Abstract
Background: A new staging system for hepatocellular carcinoma (HCC) has recently been reported from Italy (CLIP classification). It combines Child-Pugh staging with tumour criteria: tumour morphology, portal invasion, and alpha fetoprotein levels.
Aims: To validate the use of the CLIP staging in a cohort of HCC patients and compare it with Okuda staging.
Patients and methods: A retrospective analysis of patients with HCC diagnosed in the Toronto General Hospital between October 1994 and December 1998.
Results: A total of 313 patients were identified; 19 patient with insufficient data and 37 transplant patients were excluded. Hence 257 patients in whom complete data for clinical staging were available were included in the study. The median survival of the cohort was 22.8 months. The CLIP stage 0 group (23.1% of the cohort) and the Okuda stage 1 group (50.7% of the cohort) had a five year survival rate of 67% and 35%, respectively (p<0.02). The CLIP stage 0 criteria more accurately defined patients with a good prognosis. The Okuda classification failed to identify two thirds of the 37 patients with a poor prognosis, who were identified by the CLIP criteria. Patients with a CLIP score ≥4 shared a very poor prognosis (median survival 1–3 months). Further classification above stage 4 was unnecessary.
Summary: The CLIP classification for HCC is easy to implement and more accurate than the Okuda classification. Our cohort was different from the CLIP cohort (more hepatitis B) but the results were still consistent.
Keywords: hepatocellular carcinoma, classification, risk factors, prognosis, CLIP staging
Clinical staging systems for cancer provide guides to patient assessment and in making therapeutic decisions. Clinical staging is also an essential research tool which allows comparison between groups in therapeutic trials and between different studies. The current classifications which are most commonly used for hepatocellular carcinoma (HCC) are the Okuda classification,1 Child-Pugh score,2 and the tumour node metastasis (TNM) classification.3 Each has its own limitations. The Child-Pugh score was not developed for HCC patients. It considers only features related to liver function and does not include cancer parameters. The TNM classification includes only features related to the tumour and does not include liver function parameters. The TNM classification, which is widely used for hepatic resection or transplantation, has been found to be inadequate by many groups over the last few years.4–8 The Okuda classification (table 1 ▶), which was the first to include both tumour and liver function factors, does not include important tumour factors, such as whether the tumour is unifocal, multifocal, or diffuse, or whether there is vascular invasion, all of which have prognostic significance. New staging systems for HCC have recently been reported from Italy,9 France,10 and Spain (the Barcelona clinic liver cancer staging (BCLC)).11 The newer classifications have included prognostic factors which their studies showed were significant. These are: portal vein thrombosis, multifocal tumour, diffuse or massive disease, high alpha fetoprotein (AFP) levels, and performance status. Two prognostic factors which are used in the new staging systems, but which are less widely recognised as independent prognostic factors, are alkaline phosphatase in the French classification and “clinically relevant portal hypertension” in the BCLC staging. The Italian (cancer of the liver Italian program (CLIP)) (table 1 ▶) and French classifications10 have been shown to have a better predictive power than the Okuda or Child-Pugh classification. Both the CLIP and French groups have also prospectively validated their staging systems in separate cohorts.10,12 The CLIP staging does not use a subjective evaluation such as performance status whereas both the French and BCLC classifications require performance status evaluation. The BCLC staging11 was initially developed for patients undergoing surgical resection of HCC and was shown to be more accurate than the indocyanine green clearance test and the Child-Pugh score in predicting survival after surgical resection of HCC.13 The BCLC staging was then broadened to create an algorithm for the treatment of HCC. However, this system has not been prospectively validated in a large cohort of HCC patients.
Table 1.
Definitions of the CLIP and Okuda classifications
| CLIP classification | ||||||||
| Parameter | Score | |||||||
| Child-Pugh | ||||||||
| A | 0 | |||||||
| B | 1 | |||||||
| C | 2 | |||||||
| Tumour morphology | ||||||||
| Uninodular and extension <50% of tumour | 0 | |||||||
| Multinodular and extension <50% of tumour | 1 | |||||||
| Massive or extension ≥50% of tumor | 2 | |||||||
| Alpha fetoprotein | ||||||||
| <400 ng/ml | 0 | |||||||
| >400 ng/ml | 1 | |||||||
| Macro vascular invasion | ||||||||
| No | 0 | |||||||
| Yes | 1 | |||||||
| Okuda classification | ||||||||
| Tumour size | Ascites | Albumin | Bilirubin | |||||
| ≥50% | <50% | ≤30 g/l | >30 g/l | ≥3 mg% | <3 mg% | |||
| (+) | (−) | (+) | (−) | (+) | (−) | (+) | (−) | |
| Stage | ||||||||
| 1 | (−) | (−) | (−) | (−) | ||||
| 2 | 1 or 2 (+) | |||||||
| 3 | 3 or 4 (+) | |||||||
CLIP 0, 0 points; CLIP 1, 1 point; CLIP 2, 2 points, etc.
