Abstract
Background: Cirrhotic patients with hepatocellular carcinoma (HCC) frequently have impaired glucose metabolism.
Aims: To investigate whether impaired glucose metabolism affects the growth rate of the tumour.
Patients and methods: Tumour doubling time (DT), assessed by ultrasound imaging analysis, was measured in 60 patients with single small HCC (diameter <30 mm). DT was compared with plasma insulin and glucose concentrations following the oral glucose tolerance test (OGTT). The effect of continuous infusion of octreotide (a somatostatin analogue 200 μg/day) for three months on DT in five cases was assessed.
Results: The 60 patients were divided into two groups because the median DT was 140 days: rapid growth group (DT ≤140 days, n=30) and slow growth group (DT >140 days, n=30). Fasting plasma insulin concentration and area under the plasma insulin curve (AUCins) of the OGTT (10.4 (6.2) μU/ml and 262 (152) μU/ml/h, respectively; mean (SD)) in the rapid growth group were significantly higher than those in the slow growth group (7.6 (4.3) and 146 (140), respectively) (p=0.041 and p=0.0006, respectively). In contrast, fasting plasma glucose concentration and area under the plasma glucose curve (AUCgluc) in the rapid growth group were significantly lower than those in the slow growth group (p=0.0003 and p=0.0012, respectively). Univariate and multivariate analyses of logistic regression models demonstrated that AUCins was a significant factor contributing to the growth rate of HCC (p=0.001 and p=0.016, respectively). AUCins significantly decreased after octreotide treatment (p<0.02) but AUCgluc did not significantly change. DT after treatment increased in three of the five patients and could not be calculated in the remaining two patients because of no change in the diameter of the tumour.
Conclusions: These data suggest that postprandial hyperinsulinaemia is associated with accelerated HCC growth.
Keywords: hepatocellular carcinoma, doubling time, hyperinsulinaemia, impaired glucose metabolism, liver cancer
Hepatocellular carcinoma (HCC) is frequently accompanied by liver cirrhosis.1 Patients with liver cirrhosis often have impaired glucose metabolism,2 with 60–80% being glucose intolerant and 10–15% developing overt diabetes mellitus.2–4 Hepatogenous diabetes differs from type 2 diabetes mellitus.5 Its pathogenesis involves many factors, and hyperinsulinaemia and peripheral insulin resistance are thought to play major roles.6–8
The growth rate of a tumour is a critical factor for the prognosis of cancer patients. The growth of HCC can be measured by determining the doubling time (DT) with imaging analyses.9, 10 Several studies have surveyed clinical factors affecting DT in human HCC.9–13 However, no significant factors affecting the growth rate of HCC have yet been reported.
Insulin is known to be an important hepatotrophic factor and also stimulates proliferation of hepatoma cells.14, 15 However, it is not known whether the growth rate of HCC is affected by hyperinsulinaemia based on underlying chronic liver disease.
The aim of the present study was to clarify whether hyperinsulinaemia in patients with HCC and cirrhosis affects the growth rate of tumours. We investigated the relationship of plasma insulin concentrations following the oral glucose tolerance test (OGTT) with DT assessment by ultrasound (US) analyses. The effect of reduction of hyperinsulinaemia by octreotide,16, 17 a somatostatin analogue, on the DT of the tumour was also tested.
MATERIALS AND METHODS
Study patients
From January 1995 to October 2000, 205 patients with chronic liver disease were diagnosed as having single small HCC (diameter <30 mm) at Osaka University Hospital and Yamagata University Hospital. These patients were found by periodic US imaging screening, and the diagnosis of HCC was performed by computed tomography or magnetic resonance imaging techniques.
Growth of HCC in 72 of the above 205 patients was followed by two US examinations at intervals of more than 60 days (the intervals depended on the patients' schedules) between the day of HCC diagnosis and the day before surgery and other anticancer treatment. Some of the 72 patients wanted to delay treatment against HCC because of their business schedules. The others declined any anticancer therapy but later consented to undergo surgery and other anticancer treatment. DT of HCC in 60 of the 72 patients was calculated because the tumour margins were clearly visible in the two US examinations and the tumours were spherical or nearly spherical in shape, which is usually the case with small HCC. None of the patients had undergone any anticancer treatment or had received antidiabetic drugs or insulin before DT assessment.
