Abstract
Alcohol is a major aetiological factor in hepatocarcinogenesis but our understanding of its importance as a modulating factor is just beginning to emerge. In the present review, a number of possible cofactors and mechanisms are discussed by which alcohol may enhance the development of hepatoma. These include dietary or environmental carcinogens ingested along with alcoholic beverages, alcoholic cirrhosis as a precancerous condition, and the effects of alcohol metabolism.
Keywords: alcohol, hepatocellular carcinoma, hepatitis B, hepatitis C, acetaldehyde, cytochrome P450, methylation, cocarcinogen
SUMMARY
The incidence of hepatocellular carcinoma is rising worldwide. Apart from hepatitis B and C viruses, alcohol presents a major aetiological factor in hepatocarcinogenesis, as shown in numerous epidemiological studies. While the pathogenic role of alcohol in the development of liver cirrhosis has been investigated extensively, our understanding of its importance as a modulating factor in hepatocarcinogenesis is just beginning to emerge. In the present review, a number of possible cofactors and mechanisms are discussed by which alcohol may enhance the development of hepatoma. These include dietary or environmental carcinogens ingested along with alcoholic beverages, alcoholic cirrhosis as a precancerous condition, and the effects of alcohol metabolism such as the toxicity of its metabolite acetaldehyde, increased lipid peroxidation due to reactive oxygen species, activation of procarcinogens via induction of cytochrome P450 2E1, and polymorphisms of alcohol dehydrogenase. Furthermore, alterations of DNA methylation through interactions with one carbon metabolism can lead to aberrant methylation of tumour suppressor genes and oncogenes. Alcohol metabolism also reduces hepatic retinoic acid levels and may thereby enhance cell proliferation and malignant transformation via upregulation of activator protein 1 gene expression. Synergistic effects between alcohol and hepatitis B and especially C virus have been demonstrated, although the mechanisms remain unclear. Alcohol leads to malfunction of the immune system, and suppression of natural killer cells by alcohol may favour tumour development. Thus alcohol is commonly considered a tumour promoter. However, evidence from animal studies that showed preneoplastic alterations after chronic alcohol exposure indicate that alcohol may also contribute to tumour initiation.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the most frequent primary liver tumour among the commonest malignant tumours today.1 Its prevalence is increasing worldwide but differs greatly between regions, with incidences of approximately 3–4/100 000 in Western countries2–4 and up to 120/100 000 in Asia and Southern Africa. In more than 80% of European and North American cases, HCCs develop in cirrhotic livers whereas in Asia near 50% of HCCs may occur in non-cirrhotic livers.5,6 The increase in HCC is most likely due to the more widespread chronic infection with hepatotropic viruses, namely hepatitis B (HBV) and especially hepatitis C (HCV). Epidemiological studies have incriminated both viruses in hepatocarcinogenesis, and the contributory role of alcohol, a major aetiological factor of liver cirrhosis in Western countries, is undisputed.1 In the following, we summarise the evidence and discuss potential mechanisms of the cocarcinogenic effect of alcohol.
EPIDEMIOLOGY
There is compelling evidence that chronic alcohol consumption increases the risk of developing HCC.7–9 However, the exact role of alcohol in the development of HCC compared with chronic HBV and HCV infection is still incompletely defined. Numerous studies demonstrated that the incidence of HCC among alcoholics is above the expected rate.10 Thus an epidemiological survey from the UK demonstrated an eightfold increase in the risk of developing HCC among male alcoholics.11 The higher rate of alcohol related HCC worldwide may be partially explained by prolongation of survival time of patients with alcoholic cirrhosis due to improved disease management.
“Chronic alcohol consumption increases the risk of developing HCC”
The effect of abstinence in the development of HCC was discussed controversially in various studies. It was shown that cessation of alcohol consumption increased the risk of developing HCC. This was explained by alterations in cell regeneration after alcohol withdrawal, which will be discussed below. However, a major plausible argument is that abstinence allows recovery from alcohol related hepatocellular damage which by prolonging survival time may by itself increase the likelihood of developing HCC in a cirrhotic liver.
ANIMAL EXPERIMENTS
Experiments in which alcohol but no carcinogen was given continuously to rodents have shown that alcohol per se is not a carcinogen as even lifelong exposure to alcohol did not lead to more cancers than in pair fed controls.8 Most animal experiments with respect to hepatocarcinogenesis have been performed using nitrosamines as tumour inducing compounds. Unexpectedly, in almost all of these studies inhibition of hepatocarcinogenesis together with alcohol intake was shown.8 On the other hand, the rate of extrahepatic tumours, such as tumours in the nasal cavity, trachea, and oesophagus, increased. Only with additional manipulations, such as administration of a diet low in methyl donors or carbohydrates,12,13 or after partial hepatectomy,14 was alcohol able to stimulate hepatocarcinogenesis. Interestingly, striking enhancement of hepatic carcinogenesis was observed when alcohol and the procarcinogen were given on an alternating basis to avoid interactions between alcohol and carcinogen metabolism. Another important determinant in animal studies of alcohol is the route of administration. If ethanol is given with drinking water, nutrient deficiencies may occur due to interactions with their absorption that may influence carcinogenesis. Administration of ethanol as a liquid diet, a technique established by Lieber and DeCarli,15 assures constant alcohol intake and provides adequate amounts of macro and micronutrients.
PATHOMECHANISMS
For the liver, there are multiple mechanisms by which alcohol can accelerate cancer development. These include enzymes and metabolites involved in ethanol metabolism, such as cytochrome P450 2E1 (CYP 2E1) which can potentiate carcinogens, interference with methyl transfer, modulation of retinoid turnover, and the preconditioning associated with concomitant infection by HBV and HCV. Importantly, cirrhosis by itself is a precancerous condition. As no single pathomechanism can be incriminated exclusively, several must act in concert to induce HCC.
Alcohol, cirrhosis, and preneoplastic histology
Alcohol causes hepatocellular injury that can lead to enhanced fibrogenesis and finally cirrhosis, the latter being per se associated with an increased risk of developing HCC. Alcohol related HCC without pre-existing cirrhosis is rare,10 indicating that pathogenic events leading to cirrhosis precede those causing cancer or that the structural alterations of cirrhosis favour hepatocyte dedifferentiation. Although the presence of cirrhosis can be considered the major prerequisite for the development of HCC, various other pathogenic factors may contribute significantly to the malignant transformation of hepatocytes.
“Alcohol related HCC without pre-existing cirrhosis is rare”
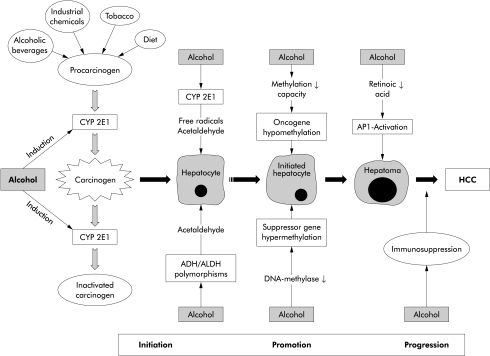
Some controversy exists as to whether alcohol is a tumour inducer in hepatocarcinogenesis (fig 1 ▶). In various animal models attempts have been made to correlate the stages of initiation, promotion, and progression in hepatocarcinogenesis with specific precancerous histological features. Thus centres of focal growth have been observed which show a number of metabolic alterations—for example, enzyme altered foci and preneoplastic nodules.16 Recently, such areas of preneoplastic tissue were also produced in rats by alternate treatment with N-nitrosodimethylamine as a cancer inducer and alcohol, strongly suggesting that ethanol may indeed act as a tumour promoter in hepatocarcinogenesis (fig 1 ▶).17 Interestingly, Mallory body (MB) formation is high in HCC and the incidence of HCC is significantly higher in cirrhosis with MBs than without.18 It was therefore hypothesised that MBs may represent an initial phenotypical alteration in the carcinogenic transformation of hepatocytes.
