Abstract
Aim: Sera of patients with autoimmune liver diseases were investigated for the presence of autoantibodies binding to human biliary epithelial cells (BECs). Furthermore, their functional capacity was investigated by testing their capacity to fix complement as well as induce expression of various adhesion molecules and production of cytokines.
Methods: Sera from patients with various stages of primary sclerosing cholangitis (PSC; n=30), primary biliary cirrhosis (PBC; n=29), autoimmune hepatitis (AIH; n=25), and normal controls (n=12) were investigated for the presence of antibodies that reacted with unstimulated and cytokine stimulated BECs isolated from a normal healthy liver. To demonstrate organ specificity, lung epithelial cells (LECs) were used as control cells. Antibodies were tested for their functional capacity.
Results: Compared with controls (8%), significantly higher numbers of PSC patients (63%, p=0.001), but not PBC (37%, NS) or AIH (16%, NS) patients, had anti-BEC antibodies. In 90% of PSC patients, the autoantibodies reacted only with cytokine stimulated target cells. Lower numbers of PSC (6%), PBC (10%), and AIH (0%) patients had LEC antibodies. Other significant findings were that anti-BEC antibodies were found in (i) PSC patients with either the HLA-DRB1*0301 or DR2 allele compared with those without (p=0.007); and (ii) in PBC patients with end stage disease compared with those without (p=0.018). Furthermore, anti-BEC antibodies from PSC and PBC but not AIH patients induced BECs to produce high levels of the cytokine interleukin 6. IgM and IgG fractions isolated from PSC but not PBC and AIH sera induced significantly increased expression of the cell adhesion molecule CD44. Sodium dodecyl sulphate-polyacrylamide gel electrophoresis and western blot analysis of BEC membranes demonstrated a specific band of 40 kDa with PSC sera and 45, 42, 30, and 33 kDa bands with PBC sera, which were absent in control groups.
Conclusion: Thus for the first time we have demonstrated the presence of functionally important autoantibodies to cell surface expressed antigens on the relevant target cells of destruction, namely BECs, in PSC and PBC. These finding have important implications for the pathogenesis of bile duct destruction in these patients.
Keywords: autoimmune liver diseases, primary sclerosing cholangitis, primary biliary cirrhosis, adhesion molecules, cytokines
Autoimmune hepatitis (AIH), primary biliary cirrhosis (PBC), and primary sclerosing cholangitis (PSC) are generally considered to be the major autoimmune liver diseases (ALDs).1 The aetiological agent is unknown in all three ALDs. They are usually considered distinct entities but mixed features of two diseases (overlap syndromes) have been described. AIH is a liver disease, mainly occurring in young and middle aged women. The main target for the inflammatory destructive process in this disease is the hepatocytes.2 The majority of patients respond well to immunosuppressive treatment. PBC is a chronic liver disease characterised by slowly progressive intrahepatic cholestasis due to inflammatory destruction of small intrahepatic bile ducts.3 PSC is a chronic cholestatic liver disease characterised by fibro-obliterative inflammation of both the intrahepatic and extrahepatic biliary tree.4 Thus the major targets in PBC are the small and middle sized bile ducts while in PSC even the large ducts are destroyed. PSC patients also have an increased risk of developing cancer of the biliary tree. Medical treatment in PBC and PSC is less effective and many of these patients need to undergo liver transplantation. Today, PSC is the most common indication for liver transplantation in the Nordic countries. In all ALDs, associations with other extrahepatic disorders are often observed, and in PSC as many as 70–90% of patients have inflammatory bowel disease.5
ALDs are characterised by circulating organ specific or non-organ specific autoantibody markers, or both.1 Autoantibodies commonly found in all three groups of patients are smooth muscle cell antibodies and antinuclear antibodies.1 However, some AIH patients are further characterised by the presence of autoantibodies to liver-kidney microsomal and soluble liver antigen.6, 7 The most sensitive and specific diagnostic immunological marker for PBC is the detection of antimitochondrial antibodies.8
It has been reported that 50% of PSC patients have hypergammaglobulinaemia with a disproportionate increase in IgM levels.9 Perinuclear cytoplasmic immunofluorescent staining of neutrophils is observed in 80% of PSC patients.10 Recently, these autoantibodies were shown to be directed against catalase and alpha-enolase.11 Serum autoantibodies reacting with colonic and portal tract antigens have been found in patients with PSC. Monoclonal antibodies to a 40 kDa colonic epithelial protein in patients with ulcerative colitis have been reported to cross react with epithelial cells lining the extrahepatic bile ducts of PSC patients with ulcerative colitis, which suggests a common antigen in the pathogenesis of these two conditions.12
The presence of autoantibodies in these patients, although considered to be good diagnostic markers, do not correlate with any clinical parameter. Moreover, so far the specificities of these autoantibodies have been shown to be directed against intracellular antigenic epitopes.1 Hence their direct contributing role in liver tissue destruction is questionable.