We have attempted to independently validate these newer staging systems. However, as our data were collected retrospectively, we could not assess the performance status of our patients. Thus we did not evaluate the French and BCLC classifications. We therefore report here our assessment of the CLIP and Okuda HCC staging systems in 257 patients with HCC in Toronto.
PATIENTS AND METHODS
We conducted a retrospective chart review of all patients with HCC diagnosed and treated at the Toronto General Hospital. Patients were identified from a prospectively collected database from a weekly HCC multidisciplinary tumour board, and from a search of hospital records (ICD-9, liver cancer primary). All patients with HCC who were newly diagnosed between 1 October 1994 and 31 December 1998 were reviewed. A total of 313 patients were identified. The diagnosis of HCC was confirmed by histology (biopsy or surgical specimen) in 221 patients (70.6%), by a typical clinical presentation and serum AFP greater then 400 ng/ml in 50 patients (16.0%), or by unequivocal clinical and radiological findings in 42 (13.4%) patients.
Exclusion criteria
Nineteen patients (6.1%) in whom data were incomplete were excluded. Thirty seven patients that underwent liver transplantation were also excluded (as they were excluded in the original CLIP model9). We analysed survival data with and without the transplant patients. For the final comparison between the three classifications, the transplant patients were excluded. Thus 257 patients in whom all three classifications (CLIP, Child-Pugh, and Okuda) were completed were eligible for application and validation of the CLIP model.
Follow up
For patients who were alive, the last follow up visit in 1999 was recorded (median follow up 23.5 (14.5) months). Mortality data were assessed as of 31 December 1999 by record linkage with mortality statistics from Cancer Care Ontario, hospital records, and family physicians. Six patients were lost to follow up (1.8%). Their data were censored at the last known date alive.
Statistics
Survival was analysed by Kaplan-Meier plots and the log rank test using SPSS software v 10.0 (Chicago, Illinois, USA). The χ2 and ANOVA on rank tests were used to compare the prevalence of HCC characteristics between the various CLIP stages.
RESULTS
A total of 257 patients in whom complete data for clinical staging were available were included in the comparison between the CLIP, Okuda, and Child-Pugh classifications. Demographics, risk factors, and baseline characteristics of the 257 CLIP validation group are described in table 2 ▶. Median age was 63 years, 73.1% were males, and 48.6% of patients were immigrants from China and other South East Asian countries. The common risk factors identified in the cohort were chronic hepatitis B in 147 subjects (58.5%), chronic hepatitis C in 54 (21.5%), and alcoholic liver disease in 39 (15.5%). At presentation, 97.2% of patients were known to have cirrhosis or chronic liver disease. Fifty one per cent of patients were asymptomatic at presentation, and 95 curative treatments (alcohol ablation n=23, resection n=72) were attempted in 95 patients (37.0%). As of December 1999, 157 patients (61.1%) have deceased.
Table 2.
Baseline characteristics of hepatocellular carcinoma in Toronto in 257 patients
| Variable | No (%) (n=257) |
| Mean age (y) | 60.1 (12.5) |
| Median age (y) | 63 |
| Sex male | 188 (73.1) |
| Country of origin | (missing data 4) |
| South-East Asia | 123 (48.6) |
| Canada | 44 (17.3) |
| Europe south | 33 (13.0) |
| Other | 53 (20.1) |
| Main risk factors | (missing data 6) |
| HBV | 147 (58.5) |
| HCV | 54 (21.5) |
| Alcohol | 39 (15.5) |
| Diagnosis | |
| Biopsy or surgical specimen | 173 (67.3) |
| Clinical/radiological+AFP >400 ng/ml | 49 (19.1) |
| Clinical/radiological | 35 (13.6) |
| Symptoms | (missing data 21) |
| Asymptomatic patients. | 122 (51.7) |
| Symptomatic patients (before diagnosis) | 114 (48.3) |
| Constitutional symptoms* | 82/122 (67.2) |
| Abdominal symptoms* | 65/114 (57.0) |
| Non-tumorous liver | (missing data 13) |
| Cirrhosis | 178 (73.0) |
| Chronic hepatitis | 59 (24.2) |
| Normal liver | 7 (2.8) |
| AFP levels | |
| >400 ng/ml | 105 (40.8) |
| 11–400 ng/ml | 92 (35.8) |
| ≤10 ng/ml | 60 (23.4) |
| Tumour type | |
| Unifocal | 107 (41.6) |
| Multifocal | 75 (29.2) |
| Diffuse massive | 75 (29.2) |
| Vascular macroscopic invasion | 43 (16.7) |
| Metastasis at diagnosis | 15 (5.8) |
| Treatments* | |
| Resection/transplantation/alcohol | 72/0/23 (37.0) |
| Chemoembolisation and experimental | 29 (11.3) |
| Palliative, Chinese herbal, tamoxiphen | 133 (51.7) |
*More than 100% due to multiple symptoms in some patients.