Figure 1.
Alcohol as a promoter of hepatocarcinogenesis. Both activation and inactivation of procarcinogens can occur. Alcohol per se is a tumour promoter but may contribute to initiation via procarcinogen activation. ADH, alcohol dehydrogenase; ALDH, aldehyde dehydrogenase; CYP 2E1, cytochrome P450 2E1; HCC, hepatocellular carcinoma.
Another histological abnormality observed in experimental hepatocarcinogenesis is the occurrence of oval cells which originate from the portal triads after long term alcohol exposure.19 These cells do also appear after administration of a choline deficient ethionine supplemented diet which is known to stimulate hepatocarcinogenesis.20 Recently, the occurrence of oval cells has also been observed in patients with chronic alcoholic liver disease.21
Alcohol and environmental carcinogens
Alcoholics may be exposed to carcinogens or procarcinogens ingested along with alcoholic beverages which may contain nitrosamines, polycyclic hydrocarbons, asbestos fibres, and fusel oils.22 In addition, many alcoholics are smokers and epidemiological surveys have shown a hyperadditive effect of alcohol and smoking in increasing the risk of developing HCC.9 Similarly, dietary carcinogens and exposure to carcinogens at the working place have to be taken into account (see fig 1 ▶). With regard to the former, aflatoxin B1 (AFB1) is a major hepatocarcinogen which is metabolised by the alcohol inducible cytochrome P450 2E1 (CYP 2E1). AFB1 can induce a mutation in codon 249 of the p53 tumour suppressor gene which is frequently found in human HCC.23
“Many alcoholics are smokers and epidemiological surveys have shown a hyperadditive effect of alcohol and smoking in increasing the risk of developing HCC”
Although animal experiments have been controversial as to whether ethanol enhances AFB1 induced hepatocarcinogenesis, an epidemiological study on AFB1 exposure demonstrated that even a moderate daily consumption of 24 g ethanol increases the risk of developing HCC induced by 4 μg of dietary AFB1 by 35-fold.24 Vinyl chloride is also metabolised by CYP 2E1 and its exposure is associated with the development of HCC which is again increased several fold by additional alcohol consumption.25
Alcohol metabolism and HCC
In the liver, ethanol is predominantly metabolised by alcohol dehydrogenase (ADH) and CYP 2E1, resulting in acetaldehyde (AA) formation. AA, the extremely toxic first intermediate of ethanol metabolism, binds rapidly to cellular proteins and also possibly to DNA. These AA adducts represent neoantigens leading to the formation of specific antibodies.26 AA has mutagenic and carcinogenic properties leading to metaplasia, inhibition of DNA repair,27 sister chromatid exchanges,28 stimulation of apoptosis, and enhanced cell injury associated with hyperregeneration.29 According to the International Agency for Research on Cancer, there is sufficient evidence to identify AA as a carcinogen in animals.
Ethanol is metabolised by the successive action of ADH and aldehyde dehydrogenase (ALDH). For both ADH and ALDH, genetic polymorphisms have been described that influence the rate of conversion of ethanol to AA and of the latter to acetate.30 It has been consistently reported that ALDH2 is the most important alcohol metabolising polymorphic enzyme affecting predisposition to alcoholism in Asian populations. With regard to ADH, the alleles ADH2*2 and ADH3*1 encode for an enzyme with a high capacity to produce AA (400 kcat/min and 87 kcat/min).31 Therefore, higher AA levels are found in patients revealing the alleles ALDH2*2, ADH2*2, and ADH3*1, either via increased AA synthesis or via decreased oxidation of AA to acetate. It has been shown that individuals with inactive ALDH2*2 or highly active ADH2*2 are at increased risk of alcoholic liver disease.32 While no association between ADH2*2 and ALDH2 genes and HCC has been found,33 preliminary data reveal a higher prevalence of ADH3*1 in patients with alcohol related HCC than controls (Stickel et al, unpublished data).
CYP 2E1 constitutes the microsomal ethanol oxidising system which is inducible by higher amounts of ethanol and other xenobiotics.34 The degree of CYP 2E1 induction can be correlated with generation of reactive oxygen species (ROS), in particular hydroxyethyl radicals and lipid peroxides.35 Moreover, experimental alcohol induced liver disease and CYP 2E1 can be modulated by CYP 2E1 inhibitors and inducers.36–38 In the setting of alcoholism, additional sources of ROS formation may be NADH dependent cytochrome C reductase, aldehyde and xanthine oxidase, neutrophil NADPH oxidase, and catalase. ROS initiate predominantly lipid peroxidation but they may also react rapidly with cell constituents, including DNA, and thereby lead to DNA damage and cancer initiation.39
Hepatic iron plays a key role as an enhancer of ROS production. Alcohol consumption increases iron absorption from the gut with its consequent accumulation in the liver, which suggests an at least additive effect of alcohol and iron in the generation of ROS.40 Under normal conditions, these toxic ROS are rapidly neutralised by reductive detoxification mechanisms, mainly glutathione, α-tocopherol, superoxide dismutase, catalase, and glutathione peroxidase. Eventually, the amount of ROS produced exceeds the neutralising capacity of these defence systems which may result in precancerous tissue and organ damage.41 Therefore, the importance of oxidative stress in alcohol related liver disease and hepatic carcinogenesis has precipitated numerous experimental studies and clinical trials.42 Although on the basis of pathophysiology the use of antioxidants in the treatment of alcoholic liver disease seems plausible, most clinical trials addressing their therapeutic effect in alcoholic liver disease have been negative.43
Induction of CYP 2E1 may also contribute to hepatocarcinogenesis by enhancing the conversion of various procarcinogens to eventual carcinogens such as dimethylnitrosamines (DMN), AFB1, vinyl chloride, and dimethylhydrazine, as previously mentioned above.44 In particular, the metabolic interaction between ethanol and nitrosamines has been investigated.45 Alcohol induces low Km-DMN demethylase activity and CYP 2E1 which both lead to increased activation of this carcinogen in animals and humans.46,47 However, ethanol is also an effective competitive inhibitor of DMN demethylase when administered simultaneously with DMN. The capacity of ethanol to both induce and inhibit DMN mediated hepatocarcinogenesis is strongly dependent on the presence or absence of alcohol at the time of carcinogen exposure. This phenomenon explains why some animal studies in which alcohol/nitrosamine interactions had been investigated have shown an increase in DMN induced hepatoma17,48 and others have not.49,50
Recently, polymorphisms of CYP 2E1 were identified and Yu and colleagues51 have suggested an association with hepatocarcinogenesis based on a higher prevalence (83.3% v 63.3%) of the CYP 2E1 c1/c1 genotype in patients with HCC compared with controls. Homozygosity of this genotype was associated with a significantly increased risk for the development of HCC in smokers. However, other investigators could not confirm the association of certain CYP 2E1 polymorphisms with the risk of HCC.52,53
TNF-α and intracellular signal transduction