Median age of the 60 patients (50 males and 10 females) with single small HCC, for whom DT was calculated, was 64 years (range 49–81). Hepatitis B surface antigen by radioimmunoassay (Abbott Laboratories, North Chicago, Illinois, USA) was detected in eight patients. Antibody to hepatitis C virus was detected in 52 patients by a second generation enzyme linked immunosorbent assay test (Abbott Laboratories). The diagnosis of HCC was confirmed histologically by examination of surgically resected tumour tissues from 35 patients or US guided needle biopsy specimens in 25. The histology of HCC was well differentiated in 27 patients and moderately/poorly differentiated in 33 patients. A diagnosis of chronic hepatitis or liver cirrhosis was carried out histologically.
Thirty five of the 60 patients underwent surgical treatment while 20 of the 60 patients were treated with percutaneous ethanol injection or microwave coagulation therapy with or without a combination of transcatheter arterial embolisation. The remaining five patients were treated with octreotide because of poor liver function reserve. DT during octreotide treatment was determined at intervals during treatment, and OGTT was also carried out at the end of treatment in five patients.
This study protocol was approved by Osaka University Medical School Ethical Committee and Yamagata University School of Medicine Ethical Committee.
Measurement of DT
For practical comparison of tumour sizes, the diameter (r) used was that for a circular tumour in the two dimensional image or an equivalent value ((a×b) 1/2), where a is the longest axis and b is the shortest axis perpendicular to it.9, 10 DT as a measure of growth was calculated in terms of volume from the radius or lengths in the largest section of the tumour. Thus the volume was either 4/3πr3 or 4/3π(a/2×b/2)2/3.
Two ultrasonographers (SI and HW) were involved in the study. The same ultrasonographer performed both volume measurements in each patient.
Oral glucose tolerance test
The 60 patients with single small HCC underwent an OGTT at admission for treatment of HCC. OGTT was performed after the second US examination for second tumour volume measurements. The test was performed at 8 am on day 2 following the recommendations of the American Diabetes Association.18 A Teflon catheter was inserted into an antecubital vein for blood sampling, and samples were obtained for plasma glucose, free insulin, and C peptide concentrations in the basal period and after an oral glucose load (75 g) at 30 minute intervals for 180 minutes.
Areas under the plasma glucose and insulin curves (AUCgluc and AUCins) for the OGTT were calculated.
Treatment with octreotide
Octreotide was subcutaneously infused at a daily dose of 200 μg with an infusion pump for three months to continuously reduce plasma insulin concentration in five patients with HCC.
Statistical analysis
Fisher's exact probability test or the Mann-Whitney test was used to compare baseline characteristics in the rapid and slow growth groups. To estimate the significant factors contributing to tumour growth, univariate and multivariate analyses by the logistic regression model were used. The variables used were sex, age, hepatitis viral infection, fasting plasma glucose and insulin concentrations, AUCgluc and AUCins of OGTT, liver function tests, renal function, haematological data, serum concentrations of α fetoprotein (AFP), underlying liver disease, and histology of HCC. The cutoff value was the median of each variable. Differences in AUCgluc and AUCins before and after octreotide treatment were analysed by the Wilcoxon (matched pair) signed ranks test. A p value <0.05 was considered statistically significant.
RESULTS
Relation of DT to postprandial hyperinsulinaemia
Baseline characteristics of patients with single small HCC are presented in table 1 ▶. The 60 patients were divided into two groups (rapid growth group, ≤140 days, n=30 and slow growth group, >140 days, n=30) because the median DT of HCC was 140 days. Sex, age, hepatitis viral infection, serum AFP, and biochemical liver function tests, including serum alanine aminotransferase, bilirubin, and albumin concentrations, were not different between the rapid and slow growth groups. Also, no difference was noted in the ratio of liver cirrhosis and histological differentiation. Fasting plasma insulin concentration and AUCins were significantly higher (10.4 (6.2) μU/ml and 262 (152) μU/ml/h, respectively; mean (SD)) in the rapid growth group compared with those in the slow growth group (7.6 (4.3) and 146 (140), respectively) (p=0.041 and p=0.0006, respectively). In contrast, fasting plasma glucose concentration and AUCgluc (100 (16.0) mg/dl and 361 (102) mg/dl/h, respectively) in the rapid growth group were significantly lower than those in the slow growth group (120 (21.7) and 439 (119), respectively) (p=0.0003 and p=0.0012, respectively).