We were interested in (i) detecting the presence of autoantibodies to surface antigens expressed on the clinically relevant target cells of destruction for PSC and PBC, namely biliary epithelial cells (BECs) and (ii) determining the possible functional role of these antibodies in the pathogenesis of these diseases.
MATERIALS AND METHODS
Patients
Sera from patients with various stages of PSC (n=30), PBC (n=29), and AIH (n=25), and normal healthy individuals (n=12) were tested for the presence and functional capacity of autoantibodies that reacted with unstimulated and cytokine stimulated BECs. The diagnoses of AIH, PBC, and PSC were based on accepted histological, clinical, and cholangiographic criteria. The diagnosis of cholangiocarcinoma was based on histological examination. All PSC patients had involvement of both the intra and extrahepatic biliary tree. Among the PSC patients, 11 were females and 19 males. Patients with end stage disease were those accepted for liver transplantation due to liver failure. Patients without end stage disease did not have signs of liver decompensation (absence of ascites, encephalopathy, variceal, haemorrhage, bilirubin >100 μmol/l) at the time of blood sampling and did not develop any signs within one year after inclusion in the study. None of the patients had an ongoing infection, were pregnant, or received blood transfusions during the 12 months prior to investigation and all were negative for hepatitis B and C infections. In patients who had undergone liver transplantation, all blood samples tested were obtained prior to transplantation. In all 22 patients with an associated inflammatory bowel disease, the colonic disease was in remission, as judged by clinical and endoscopic criteria. Among the AIH patients there were 21 females, mean age was 54 years (20–77), and they were all in remission or were being treated with steroids and/or azathioprine. In the PBC group there were 16 females and mean age was 58 years (52–71). All patients were being treated with ursodeoxycholic acid.
Isolation and characterisation of biliary epithelial cells
Human BECs were isolated from the liver of one normal healthy liver donor using a method similar to that described previously.13
Single colour fluorescence was used to phenotypically characterise the BECs. Primary antibodies for intracellular staining were directed to cytokeratin 7, 19, fluorescein isothiocynate (FITC) conjugated antibody directed to a common cytokeratin epitope (Dako, A/S, Denmark), and smooth muscle cell (α-actin) antibodies (Harlan Sera-Lab, UK). The procedure for labelling is described elsewhere.14 Appropriate FITC conjugated goat antimouse secondary antibodies (Immunotech, Marseille, France) were used. Antibodies against surface receptors included non-conjugated antifibroblast antibodies (Harlan Sera-Lab) and FITC conjugated antibodies to von Willebrand factor (Serotec, UK). To test for tissue specificity we used lung (bronchial) epithelial cells (LECs) as control target cells (Clonetics, BioWhittaker, USA).
Determination of antibodies against BECs from sera of PSC, PBC, and AIH patients by flow cytometry
Sera from healthy non-transfused blood group AB males served as negative controls. The following antibodies FITC conjugated F(ab`)2 fragments of goat antihuman IgG (Fc specific), IgM, IgA, IgE antibodies (Jackson Immunoresearch, Pennsylvania, USA), and FITC conjugated sheep antihuman IgG subclass specific IgG1, IgG2, IgG3, and IgG4 antibodies (The Binding Site, Birmingham, UK) were used.
The flow cytometric assay, using 5×105 unstimulated/stimulated BECs/LECs, was performed as described previously.15 Cells were analysed on a Becton Dickinson flow cytometer (FACSorter; Becton Dickinson, San Jose, California, USA). A shift in mean fluorescence of 15 channels in the test sample compared with negative controls was considered positive. This value was determined by repeatedly testing the same lot of normal human serum as well as 20 different batches on the same group of cells, and standard deviations were calculated as ±3 channel range.
All sera giving a positive reaction were further diluted (1:5, 1:10, 1:100) in phosphate buffered saline (PBS) to determine the titre of the antibodies.
In all experiments, one set of cell samples remained untreated while another set was stimulated with recombinant tumour necrosis factor α (TNF-α) and interferon γ (IFN-γ) (20 ng/ml, and 200 ng/ml respectively; R&D systems, Abingdon, UK) which were added to the culture medium overnight prior to harvesting of cells for analysis.