A major feature in the pathogenesis of ALD is release of tumour necrosis factor α (TNF-α) and other cytokines, mainly from Kupffer cells that are stimulated by endotoxin absorbed from the gut.54 In fact, elevated TNF-α levels and corresponding cytokines are a prominent feature of ALD compared with other liver diseases, finally resulting in hepatocyte proliferation or death, recruitment of inflammatory cells, and tissue remodelling.54,55 TNF-α binds to its cellular receptors on hepatocytes and other liver cells leading to activation of various adaptor proteins and potentially to apoptosis via the caspase cascade. Thus TNF-α can trigger jun-N terminal kinase 1 which cooperates with other mitogens such as epidermal growth factor to promote proliferation.56 On the other hand, activation of sphingomyelinase by TNF-α increases intracellular ceramide which inhibits the mitochondrial electron transport chain. Thereby, mitochondrial production of ROS is increased promoting lipid peroxidation and apoptosis independently of caspases. In addition, ROS, such as the superoxide anion as well as cytochrome C oxidase, are released from damaged mitochondria via activation of caspases 8 and 3 leading to apoptosis. However, increased levels of ROS also contribute to activation of the oxidative stress sensitive transcription factor nuclear factor κB. Nuclear factor κB is pivotal for initiation of a cell survival machinery involving antiapoptotic proteins such as Bcl-2, manganese superoxide dismutase, and nitric oxide synthase that protect the mitochondrial membrane potential.57 Interestingly, experiments in mice that lack the signal transducing type I TNF receptor have demonstrated impaired liver regeneration after partial hepatectomy.58 In summary, TNF-α may dose dependently lead to activation of cellular survival mechanisms, or to apoptosis and necrosis. This can explain why hepatocytes that are challenged by an inflammatory insult which is below the level leading to cell death may be more susceptible to proliferative stimuli and to dedifferentiation triggered by carcinogens such as AA and nitrosamines. Thus ethanol induced activation of nuclear factor κB could contribute to hepatocarcinogenesis.
Interactions with retinoids
Reduced serum and hepatic vitamin A concentrations have been shown in chronic alcoholics.59 This is of particular importance as retinoic acid (RA) is synthesised from retinol via various enzymatic steps involving microsomal and cytosolic retinol dehydrogenases, as well as via cytosolic ADH and ALDH. RA has profound effects on cellular growth and differentiation via two families of RA nuclear receptors (RAR-α, -β, and -γ, and RXR-α, -β, and -γ) which mediate RA induced gene transcription.60 In a series of experiments, the effects of alcohol on retinol and RA metabolism, on transcellular RA signalling, and on early events of carcinogenesis have been investigated. Chronic alcohol consumption affects several aspects of vitamin A metabolism, including retinol absorption, enhanced degradation in the liver, and increased mobilisation of retinol from the liver to other organs.61,62 These ethanol induced changes may result in decreased hepatic concentrations of both retinol and retinyl esters which are the metabolically active precursors of RA.
“Chronic alcohol consumption affects several aspects of vitamin A metabolism, including retinol absorption, enhanced degradation in the liver, and increased mobilisation of retinol from the liver to other organs”
Furthermore, it has been demonstrated that ethanol acts as a competitive inhibitor of retinol oxidation in the liver, thereby counteracting the biosynthesis of RA.63 Accordingly, RA levels in the liver of ethanol fed rats were decreased significantly compared with controls pair fed an isocaloric control diet containing equal amounts of vitamin A.64 It has recently been shown that ethanol causes an additional local deficiency of RA in the liver, resulting from enhanced RA catabolism due to induction of CYP 2E1.65 In the same study, treatment of ethanol fed rats with chlormethiazole, a specific CYP 2E1 inhibitor, restored both hepatic and plasma RA concentrations to normal levels. Enhancement of RA catabolism by ethanol in vitro was inhibited by CYP 2E1 antibodies and chlormethiazole, while catabolism of RA into polar metabolites was abolished completely by non-specific cytochrome P450 inhibitors. Lastly, chronic alcohol consumption resulted in a functional downregulation of RA receptors and an up to eightfold expression of the AP-1 (c-jun and c-fos) transcriptional complex.63 This explains parenchymal hyperproliferation as AP-1 is a central complex downstream of various growth factors, oncogenes, and tumour promoters.66 Most interestingly, supplementation of animals with all-trans-RA to normal RA levels not only leads to a decrease in AP-1 (c-jun and c-fos) gene expression but also to normalisation of hepatic proliferation, as expressed by proliferating cell nuclear antigen expression.65 In summary, these data suggest that low hepatic RA levels due to chronic alcohol abuse may favour proliferation and malignant transformation of hepatocytes via upregulation of AP-1 (c-jun and c-fos) gene expression.
Alcohol and methylation
Hepatocarcinogenesis is a multistep process involving genetic events such as point mutations, as well as epigenetic factors, particularly aberrant DNA methylation patterns and post-transcriptional alterations. Changes in the degree of methylation of cytosine are frequently encountered in human cancers but their relevance as an epigenetic factor in carcinogenesis is only partially understood.67 However, DNA methylation is an important determinant in controlling gene expression whereby hypermethylation has a silencing effect on genes and hypomethylation may lead to increased gene expression. In hepatocarcinogenesis, general hypomethylation may be coupled with areas of regional hypermethylation. Thus hypermethylation of tumour suppressor genes can result in decreased gene transcription of p53 and HIC-1,68 and hypomethylation of certain oncogenes such as c-myc and c-N-ras may lead to dedifferentiation and proliferation.69,70 Recently, it has been suggested that aberrant DNA hypermethylation may be associated with genetic instability, as determined by loss of heterozygosity and microsatellite instability in human HCC due to chronic viral hepatitis.71,72 Iwata et al detected hypermethylation of the 14-3-3 sigma gene which has been implicated as a key inducer of cell cycle arrest associated with p53 in 89% of investigated human HCCs.73 However, genetic alterations in animal models and human hepatocarcinogenesis differ substantially. Thus it was shown that activation of N-myc and c-myc oncogenes is frequent in woodchuck hepatitis virus associated HCC while no p53 mutations were found. This mutational pattern is reversed in humans where p53 are frequent and oncogene activation seems to play only a minor role.16,74
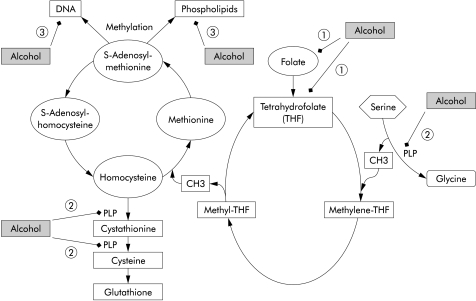
Importantly, modifications of the degree of hepatic DNA methylation have also been observed in experimental models of chronic alcoholism.75,76 Hypomethylation is a plausible consequence of metabolic alterations in the setting of ethanol consumption. In fact, alcohol has a marked impact on hepatic methylation capacity, as reflected by decreased levels of S-adenosylmethionine (SAM), an important methyl group donor, and increased levels of S-adenosylhomocystein (SAH), resulting in an up to 2.5-fold decrease in the SAM/SAH ratio.77–79 Several mechanisms have been suggested by which ethanol could interact with one carbon metabolism and DNA methylation and thereby enhance carcinogenesis (fig 2 ▶):
Figure 2.
