory effects on human pancreatic cancer cell lines. Gastroenterology 1997;112:952–9. [DOI] [PubMed] [Google Scholar]
- 16.Helander HF, Wong H, Poorkhalkali N, et al. Immunohistochemical localization of gastrin/CCK-B receptors in the dog and guinea-pig stomach. Acta Physiol Scand 1997;159:313–20. [DOI] [PubMed] [Google Scholar]
- 17.Tsutsui S, Shinomura Y, Higashiyama S, et al. Induction of heparin binding epidermal growth factor-like growth factor and amphiregulin mRNAs by gastrin in the rat stomach. Biochem Biophys Res Comm 1997;235:520–3. [DOI] [PubMed] [Google Scholar]
- 18.Miyazaki Y, Shinomura Y, Tsutsui S, et al. Gastrin induces heparin-binding epidermal growth factor-like growth factor in rat gastric epithelial cells transfected with gastrin receptor. Gastroenterology 1999;116:78–89. [DOI] [PubMed] [Google Scholar]
- 19.Wang TC, Dangler CA, Chen D, et al. Synergistic interaction between hypergastrinemia and Helicobacter infection in a mouse model of gastric cancer. Gastroenterology 2000;118:36–47. [DOI] [PubMed] [Google Scholar]
- 20.Watson F, Kiernan RS, Deavall DG, et al. Transcriptional activation of the rat vesicular monoamine transporter 2 promoter in gastric epithelial cells: Regulation by gastrin. J Biol Chem 2001;276:7661–71. [DOI] [PubMed] [Google Scholar]
- 21.Chow S, Patel H, Hedley DW. Measurement of MAP kinase activation by flow cytometry using phospho-specific antibodies to MEK and ERK: potential for pharmacodynamic monitoring of signal transduction inhibitors. Cytometry 2001;46:72–8. [DOI] [PubMed] [Google Scholar]
- 22.Dockray GJ, Varro A, Dimaline R, et al. The gastrins: their production and biological activities. Ann Rev Physiol 2001;63:119–39. [DOI] [PubMed] [Google Scholar]
- 23.Goishi K, Higashiyama S, Klagsbrun M, et al. Phorbol ester induces the rapid processing of cell surface heparin-binding EGF-like growth factor: conversion from juxtacrine to paracrine growth factor activity. Mol Biol Cell 1995;6:967–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitamura T, Higashiyama S, Taniguchi N, et al. Diphtheria toxin binds to the epidermal growth factor (EGF)-like domain of human heparin-binding EGF-like growth factor/diphtheria toxin receptor and inhibits specifically its mitogenic activity. J Biol Chem 1995;270:1015–19. [DOI] [PubMed] [Google Scholar]
- 25.Mahr S, Neumayer N, Kolb HJ, et al. Growth factor effects on apoptosis of rat gastric enterochromaffin-like cells. Endocrinology 1998;139:4380–90. [DOI] [PubMed] [Google Scholar]
- 26.Todisco A, Takeuchi Y, Urumov A, et al. Molecular mechanisms for the growth factor action of gastrin. Am J Physiol 1997;273:G891–8. [DOI] [PubMed] [Google Scholar]
- 27.Sethi T, Herget T, Wu SV, et al. CCKA and CCKB receptors are expressed in small cell lung cancer lines and mediate Ca2+ mobilization and clonal growth. Cancer Res 1993;53:5208–13. [PubMed] [Google Scholar]
- 28.Seufferlein T, Withers DJ, Broad S, et al. The human CCKB/gastrin receptor transfected into rat1 fibroblasts mediates activation of MAP kinase, p74raf-1 kinase, and mitogenesis. Cell Growth Differ 1995;6:383–93. [PubMed] [Google Scholar]
- 29.Nicke B, Detjen K, Logsdon CD. Muscarinic cholinergic receptors activate both inhibitory and stimulatory growth mechanisms in NIH3T3 cells. J Biol Chem 1999;274:21701–6. [DOI] [PubMed] [Google Scholar]
- 30.Iwase K, Evers BM, Hellmich MR, et al. Regulation of growth of human gastric cancer by gastrin and glycine-extended progastrin. Gastroenterology 1997;113:782–90. [DOI] [PubMed] [Google Scholar]
- 31.Ford MG, Valle JD, Soroka CJ, et al. EGF receptor activation stimulates endogenous gastrin gene expression in canine G cells and human gastric cell cultures. J Clin Invest 1997;99:2762–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daub H, Wallasch C, Lankenau A, et al. Signal characteristics of G protein-transactivated EGF receptor. EMBO J 1997;16:7032–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maudsley S, Pierce KL, Zamah AM, et al. The beta(2)-adrenergic receptor mediates extracellular signal-regulated kinase activation via assembly of a multi-receptor complex with the epidermal growth factor receptor. J Biol Chem 2000;275:9572–80. [DOI] [PubMed] [Google Scholar]
- 34.Daub H, Weiss FU, Wallasch C, et al. Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature 1996;379:557–60. [DOI] [PubMed] [Google Scholar]
- 35.Luttrell LM, Daaka Y, Lefkowitz RJ. Regulation of tyrosine kinase cascades by G-protein-coupled receptors. Curr Opin Cell Biol 1999;11:177–83. [DOI] [PubMed] [Google Scholar]
- 36.Prenzel N, Zwick E, Daub H, et al. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature 1999;402:884–8. [DOI] [PubMed] [Google Scholar]
- 37.Dethlefsen SM, Raab G, Moses MA, et al. Extracellular calcium influx stimulates metalloproteinase cleavage and secretion of heparin-binding EGF-like growth factor independently of protein kinase C. J Cell Biochem 1998;69:143–53. [DOI] [PubMed] [Google Scholar]
- 38.Varro A, Voronina S, Dockray GJ. Pathways of processing of the gastrin precursor in rat antral mucosa. J Clin Invest 1995;95:1642–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Varro A, Dockray GJ, Bate GW, et al. Gastrin biosynthesis in the antrum of patients with pernicious anemia. Gastroenterology 1997;112:733–41. [DOI] [PubMed] [Google Scholar]
- 40.Hansen S, Vollset SE, Ardill JES, et al. Hypergastrinaemia is a strong predictor of distal gastric adencarcinoma among Helicobacter pylori infected persons. Gastroenterology 1997;112:A575. [Google Scholar]
- 41.Schmassmann A, Reubi JC. Cholecystokinin-B/gastrin receptors enhance wound healing in the rat gastric mucosa. J Clin Invest 2000;106:1021–9. [DOI] [PMC free article] [PubMed] [Google Scholar]