HBV, hepatitis B virus; HCV, hepatitis C virus; AFP, alpha fetoprotein.
The CLIP classification
Patients were staged according to the CLIP criteria (table 1 ▶). The distribution of the HCC risk factors between the different CLIP stages is summarised in table 3 ▶. Ethnic origin was significantly different between the different CLIP stages (p<0.013 χ2). The Asian population was more commonly diagnosed at an early stage then the non-Asian population. In particular, Chinese patients were diagnosed earlier and their survival from diagnosis was longer than Canadian born patients (data not shown). As a result, alcohol was a more common risk factor in the high grade CLIP stages (30% v 10%; p<0.046) (table 3 ▶). This is probably a reflection of regular screening in hepatitis B carriers in the Chinese population in Toronto.
Table 3.
Distribution of risk factors for hepatocellular carcinoma in 257 patients in the different CLIP stages
| Stage | No | Median age (y) | Sex male (%) | Asian origin (%) | HBV (%) | HCV (%) | Alcohol (%) |
| CLIP O | 62 | 64 | 74 | 55 | 61 | 24 | 10 |
| CLIP 1 | 65 | 64 | 74 | 60 | 66 | 20 | 12 |
| CLIP 2 | 48 | 62 | 65 | 46 | 52 | 15 | 10 |
| CLIP 3 | 45 | 62 | 71 | 40 | 49 | 20 | 20 |
| CLIP 4–6 | 37 | 62 | 84 | 27 | 51 | 27 | 30 |
| p Value | — | 0.7† | 0.18* | 0.013* | 0.069* | 0.65* | 0.046* |
*χ2 test; †ANOVA test.
HBV, hepatitis B virus; HCV, hepatitis C virus.
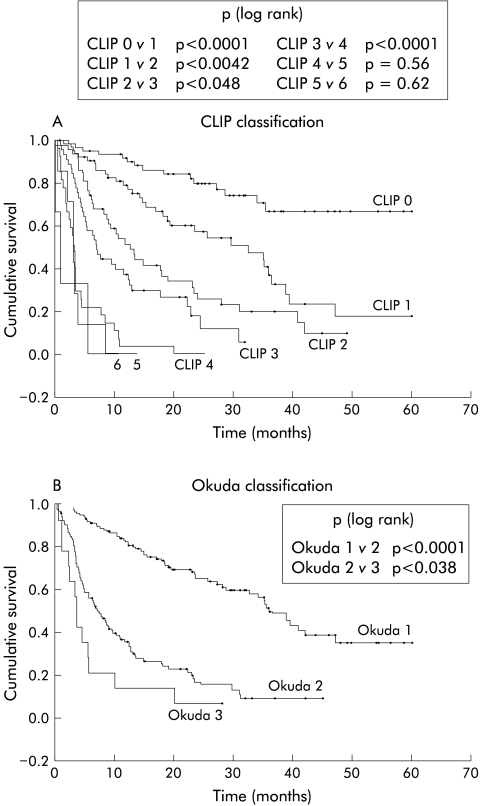
Differences between clinical stages within each classification are shown in fig 1 ▶ and table 4 ▶. Cumulative survival was significantly different between CLIP stages 1 to 4 and between the three Okuda stages. CLIP stages 4–6 shared a very poor prognosis (median survival 1–3 months) without statistically significant differences between their survival curves.
Figure 1.
Clinical classification of 257 patients with hepatocellular carcinoma. Comparison of the CLIP and Okuda classifications.