Table 1.
Clinical and laboratory characteristics of the study subjects
| Variable | Rapid growth DT ≤140 days (n=30) | Slow growth DT >140 days (n=30) | p Value |
| Sex | |||
| Males | 24 | 25 | NS |
| Females | 6 | 5 | |
| Age (y) | 64.1 (6.6) | 64.3 (5.3) | NS |
| Hepatitis virus | |||
| HBV ve+ | 2 | 6 | NS |
| HCV ve+ | 28 | 24 | |
| Haemoglobin (g/dl) | 13.1 (1.4) | 13.0 (1.0) | NS |
| Leucocytes (/μl) | 5800 (2750) | 5780 (2540) | NS |
| Platelet (103/μl) | 105 (48) | 103 (45) | NS |
| Albumin (g/dl) | 3.36 (0.54) | 3.45 (0.43) | NS |
| Total bilirubin (mg/dl) | 1.36 (0.77) | 1.64 (1.25) | NS |
| ALT (U/l) | 57.3 (23.1) | 75.8 (42.3) | NS |
| Creatinine (mg/dl) | 0.74 (0.21) | 0.73 (0.23) | NS |
| AFP (ng/ml) | 188 (684) | 237 (683) | NS |
| Fasting glucose (mg/dl) | 100 (16.0) | 120 (21.7) | 0.0003 |
| Fasting insulin (μU/ml) | 10.4 (6.2) | 7.6 (4.3) | 0.041 |
| AUCgluc (mg/dl/h) | 361 (102) | 439 (119) | 0.0012 |
| AUCins (μU/ml/h) | 263 (152) | 146 (140) | 0.0006 |
| Tumour diameter (mm) | 20.7 (4.5) | 22.2 (5.2) | NS |
| Interval of US (days) (range) | 85.0 (25.2) (62–160) | 83.2 (27.9) (61–150) | NS |
| Underlying liver disease | |||
| Chronic hepatitis | 4 | 6 | NS |
| Liver cirrhosis | 26 | 24 | |
| Histology | |||
| Well | 12 | 15 | NS |
| Moderate/poor | 18 | 15 | |
Values are mean (SD) or number.
ALT, alanine aminotransferase; AFP, α fetoprotein; AUCgluc, area under the plasma glucose curve; AUCins, area under the plasma insulin curve; DT, doubling time; HBV, hepatitis B virus; HCV, hepatitis C virus; US, ultrasound.
To identify factors contributing to shortening of the DT in HCC patients, univariate and multivariate analyses of logistic regression models were performed (table 2 ▶). AUCins for the OGTT was a significant factor contributing to HCC growth in both analyses (p=0.001 and p=0.016, respectively). These results imply that postprandial hyperinsulinaemia accelerates the growth rate of HCC. AUCgluc was a negative significant factor for HCC growth in multivariate analysis (p=0.022).
Table 2.
Factors contributing to doubling time of hepatocellular carcinoma using logistic regression model
| Univariate analysis | Multivariate analysis | |||||
| Variable | Odds ratio | 95% CI | p Value | Odds ratio | 95% CI | p Value |
| Sex | ||||||
| Male | 1 | 1 | ||||
| Female | 0.08 | 0.22–2.97 | NS | 0.650 | 0.10–4.28 | NS |
| Age (y) | ||||||
| ≤65 | 1 | 1 | ||||
| >65 | 2.10 | 0.71–6.22 | NS | 2.07 | 0.51–8.40 | NS |
| Hepatitis virus | ||||||
| HBV ve+ | 1 | 1 | ||||
| HCV ve+ | 3.50 | 0.65–18.98 | NS | 2.84 | 0.24–32.81 | NS |
| Haemoglobin (g/dl) | ||||||
| ≤13.0 | 1 | 1 | ||||
| <13.0 | 1.25 | 0.38–3.75 | NS | 0.92 | 0.37–2.60 | NS |
| Leucocyte (/μl) | ||||||
| ≤5500 | 1 | 1 | ||||
| >5500 | 1.32 | 0.43–3.91 | NS | 1.57 | 0.48–4.62 | NS |