Interaction of alcohol with methyl transfer. Alcohol impairs one carbon metabolism via interfering with (1) folate uptake and generation of tetrahydrofolate (THF); (2) degradation of pyridoxal-5`-phosphate (PLP) at several sites; and (3) inhibition of methyl transfer to DNA via inhibition of methyltransferase, resulting in hypomethylation and consequently enhanced transcription of certain oncogenes.
chronic alcohol interacts with intake, absorption, and subsequent metabolism of B vitamins involved in hepatic transmethylation reactions, namely folate and pyridoxal-5`-phosphate (vitamin B6), resulting in impaired methyl group synthesis and transfer79–83;
ethanol reduces the activity of methionine synthase which remethylates homocysteine to methionine with methyltetrahydrofolate as the methyl donor84,85;
chronic alcohol consumption decreases glutathione levels, a reductive tripeptide, which is synthesised from homocysteine via transsulphuration in the liver, and thereby enhances the susceptibility of the liver towards alcohol related peroxidative damage85,86; and
alcohol can inhibit the activity of DNA methylase which transfers methyl groups to DNA in rats,77 a finding which could not be confirmed in humans.87
To date, it is well established that dietary depletion of lipotropes, including methionine, choline, betaine, SAM, and folate, leads to DNA hypomethylation, particularly hypomethylation of oncogenes (that is, c-Ha-ras, c-Ki-ras, and c-fos) and to DNA strand breaks, all of which are associated with an increased incidence of HCC in rats.88,89 Whether chronic alcohol consumption alone is capable of inducing a lack of methylation capacity sufficient to cause hypomethylation of DNA and genes involved in hepatocarcinogenesis is not yet known.
It has been shown that DNA and site specific hypomethylation is reversible, either spontaneously90,91 or by therapeutic intervention.16 In a randomised, controlled, multicentre trial, 123 alcoholic cirrhotics received SAM or placebo for two years.92 The two groups were well matched and only six patients were lost during follow up. Mortality and the number of patients requiring liver transplantation were significantly lower in patients with Child C cirrhosis treated with SAM. However, no patient in the trial developed HCC, probably due to the short duration of surveillance. So far, a study investigating the chemopreventive effect of lipotropes—that is, SAM—in HCC has not been performed. Therefore, the role of ethanol in gene specific methylation requires further investigation.
Alcohol and hepatitis viruses
From epidemiological studies a close relationship has been noted between alcohol consumption, infection with hepatotropic viruses, and HCC. With respect to HBV, several studies have shown a high prevalence of HBV markers in patients with alcohol related HCC.93 Brechot et al screened HCC liver specimens of alcoholics for HBV-DNA to find that they were all positive.94 However, other investigators failed to confirm these data.95,96 Thus the role of alcohol and chronic HBV infection in hepatocarcinogenesis awaits further clarification.
“A close relationship has been noted between alcohol consumption, infection with hepatotropic viruses, and HCC”
In the case of chronic HCV, the role of alcohol abuse remains undisputed. A number of studies have demonstrated a high prevalence of antibodies to HCV among alcoholics with liver disease, ranging from 11% to 46%, even after confirmatory antibody tests or polymerase chain reaction were used, and after patients at risk (for example, recipients of blood transfusions and intravenous drug abusers) were excluded.97 With regard to hepatocarcinogenesis, it has been shown unambiguously by various investigators that alcohol abuse coupled with HCV infection accelerates the development of HCC.98,99 For example, Yamauchi and colleagues98 showed that the cumulative incidence of HCC after three, five, and 10 years in cirrhotic HCV infected patients with an average daily alcohol consumption of 120 g was 13.3%, 41.3%, and 80.7% versus 0%, 8.3%, and 18.5%, respectively, compared with alcoholic cirrhotics without HCV infection. A case control study by Corrao et al in 115 patients with alcoholic liver disease and chronic HCV demonstrated a clear dose dependency between long term alcohol consumption and the development of cirrhosis, a necessary precondition of HCC development in chronic HCV.100 The authors concluded that as little as 20 g/day was detrimental. While the modes of interaction between HCV and alcohol remain to be defined, there are a number of possible explanations.
Chronic alcohol consumption and coinfection with HCV synergistically aggravate histological damage resulting in faster progression.98,101,102
Alcohol appears to enhance HCV replication with subsequent direct cytopathic damage.103,104
Alcohol may compromise the host's immune response to HCV infection, as demonstrated by Oshita and colleagues103 who measured serum neopterin levels, a suggested indirect marker of macrophage activation. Thus in heavy drinkers coinfected with HCV, neopterin levels were significantly lower than in non-alcoholic HCV infected individuals. In addition, CD4 cells, which are important in the antiviral immune response, are particularly susceptible to alcohol related functional impairment.105
Alcoholic patients with chronic hepatitis C show higher hepatic iron levels than patients with HCV infection alone and iron excess is an important factor in liver damage and may increase HCV replication.106
Alcohol and immune surveillance
Chronic alcohol consumption results in a complex alteration of the unspecific (innate) and specific (acquired) immune response.107 Numerous studies and clinical experience have shown that chronic alcoholics are more susceptible to infections and to certain neoplasms.108 Thus alcohol related alterations of immune surveillance could contribute to the development of cancer. Among the factors affecting the immune system in the setting of alcoholism are malnutrition, vitamin deficiencies, established cirrhosis, and alcohol itself. In this respect, the influence of alcohol on natural killer (NK) cells, which are implicated in the control of tumour development and growth, is of particular importance. Interactions between alcohol and this subset of cytotoxic cells have been investigated in cell culture, animal studies, and in human alcoholics. However, the data are conflicting which is mainly due to discrepancies in analysis of lymphoid subsets and NK cell cytotoxic activity, the presence or absence of active alcohol consumption, biased patient selection, and different nutritional status and comorbidity variables, such as coinfection with hepatitis viruses.109
“Chronic alcoholics are more susceptible to infections and to certain neoplasms”
Studies in mice have demonstrated that chronic alcohol administration inhibits NK cell activity110 and reduces NK cell number and lytic activity following a single binge equivalent of alcohol.111 A more recent study in rats has shown that acute alcohol intoxication may lead to an up to 10-fold increase in the number of lung metastases of the NK cell controlled adenocarcinoma cell line MADB106.112 Few studies in humans have so far been performed. In a study by Laso et al, alcoholic cirrhotics revealed both diminished NK cell numbers and reduced lytic activity, even when stimulated by interleukin 2, a powerful NK cell stimulating cytokine.113 NK cell numbers were also found to be decreased in actively drinking individuals without established alcoholic liver disease.114 Pathomechanisms are not fully understood but it has been suggested that transforming growth factor β1, which is a key profibrogenic cytokine in liver fibrogenesis and which is markedly elevated in alcoholic liver disease,115 suppresses immune function in general and NK cell activity in particular.116 However, there are no data on how far antigen specific lymphocyte reactivity, HLA class I and II expression, or organ specific lymphocyte subsets are altered in alcoholism.