Table 4.
Survival of patients in different stages, according to the different staging systems: CLIP, Okuda, and Child-Pugh classifications (n=257 patients)
| Classification system | No (%) | Median (95% CI) survival (months)* | One year survival (%) | Three year survival (%) | Five year survival (%) |
| CLIP | |||||
| 0 | 62 (24.1) | — | 92 | 67 | 67 |
| 1 | 65 (25.3) | 32.6 (19–46) | 80 | 37 | 17 |
| 2 | 48 (18.7) | 12.7 (9–17) | 52 | 20 | 0 |
| 3 | 45 (17.5) | 7.0 (5–9) | 37 | 0 | 0 |
| 4 | 27 (10.5) | 3.2 (2.6 –3.8) | 4 | 0 | 0 |
| 5 | 7 (2.7) | 3.2 (2.9–3.5) | 0 | 0 | 0 |
| 6 | 3 (1.2) | 1.0 (0–2.4) | 0 | 0 | 0 |
| Okuda | |||||
| 1 | 132 (51.3) | 36.3 (32–40) | 82 | 50 | 35 |
| 2 | 111 (43.2) | 7.0 (5–9) | 36 | 9 | 0 |
| 3 | 14 (5.5) | 3.5 (2.7–4.2) | 14 | 0 | 0 |
| Child-Pugh | |||||
| A | 191 (74.3) | 27.9 (19–37) | 67 | 38 | 29 |
| B | 49 (19.1) | 8.5 (4–13) | 37 | 5 | 0 |
| C | 17 (6.6) | 3.5 (0–7.7) | 18 | 0 | 0 |
*Median survival could not be calculated for the CLIP stage 0 as the last cumulative survival in this group was 67%. Median survival is the first observed time when cumulative survival is 50% or less.
The CLIP classification was more accurate then the Okuda classification in identifying patients with a good prognosis. The CLIP stage 0 group (24.1% of the cohort) and the Okuda stage 1 group (51.3% of the cohort) had a five year survival of 67% and 35%, respectively (p<0.02 log rank). The CLIP stage 0 group excluded patients with adverse tumour prognostic factors, and more accurately defined the subgroup of patients with the best prognosis. The Okuda stage 1 group and the Child-Pugh stage A group contained patients with a good prognosis, mixed with patients with adverse tumour risk factors who had a less favourable prognosis. There were 132 patients in Okuda stage 1 and their CLIP staging (stages 0–3) was: 62 (47%), 51 (38.0%), 16 (12.1%), and 3 (2.3%), respectively.
The CLIP classification also had an advantage in patients with a poor prognosis. There were 37 patients in CLIP stages 4–6 and 14 patients in Okuda stage 3 (table 4 ▶). Among the 37 patients in CLIP stages 4–6, only 10 were in Okuda stage 3 (27%) and 27 were in Okuda stage 2. Median survival of CLIP stage 4–6 patients whether in Okuda stage 2 or 3 was similar, at 3.2 months (95% confidence interval (CI) 2.8–3.5) and at 3.1 months (95% CI 1.0–5.1), respectively (p=0.43, log rank). The Okuda classification does not account for portal vein invasion, AFP levels, or diffuse or multifocal disease, and therefore misclassified more than two thirds of the CLIP 4–6 patients who had these poor prognostic factors. Looked at from the opposite perspective, there were 14 patients in Okuda stage 3, one of whom had a CLIP score of 2. This patient was still alive after 28 months of follow up. All other patients in Okuda stage 3 had high CLIP scores (three patients in CLIP stage 3 and 10 patients in CLIP stages 4–6). Thus the CLIP criteria correctly identified patients with a poor Okuda score but the Okuda staging failed to assign an appropriately poor prognosis to two thirds of patients with high CLIP scores.
The Child-Pugh staging was less accurate than the Okuda stages. The survival plots of Child-Pugh B and C curves were not statistically different (p=0.06, log rank), even after exclusion of transplant patients (plots not shown). The Child-Pugh staging was not accurate and is not suitable for classification of HCC patients.