| Platelet (103/μl) | ||||||
| ≤100 | 1 | 1 | ||||
| >100 | 0.86 | 0.32–2.70 | NS | 1.15 | 0.37–3.63 | NS |
| Albumin (g/dl) | ||||||
| ≤3.3 | 1 | 1 | ||||
| >3.3 | 1.24 | 0.49–3.15 | NS | 1.35 | 0.47–3.95 | NS |
| Total bilirubin (mg/dl) | ||||||
| ≤1.2 | 1 | 1 | ||||
| >1.2 | 0.95 | 0.23–3.60 | NS | 0.73 | 0.23–2.39 | NS |
| ALT (U/l) | ||||||
| ≤60 | 1 | 1 | ||||
| >60 | 1.60 | 0.43–5.82 | NS | 1.31 | 0.44–4.03 | NS |
| Creatinine (mg/dl) | ||||||
| ≤0.8 | 1 | 1 | ||||
| >0.8 | 1.42 | 0.41–4.01 | NS | 0.82 | 0.31–2.20 | NS |
| AFP (ng/ml) | ||||||
| ≤35 | 1 | 1 | ||||
| >35 | 1.00 | 0.35–2.85 | NS | 0.61 | 0.16–2.37 | NS |
| Fasting glucose (mg/dl) | ||||||
| ≤110 | 1 | 1 | ||||
| >110 | 0.38 | 0.13–1.09 | NS | 1.25 | 0.21–7.53 | NS |
| Fasting insulin (μU/ml) | ||||||
| ≤8 | 1 | 1 | ||||
| >8 | 2.67 | 0.92–7.70 | NS | 0.30 | 0.04–2.16 | NS |
| AUCglu (mg/dl/h) | ||||||
| ≤400 | 1 | 1 | ||||
| >400 | 0.38 | 0.12–19.76 | NS | 0.17 | 0.04–0.77 | 0.022 |
| AUCins (μU/ml/h) | ||||||
| ≤205 | 1 | 1 | ||||
| >205 | 6.42 | 2.08–19.76 | 0.001 | 22.23 | 1.79–276.7 | 0.016 |
| Underlying liver disease | ||||||
| Chronic hepatitis | 1 | 1 | ||||
| Liver cirrhosis | 1.63 | 0.41–6.47 | NS | 0.96 | 0.12–7.76 | NS |
| Histology | ||||||
| Well | 1 | 1 | ||||
| Moderate/poor | 1.50 | 0.54–4.17 | NS | 3.43 | 0.80–14.63 | NS |
ALT, alanine aminotransferase; AFP, α fetoprotein; AUCgluc, area under the plasma glucose curve; AUCins, area under the plasma insulin curve; CI, confidence interval.
Correlations of DT with fasting plasma glucose and insulin concentrations, AUCgluc, and AUCins were assessed. DT was inversely correlated with AUCins (r=−0.441, p<0.01). DT was also significantly correlated with fasting plasma glucose concentration (r=0.496, p<0.01) but showed no correlation with AUCgluc or fasting plasma insulin concentration (r=0.319 and r=−0.268, respectively).
Effect of reduction of hyperinsulinaemia on DT
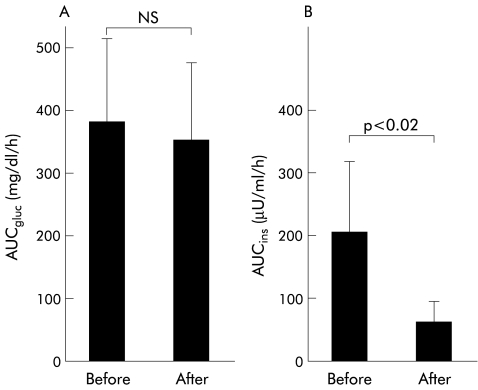
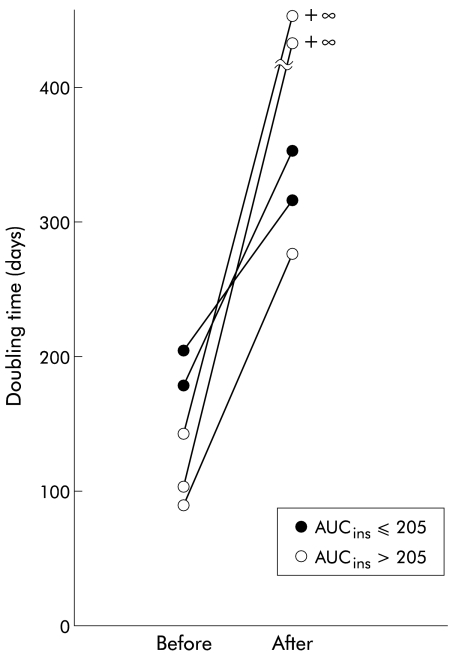
Five of the 60 patients were treated with octreotide for three months with no significant adverse effects. AUCgluc values in the five patients were not different before and after treatment (380 (133) mg/dl/h v 350 (126)) (fig 1 ▶). AUCins after treatment significantly decreased in comparison with before treatment (65 (34) μU/ml/h v 205 (112); p<0.02, Wilcoxon signed ranks test). DT after octreotide treatment increased in three of the five patients (from about 1.5-fold to threefold) and could not be calculated because of no change in the diameters of the tumour for three months during octreotide treatment in the remaining two patients (fig 2 ▶). DT in the three patients with an AUCins >205 μU/ml/h became longer than that in the two patients with an AUCins <205 μU/ml/h.