In summary, a major impact of alcohol on the immune system is undisputed which may favour tumour development and expansion but mechanisms by which alcohol compromises antitumour immune surveillance are not yet completely understood.
Abbreviations
AA, acetaldehyde
ADH, alcohol dehydrogenase
AFB1
aflatoxin B1
ALD, alcoholic liver disease
ALDH, aldehyde dehydrogenase
DMN, dimethyl-nitrosamine
CYP 2E1, cytochrome P450 2E1
HBV, hepatitis B virus
HCC, hepatocellular carcinoma
HCV, hepatitis C virus
MB, Mallory body
NK, natural killer
RA, retinoic acid
ROS, reactive oxygen species
SAH, S-adenosylhomocysteine
SAM, S-adenosylmethionine
TNF-α, tumour necrosis factor α
REFERENCES
- 1.El-Serag HB, Mason AC. Risk factors for the rising rates of primary liver cancer in the United States. Arch Intern Med 2000;160:3227–30 [DOI] [PubMed] [Google Scholar]
- 2.Muir C, Waterhouse J, Mack T, et al. Cancer incidence in five continents. In: World Health Organization. New York: IARC Scientific Publications 1987:5:88.
- 3.Becker N, Wahrendorf J. Atlas of cancer mortality in the Federal Republic of Germany. Berlin: Springer, 1998.
- 4.Taylor-Robinson SD, Foster GR, Arora S, et al. Increase in primary liver cancer in the UK, 1979–94. Lancet 1997;350:1142–3. [DOI] [PubMed] [Google Scholar]
- 5.Oka H, Kurioka N, Kim K, et al. Prospective study of early detection of hepatocellular carcinoma in patients with cirrhosis. Hepatology 1990;12:680–7. [DOI] [PubMed] [Google Scholar]
- 6.Simonetti RG, Liberati A, Angiolini C, et al. Treatment of hepatocellular carcinoma: a systematic review of randomized controlled trials. Ann Oncol 1997;8:117–36. [DOI] [PubMed] [Google Scholar]
- 7.Caselmann WH, Alt M. Hepatitis C virus infection as a major risk factor for hepatocellular carcinoma. J Hepatol 1996;24:61–6. [PubMed] [Google Scholar]
- 8.Seitz HK, Poeschl G, Simanowski UA. Alcohol and cancer. In: Galanter M, ed. Recent developments in alcoholism. New York: Plenum Press, 1998:67–95. [DOI] [PubMed]
- 9.Kuper H, Tzonou A, Kaklamani E, et al. Tobacco smoking, alcohol consumption and their interaction in the causation of hepatocellular carcinoma. Int J Cancer 2000;85:498–502. [PubMed] [Google Scholar]
- 10.Ohnishi K. Alcohol and hepatocellular carcinoma. In: Watson RR, ed. Alcohol and cancer. Boca Raton: CRC Press, 1992:179–202.
- 11.Prior P. Long-term cancer risk in alcoholism. Alcohol Alcohol 1988;23:163–71. [PubMed] [Google Scholar]
- 12.Porta EA, Markell N, Dorado RD. Chronic alcohol enhances hepatocarcinogenicity of diethylnitrosamine in rats fed a marginally methyl-deficient diet. Hepatology 1985;5:1120–5. [DOI] [PubMed] [Google Scholar]
- 13.Wainfan E, Poirier LA. Methyl groups in carcinogenesis: effects on DNA methylation and gene expression. Cancer Res 1992;52(suppl 7):2071–7. [PubMed] [Google Scholar]
- 14.Takada A, Neii J, Takase S, et al. Effects of ethanol on experimental hepatocarcinogenesis. Hepatology 1986;6:65–72. [DOI] [PubMed] [Google Scholar]
- 15.Lieber CS, DeCarli LM, Sorrell MF. Experimental methods of ethanol administration. Hepatology 1989;10:501–10. [DOI] [PubMed] [Google Scholar]
- 16.Pascale RM, Simile MM, Feo F. Genomic abnormalities in hepatocarcinogenesis. Implications for a chemopreventive strategy. Anticancer Res 1993;13:1341–56. [PubMed] [Google Scholar]
- 17.Tsutsumi M, Matsuda Y, Takada A. Role of ethanol-inducible cytochrome P-450 2E1 in the developments of hepatocellular carcinoma by the chemical carcinogen, N-nitrosodimethylamine. Hepatology 1993;18:1483–9. [PubMed] [Google Scholar]
- 18.Nakanuma Y, Ohta G. Is Mallory body formation a preneoplastic change? A study of 181 cases of liver bearing hepatocellular carcinoma and 82 cases of cirrhosis. Cancer 1985;55:2400–5. [DOI] [PubMed] [Google Scholar]
- 19.Smith P, Tee LBG, Yeoh GCT. Appearance of oval cells in the liver of rats after long-term exposure to ethanol. Hepatology 1996;23:145–54. [DOI] [PubMed] [Google Scholar]
- 20.Shinozuka H, Lombardi B, Sell S, et al. Early histological and functional alterations of ethionine liver carcinogenesis in rats fed a choline-deficient diet. Cancer Res 1978;38:1092–98. [PubMed] [Google Scholar]
- 21.Lowes KN, Brennan BA, Yeoh GC, et al. Oval cell numbers in human chronic liver diseases are directly related to disease severity. Am J Pathol 1999;154:537–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Craddock VM. Metabolism of ethanol and higher alcohols present in alcoholic drinks and their corresponding aldehydes in subcellular components of rat esophageal mucosa, and relevance for esophageal cancer in man. In: Palmer TN, ed. Alcoholism: a molecular perspective. New York: Plenum Press, 1991:297–301.