The transplant effect
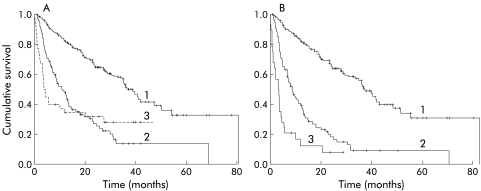
When patients who were transplanted were included in the analysis, the correlation between disease severity and survival was less clear. In the Okuda and Child-Pugh staging systems, patients classified in advanced stages (Okuda 3 or Child C) who were transplanted have extended survival. This shifts the survival curves of the Okuda 3 group to the right, as shown in fig 2 ▶ (the Child-Pugh plot undergoes similar changes, not shown). However, because these patients had unifocal disease, low AFP levels, and no vascular invasion, when classified by the CLIP staging system their inclusion did not alter the CLIP survival curves (plot not shown). Only two of the 37 transplant patients were classified as CLIP stage 4 and none in grades 5–6. Thus when attempting to make comparisons between the various classifications it was necessary to exclude the transplant group.
Figure 2.
Kaplan-Meier plots. Cumulative survival of patients with hepatocellular carcinoma in different Okuda stages: (A) 294 patients, including transplant patients; (B) 257 patients (transplant patients excluded). 1=Okuda stage 1; 2=Okuda stage 2; 3=Okuda stage 3.
DISCUSSION
We have evaluated the CLIP classification for HCC in a cohort of 257 patients with HCC in Toronto. To our knowledge this is the first independent study examining the CLIP classification in a large non-Italian population. Considering the difference between the Canadian and Italian populations, we were impressed at how similar our results were to those of the original CLIP studies.9,12 The CLIP criteria combine the Child-Pugh criteria with tumour characteristics: the nature of the tumour, whether unifocal, multifocal, or diffuse, whether there is vascular invasion, or high levels of AFP. All have been separately described as adverse prognostic characteristics in patients with HCC.14–17 As these tumour criteria are assessed today in the primary evaluation of new patients, it is very easy to implement this scoring system.
The process of developing a staging system involves identifying potential factors in a cohort (the training cohort). Multivariate analysis is the statistical tool of choice. This requires that the sample size be sufficiently large to allow for 10–20 events (deaths) for each prognostic variable. The staging system so developed must then be validated in an independent cohort, both by the initial authors and by independent studies. Of the more recent HCC staging systems the CLIP and French systems are the only ones that are close to meeting all of these criteria. Both have a relatively large number of patients in the training cohort and both have been tested in another independent prospective cohort.
Our analysis of 257 patients staged by the CLIP system confirms that this is a useful system. The addition of tumour specific prognostic markers improved the prognostic accuracy of the CLIP stages compared with the Okuda and Child-Pugh scores. This difference was noticeable in two areas. Firstly, the CLIP score adequately separated patients with no adverse tumour prognostic factors (CLIP 0) who have the best prognosis from patients with even one adverse prognostic factor who showed a significantly reduced prognosis (fig 1 ▶). Secondly, the CLIP score was better in identifying patients with a poor prognosis. Two thirds of the 37 patients with CLIP scores 4–6 were classified in Okuda stage 2 only. However, all but one of the 17 Okuda stage 3 patients were classified into CLIP score groups 3–6.
The Okuda classification uses a level of bilirubin of above 51 mmol/l (3mg%, three times the upper limit of normal) to record an increase in score. This level may be too high as a rise in bilirubin to double normal in patients with HCC may be an adverse prognostic factor. The BCLC staging11 recently emphasised a lower bilirubin level of 17 mmol/l as a threshold for change in clinical stage. In this scoring system a bilirubin level >17 mmol/l (1mg%) advances the score. This number was determined from a prospective study of liver resection for HCC.13 Okuda's criteria lack sensitivity to important tumour factors and have a high threshold for bilirubin. This system is probably unsuitable for the current population of HCC patients, many of whom are diagnosed early in an asymptomatic stage of disease.
Staging systems need to be simple; the CLIP classification satisfies this demand but yet it uses eight clinical parameters. The French10 and BCLC11 studies have used other staging parameters such as alkaline phosphatase, portal hypertension, and performance status. These further complicate the staging procedure. The CLIP study clearly showed that patients with CLIP scores of 4 and above all share less than three month median survival and further subdivision is not relevant. A patient with portal vain invasion and a very high AFP is likely to also present with diffuse tumour, high alkaline phosphatase, and will rapidly develop liver failure and a low performance status. The risk factors progress simultaneously and not all are needed for accurate staging. The CLIP system uses the Child-Pugh score. It may be that some of the Child-Pugh components are unnecessary. Indeed, when we combined the three tumour criteria of the CLIP classification with only two liver synthetic function tests (albumin <35 g/l and bilirubin >35 mmol/l (2mg%)) the survival curves were virtually superimposable on the CLIP curves (plot not shown). This suggests that altogether no more than 5–6 (tumour and liver synthetic tests) criteria are needed to adequately stage HCC.