Purification of IgM and IgG antibodies from sera of PSC, PBC, and AIH patients with anti-BEC antibodies
In order to demonstrate that it was the immunoglobulin (Ig) fraction in serum that had the functional properties investigated in this study, we used purified IgM and IgG antibodies in some of the assays. IgM and IgG fractions were purified from sera of AIH, PBC, PSC, and normal individuals with anti-BEC antibodies using goat antihuman IgM or IgG (μ chain and Fc chain specific, respectively; Sigma, St Louis, Missouri, USA) agarose beads. Lyophilised immunoglobulins were resuspended in distilled water and concentrations adjusted to 1 mg/ml for IgM and 6 mg/ml for IgG.
As the screening procedure demonstrated that PSC sera reacted with only cytokine stimulated BECs, all subsequent experiments were performed using cytokine stimulated cells.
Functional studies
Microcytotoxicity assay
To study the functional capacity of the antibodies directed against BECs, we tested the in vitro ability of the anti-BEC antibodies to fix complement using the microcytotoxicity assay.16 Reactions were considered positive when there was lysis of more than 10% above background compared with the negative control. Negative control consisted of serum from two normal healthy individuals.
Purified Ig fractions from PSC, PBC, and AIH on adhesion molecule expression of BECs
As BECs are known to be targets of immune destruction during various chronic liver diseases, we decided to investigate whether the Ig fractions from the three groups of patients with ALDs could induce expression of immune recognition elements known to facilitate T cell activation. For this purpose, BECs at the third passage were grown to confluence in six well culture plates (Falcon, Becton Dickinson). Cells were incubated overnight in the presence of medium alone or stimulated with recombinant TNF-α and IFN-γ at a final volume of 2 ml.
Parallel experiments with cytokine stimulated BECs incubated with IgM/IgG fractions from AIH, PBC, PSC, or normal human serum (0.5 mg/ml for IgM and 3 mg/ml for IgG) at the same final volume were performed. After incubation, cells were detached by trypsinisation, washed, and incubated with the following FITC conjugated monoclonal antibodies: anti-HLA-DR, anti-ICAM-1, anti-CD80, anti-CD44, anti-CD58, and anti-CD40. Isotype control antibodies were used as negative controls. After incubation on ice at 4°C, cells were washed and analysed by flow cytometry.
Cytokine production by BECs cultures
Purified PSC, PBC, AIH, and normal IgM fractions diluted in medium, as stated above, were added to cytokine stimulated BECs and the culture supernatants were collected after 12 hours, sterile filtered, and kept frozen at −70°C until assayed. The cytokines TNF-α, transforming growth factor β, IFN-γ, interleukins (IL)-1β, IL-2, IL-4, IL-6, and IL-12 were measured by standard sandwich ELISA techniques using the Quantikine sandwich enzyme immunoassay from R&D systems (Minnesota, USA). Assays were performed according to the manufacturer's instructions.
Molecular characterisation of antigens recognised by anti-BEC antibodies
Isolation of biliary epithelial cell membranes
Activated BECs were trypsinised and washed once with PBS containing 1 mM phenylmethanesulphonylfluoride and kept frozen at −70 oC until use. Cell membranes were prepared using a method described previously.17
SDS-PAGE and western blotting
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) was run using the method of Laemmli18 with 5% stacking gels and 10% resolving gels with a vertical Mini-Protean II electrophoresis system (Bio-Rad, Herculus, California, USA). Western blotting was performed according to the standard assay.
Blocking of binding of patient antibodies to BECs by antitropomyosin monoclonal antibodies
Autoantibodies to tropomyosin (a 40 kDa protein) have been reported in the sera of PSC patients which have been shown to react with BECs. We therefore decided to test whether the anti-BECs antibodies detected in the sera of our PSC patients were also directed to tropomyosin. For this purpose, 5×105 BECs were incubated with 20 μl (0.5 mg/ml) of anti-tropomyosin specific antibodies (Sigma) for one hour at room temperature and washed once with PBS. The patient's serum was then added to the pretreated cells and the procedure carried out as described previously. Cells were then analysed in the flow cytometer.
Statistical analysis
The Mann-Whitney U test was used to compare quantitative variables between the two groups and the Kruskal-Wallis test was used when comparisons between more than two groups were done. The κ2 test and, when appropriate, Fisher's exact were used to compare categorical parameters. Differences were considered significant if p<0.05.