- 23.Aguilar F, Hussain SP, Cerutti P. Aflatoxin B1 induces the transversion of G→T in codon 249 of the p53 tumor suppressor gene in human hepatocytes. Proc Natl Acad Sci USA 1993;90:8586–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bulatao-Jayme J, Almero EM, Castro CA, et al. A case-control dietary study of primary liver cancer risk from aflatoxin exposure. Int J Epidemiol 1982;11:112–19. [DOI] [PubMed] [Google Scholar]
- 25.Tamburro CH, Lee HM. Primary hepatic cancer in alcoholics. Clin Gastroenterol 1981;10:457–77. [PubMed] [Google Scholar]
- 26.Fang JL, Vaca CE. Develpoment of a 32P-postlabelling method for the analysis of adducts arising through the reaction of acetaldehyde with 2`-deoxyguanosin-3`-monophosphate and DNA. Carcinogenesis 1995;16:2177–85. [DOI] [PubMed] [Google Scholar]
- 27.Garro AJ, Espina N, Farinati F, et al. The effect of chronic ethanol consumption on carcinogen metabolism and on O6-methylguanine transferase-mediated repair of alkylated DNA. Alcohol Clin Exp Res 1986;10(suppl):73–7. [DOI] [PubMed] [Google Scholar]
- 28.Obe G, Ristow H. Mutagenic, carcinogenic and teratogenic effects of alcohol. Mutat Res 1979;65:229–59. [DOI] [PubMed] [Google Scholar]
- 29.International Agency for Research on Cancer. Working Group on the evaluation of the carcinogenic risk of chemicals to humans: Acetaldehyde. IARC Monographs 1985;36:101–32. [PubMed] [Google Scholar]
- 30.Thomasson HR, Crabb DW, Edenberg HJ, et al. Alcohol and aldehyde dehydrogenase polymorphism and alcoholism. Behav Gen 1993;23:131–6. [DOI] [PubMed] [Google Scholar]
- 31.Bosron WF, Li TK. Catalytic properties of human liver alcohol dehydrogenase isoenzymes. Enzyme 1987;37:19–28. [DOI] [PubMed] [Google Scholar]
- 32.Borras E, Coutelle C, Rosell A, et al. Genetic polymorphism of alcohol dehydrogenase in Europeans: the ADH2*2 allel decreases the risk of alcoholism and is associated with ADH3*1. Hepatology 2000;31:984–9. [DOI] [PubMed] [Google Scholar]
- 33.Takeshita T, Yang X, Inoue Y, et al. Relationship between alcohol drinking, ADH2 and ALDH2 genotypes, and risk for hepatocellular carcinoma in Japanese. Cancer Lett 2000;149:69–76. [DOI] [PubMed] [Google Scholar]
- 34.Koop DR, Crump BL, Nordblom GD, et al. Immunochemical evidence for induction of the alcohol-oxidizing cytochrome P450 of rabbit liver microsomes by diverse agents: ethanol, imidazole, trichloroethylene, acetone, pyrazole and isoniazid. Proc Natl Acad Sci USA 1985;82:4065–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Albano E, Clot P, Morimoto M, et al. Role of cytochrome P4502E1-dpendent formation of hydroxyethyl free radical in the develpoment of liver damage in rats intragastrically fed with ethanol. Hepatology 1996;23:155–63. [DOI] [PubMed] [Google Scholar]
- 36.French SW, Albano E, Hagbjörk AL, et al. Effect of isoniazid (INH) on ethanol-induced lipid peroxiadtion and serum protein adduct formation. Hepatology 1992;16:534A. [Google Scholar]
- 37.Gouillon Z, Lucas D, Hagbjork AL, et al. Inhibition of ethanol-induced liver disease in the intragastric feeding rat model by chlormethiazole. Proc Soc Exp Biol Med 2000;224:302–8. [DOI] [PubMed] [Google Scholar]
- 38.Gebhardt AC, Lucas D, Menez JF, et al. Chlormethiazole inhibition of cytochrome P450 2E1 as assessed by chlorzoxazone hydroxylation in humans. Hepatology 1997;26:957–61. [DOI] [PubMed] [Google Scholar]
- 39.Nair J, Sone H, Nagao M, et al. Copper dependent formation of miscoding etheno-DNA adducts in the liver of Long Evans Cinnamon (LEC) rats developping hereditary hepatitis and hepatocellular carcinoma. Cancer Res 1996;56:1267–71. [PubMed] [Google Scholar]
- 40.Tsukamoto H, Horne W, Kamimura S, et al. Experimental liver cirrhosis induced by alcohol and iron. J Clin Invest 1995;98:620–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seitz HK, Suter P. Ethanol toxicity and nutritional status. In: Kotsonis FM, McKey M, eds. Nutritional toxicology. London: Taylor and Francis, 2001:122–54.
- 42.Seitz HK, Arslic T. Treatment of alcoholic liver disease. In: Agarwal DP, Seitz HK, eds. Alcohol in health and disease. New York: Marcel Dekker, 2001:453–72.
- 43.Seitz HK, Pöschl G. Antioxidant drugs and colchicine in the treatments of alcoholic liver disease. In: Arroyo V, Bosch J, Rodes J, eds. Treatments in hepatology. Barcelona: Masson SA, 1995:271–6.
- 44.Seitz HK, Osswald BR. Effect of ethanol on procarcinogen activation. In: Watson RR, ed. Alcohol and the gastrointestinal tract. Boca Raton: CRC Press, 1992:55–72.
- 45.Anderson LM. Modulation of nitrosamine metabolism by ethanol: implications of cancer risk. In: Watson RR, ed. Alcohol and cancer. Boca Raton: CRC Press, 1992:17–54.
- 46.Garro AJ, Seitz HK, Lieber CS. Enhancement of dimethylnitrosamine metabolism and activation to a mutagen following chronic ethanol consumption in the rat. Cancer Res 1981;41:120–4. [PubMed] [Google Scholar]
- 47.Amelizad S, Appel KE, Schoepke M, et al. Enhanced dimethylase and dinitrosation on N-nitrosodimethylamine by human liver microsomes from alcoholics. Cancer Lett 1989;46:43–8. [DOI] [PubMed] [Google Scholar]
- 48.Driver HE, McLean AEM. Dose-response relationship for initiation of rat liver tumors by diethylnitrosamine and promotion by phenobarbitone or alcohol. Food Chem Toxicol 1986;24:241–5. [DOI] [PubMed] [Google Scholar]
- 49.Teschke R, Minzlaff M, Oldiges H, et al. Effect of chronic ethanol consumption on tumor incidence due to dimethylnitrosamine administration. J Cancer Res Clin Oncol 1983;106:58–64. [DOI] [PubMed] [Google Scholar]
- 50.Griciute L, Castegnaro M, Bereziat JC. Influence of ethyl alcohol on carcinogenesis induced by volatile N-nitrosamines detected in alcoholic beverages. IARC Sci Publ 1987;84:264–8. [PubMed] [Google Scholar]
- 51.Yu MW, Gladek-Yarborough A, Chiamprasert S, et al. Cytochrome P450 2E1 and glutathione S-transferase M1 polymorphisms and susceptibility to hepatocellular carcinoma. Gastroenterology 1995;109:1266–73. [DOI] [PubMed] [Google Scholar]
- 52.Lee HS, Yoon JH, Kamimura S, et al. Lack of association of cytochrome P4502E1 genetic polymorphism with the risk of human hepatocellular carcinoma. Int J Cancer 1997;71:737–40. [DOI] [PubMed] [Google Scholar]