Our study supports the findings of the CLIP group. The CLIP staging is simple, uses common clinical criteria, and is more accurate than the Okuda and Child-Pugh staging systems. Until a better system comes along it should be implemented as a useful staging system for HCC. Other systems, such as the BCLC and French systems, may also be useful but we were unable to evaluate them adequately.
Abbreviations
HCC, hepatocellular carcinoma
CLIP, cancer of the liver Italian program
TMN, tumour node metastasis
BCLC, Barcelona clinic liver cancer staging
AFP, alpha fetoprotein
REFERENCES
- 1.Okuda K, Ohtsuki T, Obata H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Cancer 1985;56:918–28. [DOI] [PubMed] [Google Scholar]
- 2.Pugh RNH, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973;60:646–64. [DOI] [PubMed] [Google Scholar]
- 3.International Union Against Cancer (UICC). In: LH Sobin, Ch Wittekind, eds. TMN classification of malignant tumours, 5 edn. New York: Wiley-Liss, 1997:74–7.
- 4.Izumi R, Shimizu K, II T, et al. Prognostic factors of hepatocellular carcinoma in patients undergoing hepatic resection. Gastroenterology 1994;106:720–7. [DOI] [PubMed] [Google Scholar]
- 5.Staudacher C, Chiappa A, Biella F, et al. Validation of the modified TMN-Izumi classification for hepatocellular carcinoma. Tumori 200;86:8–11. [DOI] [PubMed]
- 6.Marsh JW, Dvorchik I, Bonham CA, et al. Is the pathologic TMN staging system for patients with hepatoma predictive of outcome? Cancer 2000;88:538–43. [DOI] [PubMed] [Google Scholar]
- 7.Llovet JM, Bruix J, Fuster J, et al. Liver transplantation for small hepatocellular carcinoma: the tumor-node metastasis classification does not have prognostic power. Hepatology 1998;27:1572–7. [DOI] [PubMed] [Google Scholar]
- 8.Lui WY, Chiu JH, Loong CC, et al. Evaluation of simplified staging system for prognosis of hepatocellular carcinoma. J Formos Med Assoc 1999;98:248–53. [PubMed] [Google Scholar]
- 9.The Cancer of the Liver Italian Program (CLIP) investigators. A new prognostic system for hepatocellular carcinoma: A retrospective study of 435 patients. Hepatology 1998;28:751–5. [DOI] [PubMed] [Google Scholar]
- 10.Group de'etude et de Traitement du Carcinoma Hepatocellulair. A new prognostic classification for predicting survival in patients with hepatocellular carcinoma. J Hepatol 1999;31:133–41. [DOI] [PubMed] [Google Scholar]
- 11.Llovet JM, Bru C, Broux J. Prognosis of hepatocellular carcinoma: The Barcelona Clinic Liver Cancer staging Classification. Semin Liver Dis 1999;3:329–37. [DOI] [PubMed] [Google Scholar]
- 12.The Cancer of the Liver Italian Program (CLIP) investigation. Prospective validation of the CLIP score: a new prognostic system for patients with cirrhosis and hepatocellular carcinoma. Hepatolgy 2000;31:840–5. [DOI] [PubMed] [Google Scholar]
- 13.Bruix J, Castellas A, Bosch J, et al. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology 1996;11:1018–22. [DOI] [PubMed] [Google Scholar]
- 14.Stuart KE, Anand AJ, Jenkins RL. Hepatocellular carcinoma in the United States. Prognostic features, treatment outcome, and survival. Cancer 1996;77:2217–22. [DOI] [PubMed] [Google Scholar]
- 15.Calvet X, Bruix J, Bru C, et al. Natural history of hepatocellular carcinoma in Spain. Five year's experience in 249 cases. J Hepatol 1990;10:311–17. [DOI] [PubMed] [Google Scholar]
- 16.Llovet JM, Bustemante J, Castellas A, et al. Natural history of non surgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology 1999;29:62–7. [DOI] [PubMed] [Google Scholar]
- 17.Hamy A, Savigny JL, Paineau J, et al. Uni and multivariate analysis of predictive survival factors in hepatocellular carcinoma. A study series of 122 patients. J Chir (Paris) 1997;134:417–22. [PubMed] [Google Scholar]