Figure 1.
Effects of octreotide on plasma insulin and glucose concentrations during the oral glucose tolerance test (OGTT) in five patients with hepatocellular carcinoma (mean (SD)). (A) Comparison of the areas under the plasma glucose curve (AUCgluc) for the OGTT before and after octreotide treatment (NS). (B) Comparison of the areas under the plasma insulin curve (AUCins) for the OGTT before and after octreotide treatment (p<0.02, Wilcoxon signed ranks test).
Figure 2.
Changes in doubling time of hepatocellular carcinoma before and after octreotide treatment in five patients with an area under the plasma insulin curve (AUCins) of ≤205 μU/ml/h or AUCins >205 μU/ml/h before octreotide treatment.
DISCUSSION
The growth rate of a tumour is a critical factor for the prognosis of cancer patients. The DT of human HCC has been measured in an attempt to clarify the clinical factors affecting tumour growth rate.9, 12, 13 However, no clinical factor, including age, sex, liver function, and hepatitis viral infection, was found to correlate with the growth rate of HCC. DT was also independent of the stage of liver cirrhosis, histological type, and grade of malignancy. Only vascularity showed a correlation with growth rate in well differentiated HCC.13 The present study showed that hyperinsulinaemia after oral glucose intake was a significant factor contributing to the growth rate of HCC.
Glucose intolerance, overt diabetes mellitus, and insulin resistance are characteristic features of patients with cirrhosis.2–8, 19 A recent study suggested that liver cirrhosis is a condition characterised by marked peripheral insulin resistance, and diabetes develops only in patients in which β cells cannot compensate with increased secretory performance.20 In fact, insulin resistance in cirrhosis is normalised after liver transplantation.20, 21 Several lines of evidence suggest that hyperinsulinaemia in cirrhotic patients results from three abnormalities: impaired hepatic insulin clearance, portosystemic/intrahepatic shunting, and enhanced insulin secretion related to insulin resistance.19, 22
Insulin functions as a potent growth factor via phosphorylation of insulin receptor substrate 1 and activation of mitogen activated protein kinases.23, 24 Human and rat hepatoma derived cell lines have insulin receptors and their proliferation is dose dependently stimulated by addition of physiological concentrations of insulin into the medium.24, 25 Thus high concentrations of insulin after a meal in cirrhotic patients may stimulate growth of HCC. In this study, peripheral insulin was measured in contrast with portal insulin which is more likely to exert any growth stimulating effects on liver tumours. Further study is needed to clarify whether insulin can sufficiently act to stimulate growth of hepatoma cells in vivo.
AUCgluc was a negative factor for the growth of HCC in multivariate analysis. Fasting plasma glucose also correlated with DT. However, this result is difficult to explain. Cirrhotic patients with elevated fasting plasma glucose concentrations and higher AUCgluc values appear to have lower AUCins values due to development of overt diabetes and reduced insulin secretion. It is possible that the relatively slow growth of HCC results from lower AUCins values.