- 53.Wong NA, Rae F, Simpson KJ, et al. Genetic polymorphism of cytochrome P450 2E1 and susceptibility to alcoholic liver disease and hepatocellular carcinoma in a white population: a study and literature review, including meta-analysis. Mol Pathol 2000;53:88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Urbaschek R, McCuskey RS, Rudi V, et al. Endotoxin, endotoxin-neutralizing-capacitiy, sCD14, sICAM, and cytokines in patients with various degrees of alcoholic liver disease. Alcohol Clin Exp Res 2001;25:261–7. [PubMed] [Google Scholar]
- 55.Khoruts A, Stahnke L, McClain CI, et al. Circulating tumor necrosis factor, interleukin-1 and interleukin-6 concentrations in chronic alcoholic patients. Hepatology 1991;13:267–76. [PubMed] [Google Scholar]
- 56.Sheron N, Bird G, Koskinas J, et al. Circulating and tissue levels of neutrophil chemotaxin interleukin-8 are elevated in severe alcoholic hepatitis, and tissue levels correlate with neutrophil infiltration. Hepatology 1993;18:41–6. [PubMed] [Google Scholar]
- 57.Tilg H, Diehl AM. Cytokines in alcoholic and nonalcoholic steatohepatitis. N Engl J Med 2000;343:1467–76. [DOI] [PubMed] [Google Scholar]
- 58.Yamada Y, Kirillova I, Peschon JJ, et al. Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proc Natl Acad Sci USA 1997;94:1441–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leo MA, Lieber CS. Hepatic vitamin A depletion in alcoholic liver injury. N Engl J Med 1982;304:597–600. [DOI] [PubMed] [Google Scholar]
- 60.Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J 1996;10:940–53. [PubMed] [Google Scholar]
- 61.Seitz HK. Alcohol and retinoid metabolism. Gut 2000;47;748–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leo MA, Lieber CS. Alcohol, vitamin A, and beta-carotene: adverse interactions including hepatotoxicity and carcinogenensis. Am J Clin Nutr 1999;69:1071–85. [DOI] [PubMed] [Google Scholar]
- 63.Wang XD, Liu C, Jayong C, et al. Chronic alcohol intake reduces retinoic acid concentration and enhances AP-1 (c-jun and c-fos) expression in rat liver. Hepatology 1998;28:744–50. [DOI] [PubMed] [Google Scholar]
- 64.Liu C, Russell RM, Seitz HK, et al. Ethanol enhances retinoic acid metabolism into polar metabolites in rat liver via induction of cytochrome P4502E1. Gastroenterology 2001;120:179–89. [DOI] [PubMed] [Google Scholar]
- 65.Chung JY, Liu C, Smith D, et al. Restoration of retinoic acid concentration suppresses ethanol-enhanced c-jun expression and hepatocyte proliferation in rat liver. Carcinogenesis 2001;22:1213–19. [DOI] [PubMed] [Google Scholar]
- 66.Chiu R, Boyle WJ, Meek J, et al. The c-fos protein interacts with c-jun/AP-1 to stimulate transcription of AP-1 responsive genes. Cell 1988;54:541–52. [DOI] [PubMed] [Google Scholar]
- 67.Counts JL, Goodman JI. Alterations in DNA methylation may play a variety of roles in carcinogenesis. Cell 1995;83:13–15. [DOI] [PubMed] [Google Scholar]
- 68.Kanai Y, Hui AM, Sun L, et al. DNA hypermethylation at the D17S5 locus and reduced HIC-1 mRNA expression are associated with hepatocarcinogenesis. Hepatology 1999;29:703–9. [DOI] [PubMed] [Google Scholar]
- 69.Wainfan E, Dizik M, Stender M, et al. Rapid appearance of of hypomethylated DNA in livers of rats fed cancer-promoting methyl-defient diets. Cancer Res 1989;49:4094–7. [PubMed] [Google Scholar]
- 70.Shen L, Fang J, Qui D, et al. Correlation between DNA methylation and pathological changes in human hepatocellular carcinoma. Hepatogastroenterology 1998;45:1753–9. [PubMed] [Google Scholar]
- 71.Kondo Y, Kanai Y, Sakamoto M, et al. Genetic instability and aberrant DNA methylation in chronic hepatitis and cirrhosis—A comprehensive study of loss of heterozygosity and microsatellite instability at 39 loci and DNA hypermethylation on CpG islands in microdissected specimens from patients with HCC. Hepatology 2000;32:970–9. [DOI] [PubMed] [Google Scholar]
- 72.Kanai Y, Ushijima S, Tsuda H, et al. Aberrant methylation precedes loss of heterozygosity on chromosome 16 in chronic hepatitis and liver cirrhosis. Cancer Lett 2000;148:73–80. [DOI] [PubMed] [Google Scholar]
- 73.Iwata N, Yamamoto H, Sasaki S, et al. Frequent hypermethylation of CpG islands and loss of expression of the 14–3–3 sigma gene in human hepatocellular carcinoma. Oncogene 2000;19:5298–302. [DOI] [PubMed] [Google Scholar]
- 74.Hui AM, Makuuchi M. Molecular basis of multistep hepatocarcinogenesis: genetic and epigenetic events. Scand J Gastroenterol 1999;8:737–42. [DOI] [PubMed] [Google Scholar]
- 75.Garro AJ, McBeth DL, Lima V, et al. Ethanol consumption inhibits fetal DNA methylation in mice: implications for the fetal alcohol syndrome. Alcohol Clin Exp Res 1991;15:395–8 [DOI] [PubMed] [Google Scholar]
- 76.Choi SW, Stickel F, Baik HW, et al. Chronic alcohol consumption induces genomic but not p53-specific DNA hypomethylation in rat colon. J Nutr 1999;129:1945–50. [DOI] [PubMed] [Google Scholar]
- 77.Lieber CS, Casini A, DeCarli L, et al. S-adenosyl-L-methionine attenuates alcohol-induced liver injury in the baboon. Hepatology 1990;11:165–72. [DOI] [PubMed] [Google Scholar]
- 78.Trimble KC, Molloy AM, Scott JM, et al. The effect of ethanol on one-carbon metabolism: increased methionine catabolism and methyl-group wastage. Hepatology 1993;18:984–9. [DOI] [PubMed] [Google Scholar]
- 79.Stickel F, Choi SW, Kim YI, et al. Effect of chronic alcohol consumption on total plasma homocysteine levels in rats. Alcohol Clin Exp Res 2000;24:259–64. [PubMed] [Google Scholar]
- 80.Lumeng L, Li TK. Vitamin B6 metbolism in chronic alcohol abuse. J Clin Invest 1974;53:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Labadarios D, Rossouw JE, McConnell JB, et al. Vitamin B6 deficiency in chronic liver disease—evidence for decreased degradation of pyridoxal-5`-phosphate. Gut 1977;18:23–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Savage D, Lindenbaum J. Anemia in alcoholics. Medicine 1986;65:322–8. [DOI] [PubMed] [Google Scholar]
- 83.Gloria L, Cravo M, Camilo ME, et al. Nutritional deficiencies in chronic alcoholics: relation to dietary intake and alcohol consumption. Am J Gastroenterol 1997;92:485–9. [PubMed] [Google Scholar]
- 84.Barak AJ, Beckenhauer HC, Hidiroglu N, et al. The relationship of ethanol feeding to the methyl folate trap. Alcohol 1993;10:495–7. [DOI] [PubMed] [Google Scholar]
- 85.Lieber CS. Alcohol and the liver: 1994 update. Gastroenterology 1994;106:1085–105. [DOI] [PubMed] [Google Scholar]