RESULTS
Phenotyping of biliary epithelial cells
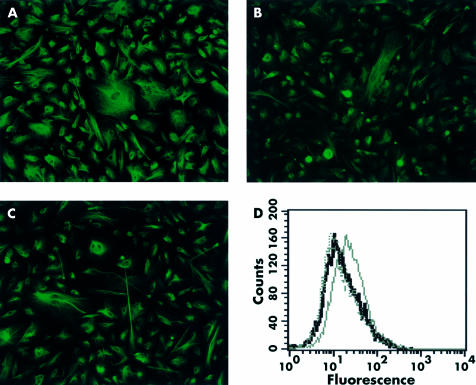
BECs were routinely characterised during growth by immunofluorescence labelling and flow cytometry. Results indicated that the cultures were more than 95% pure for BECs on the basis of expression of cytokeratins 19 and 7 and an FITC conjugated antibody directed to a common cytokeratin epitope (fig 1A–C ▶). The cells stained negative for antibodies against von Willebrand factor (endothelial cells) and a fibroblast and smooth muscle cell (α-actin) marker (fig 1D ▶). Normal BECs showed the typical epitheloid morphology.
Figure 1.
Isolated biliary epithelial cells stained positive for cytokeratin 7 (A), cytokeratin 19 (B), and a common cytokeratin epitope (C). Flow cytometric analysis indicated that the same cells stained negative for von Williebrand factor (dotted curve) (endothelial cells), smooth muscle (dashed curve), and antifibroblast antibodies (light grey curve). A negative control using only secondary antibodies was also included (black curve) (D).
Detection of binding of autoantibodies in sera of ALD patients to biliary and lung epithelial cells
A significantly high number of PSC patients (19/30 (63.3%), p=0.0017), 11/29 PBC patients (37%, NS), and 4/25 (16%, NS) AIH patients compared with 1/12 (8%) normal controls had autoantibodies to BECs (PSC v PBC (p=0.05); PSC v AIH (p=0.003); and PBC v AIH (NS)). Importantly, binding of anti-BEC antibodies in 90% (17/19) of PSC patients was detected using only cytokine stimulated BECs. In PBC and AIH patients, antibody binding was detected in both unstimulated and stimulated cells (table 1 ▶). Patients sera' were further tested for tissue specificity using LECs. We found that significantly lower numbers of PSC (2/30 (6%), p<0.001) and PBC (2/19 (10%), p<0.05) patients had antibodies against LECs compared with BECs. In normal controls and patients with AIH, no anti-LEC antibodies were detected. In addition, sera taken from five rheumatoid arthritis, five systemic lupus erythematosus, and five Wegener's granulomatosis patients with no liver complications showed no reactivity with BECs.
Table 1.
Binding of autoantibodies in the sera of patients with autoimmune liver diseases to biliary epithelial cells
| Patient group | Total No of sera with positive reactivity to BECs | Positive with unstim+stim* BECs | Positive with only stim BECs |
| PSC (n=30) | 19 (63%) | 2 | 17 |
| PBC (n=29) | 11 (37%) | 11 | 0 |
| AIH (n=25) | 4 (16%)† | 1 | 2 |
| RA (n=5) | 0 | 0 | 0 |
| SLE (n=5) | 0 | 0 | 0 |
| WG (n=5) | 0 | 0 | 0 |
| Normals (n=12) | 1 (8%) | 1 | 0 |
PSC, primary sclerosing cholangitis; PBC, primary biliary cirrhosis; AIH, autoimmune hepatitis; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; WG, Wegeners granulomatosis (PSC v normals, p=0.001); BECs, biliary epithelial cells.
*BECs were stimulated (stim) overnight with interferon γ and tumour necrosis factor α.
†One patient had anti-BEC antibodies that reacted with only unstimulated (unstim) BECs.
Immunoglobulin class and titres of anti-BEC antibodies in sera of ALD patients
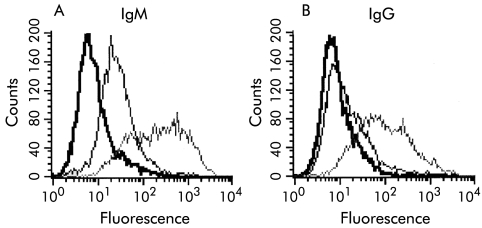
Table 2 ▶ summarises the various immunoglobulin classes and titres of anti-BEC antibodies detected in the sera of patients with PSC, PBC, and AIH. In general, patients with PBC, AIH, and normal controls had anti-BEC antibodies that belonged mainly to the IgM class. The initial antibody screening procedure using sera from PSC patients indicated that many without end stage disease had IgM antibodies while patients with end stage disease had mainly IgG antibodies (fig 2A ▶). Interestingly, sera from those patients whose unseparated serum showed binding of only IgM but not IgG antibodies, when fractionated into IgG and IgM using affinity chromatography, showed binding of the separated IgG fraction to BECs (fig 2B ▶). In addition, titres of fractionated IgG antibodies were >1:20.