Recently, Kouroumalis and colleagues16 reported that octreotide administration significantly improved quality of life and survival in patients with inoperable HCC in a randomised controlled trial. Continuous infusion of octreotide at a daily dose of 200 μg reduces hyperinsulinaemia and restores plasma insulin concentrations to the normal range during OGTT without changes in plasma glucose concentration in patients with cirrhosis.17 In this study, we observed a reduction in hyperinsulinaemia and a decrease in HCC growth rate in five patients during octreotide treatment. Furthermore, the reduction in growth rate was more marked in the three patients with hyperinsulinaemia with an AUC for OGTT of >205 μU/ml/h (fig 2 ▶). These data support the fact that postprandial hyperinsulinaemia may participate in HCC growth although there is no direct evidence that the reduction in hyperinsulinaemia during octreotide treatment caused the decreased HCC growth rate in these patients. Insulin-like growth factors 1 (IGF-1) and 2 (IGF-2) and their receptor (IGF-1R) are expressed by human hepatoma cells and have been implicated in the pathogenesis of liver cancer.26–29 Octreotide is also reported to inhibit growth of liver tumours via suppression of circulating IGF-1 levels and induction of insulin-like growth factor 1 binding protein (IGFBP-1).30 In this study, IGF-1 and IGFBP-1 levels were not measured, even in the five patients who received octreotide. Examination of IGF-1 and IGFBP-1 levels should be done in further studies.
The present study demonstrated that postprandial hyperinsulinaemia was associated with accelerated HCC growth. Treatment of postprandial hyperinsulinaemia may be beneficial for therapeutic management of cirrhotic patients with HCC. However, this study could not clarify whether the presence of postprandial hyperinsulinaemia predicts the prognosis of patients with HCC. Further study is needed to examine whether hyperinsulinaemia affects the prognosis of patients with HCC.
Abbreviations
AFP, α fetoprotein
AUCgluc, area under the plasma glucose curve
AUCins, area under the plasma insulin curve
DT, doubling time
HCC, hepatocellular carcinoma
IGF, insulin-like growth factor
IGF-1R, insulin-like growth factor 1 receptor
IGFBP-1, insulin-like growth factor 1 binding protein
OGTT, oral glucose tolerance test
US, ultrasound
REFERENCES
- 1.Okuda K. Hepatocellular carcinoma: recent progress. Hepatology 1992;15:948–63. [DOI] [PubMed] [Google Scholar]
- 2.Megyesi C, Samols E, Matks V. Glucose tolerance and diabetes in chronic liver disease. Lancet 1967;2:1051–6. [DOI] [PubMed] [Google Scholar]
- 3.Conn HO, Schreiber W, Elkington SG, et al. Cirrhosis and diabetes. Increased incidence of diabetes in patients with Laennec's cirrhosis. I. Am J Dig Dis 1969;14:837–52. [DOI] [PubMed] [Google Scholar]
- 4.Conn HO, Schreiber W, Elkington SG, et al. Cirrhosis and diabetes. Association of impaired glucose tolerance with potal-systemic shunting in Laennec's cirrhosis. Am J Dig Dis 1971;16:227–39. [DOI] [PubMed] [Google Scholar]
- 5.Nolte W, Hartmann H, Ramadori G. Glucose metabolism in liver cirrhosis. Exp Clin Endocrinol 1995;103:63–74. [DOI] [PubMed] [Google Scholar]
- 6.Johnston DG, Alberti KGMM, Baber OK, et al. Hyperinsulinism of hepatic cirrhosis: diminished degradation or hypersecretion? Lancet 1977;i:10–13. [DOI] [PubMed] [Google Scholar]
- 7.Petrides AS, De Fronzo RA. Glucose metabolism in cirrhosis: a review with some perspectives for the future. Diabetes Metab Rev 1989;5:691–709. [DOI] [PubMed] [Google Scholar]
- 8.Petrides AS, Groop LC, Riely CA, et al. Effect of physiologic hyperinsulinemia on glucose and lipid metabolism in cirrhosis. J Clin Invest 1991;88:561–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheu J-C, Sung J-L, Chen D-S, et al. Growth rate of aymptomatic hepatocellular carcinoma and its clinical implication. Gastroenterology 1985;89:259–66. [DOI] [PubMed] [Google Scholar]
- 10.Yamagata M, Masaki T, Okudaira T, et al. Small hyperechoic nodules in chronic liver diseases include hepatocellular carcinomas with low cyclin D1 and Ki-67 expression. Hepatology 1999;30:1722–9. [DOI] [PubMed] [Google Scholar]