- 86.Speisky H, MacDonald A, Giles G, et al. Increased loss and decreased synthesis of hepatic glutathione after acute ethanol administration. Biochem J 1985;225:565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Miyakawa H, Liu J, Noguchi O, et al. Effect of alcohol drinking on gene expression of hepatic O6-methylguanine DNA methyltransferase in chronic liver diseases. Alcohol Clin Exp Res 1996;20(suppl):297–300. [PubMed] [Google Scholar]
- 88.Zapisek WF, Cronin GM, Lyn-Cook BD, et al. The onset of oncogene hypomethylation in the livers of rats fed methyl-deficient, amino acid-defined diets. Carcinogenesis 1992;13:169–72. [DOI] [PubMed] [Google Scholar]
- 89.Pogribny IP, Basnakian AG, Miller BJ, et al. Breaks in genomic DNA and within the p53 gene are associated with hypomethylation in livers of folate/methyl-deficient rats. Cancer Res 1995;55:1894–901. [PubMed] [Google Scholar]
- 90.Ramchandani S, Bhattacharya SK, Cervoni N, et al. DNA methylation is a reversible biological signal. Proc Natl Acad Sci USA 1999;96:6107–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Christman JK, Sheiknejad G, Dizik M, et al. Reversibility of changes in nucleic acid methylation and gene expression induced in rat liver by severe dietary methyl deficiency. Carcinogenesis 1993;14:551–7. [DOI] [PubMed] [Google Scholar]
- 92.Mato JM, Camara J, Fernandez de Paz J, et al. S-adenosylmethionine in alcoholic liver cirrhosis: a randomized, placebo-controlled, double-blind, multicenter trial. J Hepatol 1999;30:1081–9. [DOI] [PubMed] [Google Scholar]
- 93.Inoue H, Seitz HK. Viruses and alcohol in the pathogenesis of primary hepatic carcinoma. Eur J Cancer Prev 2001;10:1–4. [DOI] [PubMed] [Google Scholar]
- 94.Brechot C, Nalpas B, Courouce AM, et al. Evidence that hepatitis B virus has a role in liver-cell carcinoma in alcoholic liver disease. N Engl J Med 1982;306:1384–7. [DOI] [PubMed] [Google Scholar]
- 95.Horiike N, Michika K, Onji M, et al. HBV-DNA hybridization in hepatocellular carcinoma associated with alcohol in Japan. J Med Virol 1989;28:189–92. [DOI] [PubMed] [Google Scholar]
- 96.Walter E, Blum HE, Meier KP, et al. Hepatocellular carcinoma in alcoholic liver disease: no evidence for a pathogenic role of hepatitis B virus infection. Hepatology 1988;8:745–8. [DOI] [PubMed] [Google Scholar]
- 97.Degos F. Hepatitis C and alcohol. J Hepatol 1999;31(suppl 1):113–18. [DOI] [PubMed] [Google Scholar]
- 98.Yamauchi M, Nakahara M, Maezawa Y, et al. Prevalence of hepatocellular carcinoma in patients with alcoholic cirrhosis and prior exposure to hepatitis C. Am J Gastroenterol 1993;88:39–43. [PubMed] [Google Scholar]
- 99.Miyakawa H, Sato C, Izumi N, et al. Hepatitis C virus infection in alcoholic liver cirrhosis in Japan: its contribution to the development of hepatocellular carcinoma. Alcohol Alcohol 1993;28(suppl):85–90. [DOI] [PubMed] [Google Scholar]
- 100.Corrao G, Carle F, Lepore AR, et al. Interaction between alcohol consumption and positivity for antibodies to hepatitis C virus on the risk of liver cirrhosis: a case-control study. Eur J Epidemiol 1992;8:634–9. [DOI] [PubMed] [Google Scholar]
- 101.Tanaka T, Yabusako T, Yamashita T, et al. Contribution of hepatitis C virus to the progression of alcoholic liver disease. Alcohol Clin Exp Res 2000;24(suppl 4):112–16S. [PubMed] [Google Scholar]
- 102.Pessione F, Degos F, Marcellin P, et al. Effect of alcohol consumption on serum hepatitis C virus RNA and histological lesions in chronic hepatitis C. Hepatology 1998;27:1717–22. [DOI] [PubMed] [Google Scholar]
- 103.Oshita M, Hayashi N, Kashahara A, et al. Increased serum hepatitis C RNA levels among alcoholic patients with chronic hepatitis C. Hepatology 1994;20:1115–20. [PubMed] [Google Scholar]
- 104.Cromie SL, Jenkins PJ, Bowden DS, et al. Chronic hepatitis C: effect of alcohol on hepatitic activity and viral titre. J Hepatol 1996;25:821–6. [DOI] [PubMed] [Google Scholar]
- 105.Bagasra O, Howeedy A, Dorio R, et al. Functional analysis of T-cell subsets in chronic experimental alcoholism. Immunology 1987;61:63–9. [PMC free article] [PubMed] [Google Scholar]
- 106.Izumi N, Enomoto N, Uchihara M, et al. Hepatic iron contents and response to to interferon-α in patients with chronic hepatitis C. Dig Dis Sci 1996;41:989–94. [DOI] [PubMed] [Google Scholar]
- 107.Cook RT. Alcohol abuse, alcoholism, and damage to the immune system—a review. Alcohol Clin Exp Res 1998;22:1927–42. [PubMed] [Google Scholar]
- 108.Roselle G, Mendenhall CL, Grossmann C. Effects of alcohol on immunity and cancer. In: Yirmiya R, Taylor AN eds. Alcohol, immunity and cancer. Boca Raton: CRC Press, 1993:3–22.
- 109.Szabo G. Consequences of alcohol consumption on host defence. Alcohol Alcohol 1999;34:830–41. [DOI] [PubMed] [Google Scholar]
- 110.Gallucci RM, Pfister LJ, Meadows GG. Effects of ethanol consumption on enriched natural killer cells from C57BL/6 mice. Alcohol Clin Exp Res 1994;18:625–31. [DOI] [PubMed] [Google Scholar]
- 111.Wu WJ, Wolcott RM, Pruett SB. Ethanol decreases the number and activity of splenic natural killer cells in a mouse model for binge drinking. J Pharmacol Exp Ther 1994;271:722–9. [PubMed] [Google Scholar]
- 112.Ben-Eliyahu S, Page GG, Yirmiya R, et al. Acute alcohol intoxication suppresses natural killer cell activity and promotes tumor metastasis. Nat Med 1996;2:457–60. [DOI] [PubMed] [Google Scholar]
- 113.Laso FJ, Madruga JI, Giron JA, et al. Decreased natural killer cytotoxic activity in chronic alcoholism is associated with alcoholic liver disease but not active ethanol consumption. Hepatology 1997;25:1096–100. [DOI] [PubMed] [Google Scholar]
- 114.Cook RT, Garvey MJ, Booth BM, et al. Activated CD-8 cells and HLA DR expression in alcoholics without overt liver disease. J Clin Immunol 1991;11:246–53. [DOI] [PubMed] [Google Scholar]
- 115.Stickel F, Urbaschek R, Schuppan D, et al. Serum collagen type VI and XIV, and hyaluronic acid as early indicators for altered connective tissue turnover in alcoholic liver disease. Dig Dis Sci 2001;46:2025–32. [DOI] [PubMed] [Google Scholar]
- 116.Rook AH, Kehrl JH, Wakefield LM, et al. Effects of tranforming growth factor beta on the functions of natural killer cells: depressed cytolytic activity and and blunting of interferon responsiveness. J Immunol 1986;136:3916–20. [PubMed] [Google Scholar]