- 11.Ebara M, Ohto M, Shinagawa T, et al. Natural history of minute hepatocellular carcinoma smaller than three centimeter complicating cirrhosis. A study of 22 patients. Gastroenterology 1986;90:289–98. [DOI] [PubMed] [Google Scholar]
- 12.Barbara L, Benzi G, Galani S, et al. Natural history of small untreated hepatocellular carcinoma in cirrhosis: a multivariate analysis of prognostic factors of tumor growth rate and patient survival. Hepatology 1992;16:132–7. [DOI] [PubMed] [Google Scholar]
- 13.Saitoh S, Ikeda K, Koida I, et al. Serial hemodynamic measurements in well-differentiated hepatocellular carcinomas. Hepatology 1995;21:1530–4. [PubMed] [Google Scholar]
- 14.Starzl TE, Francavilla A, Halgrimson CG, et al. The origin, hormonal nature, and action of hepatotrophic substances in portal venous blood. Surg Gynecol Obstet 1973;137:179–99. [PMC free article] [PubMed] [Google Scholar]
- 15.Sasaki Y, Zhang XF, Nishiyama M, et al. Expression and phosphorylation of insulin receptor substrate 1 during rat liver regeneration. J Biol Chem 1993;268:3805–8. [PubMed] [Google Scholar]
- 16.Kouroumalis E, Skordilis P, Thermos K, et al. Treatment of hepatocellular carcinoma with octreotide: a randomized controlled study. Gut 1998;42:442–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petrides AS, Stanley T, Matthews DE, et al. Insulin resistance in cirrhosis: prolonged reduction of hyperinsulinemia normalizes insulin sensitivity. Hepatology 1998;28:141–9. [DOI] [PubMed] [Google Scholar]
- 18.National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories intolerance. Diabetes 1979;28:1039–57. [DOI] [PubMed] [Google Scholar]
- 19.Petrides AS, DeFronzo RA. Glucose and insulin metabolism in cirrhosis. J Hepatol 1989;8:107–14. [DOI] [PubMed] [Google Scholar]
- 20.Perseghin G, Mazzaferro V, Sereni LP, et al. Contribution of reduced insulin sensitivity and secretion to the pathogenesis of hepatogenous diabetes: effect of liver transplantation. Hepatology 2000;31:694–703. [DOI] [PubMed] [Google Scholar]
- 21.Merli M, Leonetti F, Riggio O, et al. Glucose intolerance and insulin resistance in cirrhosis are normalized after liver transplantation. Hepatology 1999;30:649–54. [DOI] [PubMed] [Google Scholar]
- 22.Smith-Laing G, Sherlock S, Farber OK. Effect of spontaneous portal-systemic shunting on insulin metabolism. Gastroenterology 1979;76:685–90. [PubMed] [Google Scholar]
- 23.Rose DW, Saltiel AR, Majumdar M, et al. Insulin receptor substrate 1 is required for insulin-mediated mitogenic transduction. Proc Natl Acad Sci USA 1994;91:797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skolnik EY, Batzer A, Li N, et al. The function of GRB2 in linking the insulin receptor to Ras signaling pathways. Science 1993;260:1953–5. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka S, Wands JR. A carboxy-terminal truncated insulin receptor substrate-1 dominant negative protein reverses the human hepatocellular carcinoma malignant phenotype. J Clin Invest 1996;98:2100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koontz JW, Iwahashi M. Insulin as a potent, specific growth factor in a rat hepatoma cell line. Science 1981;211:947–9. [DOI] [PubMed] [Google Scholar]
- 27.Cariani JM, Lasserre C, Kemeny F, et al. Expression of insulin-like growth factor-like growth factor-II, alpha-fetoprotein and hepatitis B virus transcripts in human primary liver cancer. Hepatology 1991;13:644–9. [PubMed] [Google Scholar]
- 28.Kim SO, Park JG, Lee YI. Increased expression of the insulin-like growth factor I (IGF-1) receptor gene in hepatocellular carcinoma cell lines; implications of IGF-1 receptor gene activation by hepatitis B virus X gene product. Cancer Res 1996;56:3831–6. [PubMed] [Google Scholar]
- 29.Lamas E, Le Bail B, Housset C, et al. Localization of insulin-like growth factor-II and hepatitis B virus mRNAs and proteins in human hepatocelular carcinomas. Lab Invest 1991;64:98–104. [PubMed] [Google Scholar]
- 30.Ren S-G, Ezzat S, Melmed S, et al. Somatostatin analog induces insulin-like growth factor binding protein-1 (IGFBP-1) expression in human hepatoma cells. Endocrinology 1992;131:2479–81. [DOI] [PubMed] [Google Scholar]