Table 2.
Detection of immunoglobulin class, titres, and complement fixation capacity of antibiliary epithelial cell antibodies in sera of patients with autoimmune liver diseases
| Group | Only IgM | IgM+ IgA | IgM+ IgE | IgM+ IgG1 | IgM+IgG3 +IgG4 | Titres | Microcytotoxicity positive (% lysis of BECs) |
| PSC (n=19)** | 9† | 4 | 3 | 1 | 2 | 1:10–1:50 | 5 (20–25%) |
| PBC (n=11) | 5 | 4 | 2 | 1:10–>1:50 | 1 (20%) | ||
| AIH (n=4) | 4 | 1:5–1:10 | 3 (20–40%) | ||||
| Normals (n=1) | 1 | <1:5 | 0 |
PSC, primary sclerosing cholangitis; PBC, primary biliary cirrhosis; AIH, autoimmune hepatitis.
**PSC versus normals, p=0.001.
†All of these patients in addition had IgG antibodies which were detected only after separation of the IgG fraction from the sera of these patients (see also results section).
Figure 2.
(A) Histograms showing positive binding of IgM autoantibodies in serum from two patient with primary sclerosing cholangitis to cytokine stimulated biliary epithelial cells (BECs). An example of strong binding (light grey curve) and relatively lower binding (dark grey curve) is shown. (B) Binding of IgG antibodies to BECs was observed in many patients, only after separation of the IgG fractions from the sera of these patients (light grey curve). Binding of IgG antibodies to BECs using unseparated serum from the same patient was not detected (dark grey curve). Appropriate negative controls (black curves) were included.
Anti-BEC antibodies in some ALD patients can fix complement and cause low level lysis of BECs
Anti-BEC antibodies in the sera of 5/19 PSC patients lysed 20–25% of BECs while anti-BEC antibodies from 1/5 PBC and 3/4 AIH lysed BECs. However, sera from normal controls did not lyse BECs. All five PSC patients with cytotoxic anti-BEC antibodies had antibody titres >1:10 (table 2 ▶).
Anti-BEC autoantibodies from PSC patients induce increased expression of CD44 on BECs
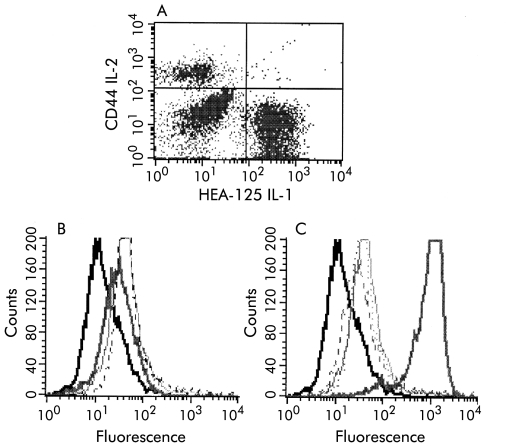
As only a small fraction of PSC patients had complement fixing antibodies, we investigated an additional role for anti-BEC antibodies in the sera of PSC and PBC patients, namely if these antibodies could induce expression of immune recognition molecules important in T cell activation. We initially tested for expression of CD44 on BECs and found that freshly isolated BECs do not express CD44 (fig 3A ▶) but CD44 expression appeared on these cells during in vitro cultivation. Treatment of BECs with only cytokines IFN-γ and TNF-α or IL-6 (100 ng/ml) did not alter expression of CD44 (fig 3B ▶). We found that anti-BEC IgM/IgG fractions from (15/19) PSC patients induced increased expression of CD44 on stimulated BECs while PBC IgM/IgG fractions induced only a slight increase (fig 3C ▶). No increased expression of CD44 was observed with IgM/IgG fractions from AIH patients and normal controls (fig 3C ▶). Furthermore, expression of other molecules such as HLA-DR, ICAM, CD80, CD58, and CD40 was not altered by treatment with PSC, PBC, AIH, or normal IgM/IgG fractions (table 3 ▶).
Figure 3.
(A) No expression of CD44 was observed on freshly isolated (Percoll separated) biliary epithelial cell (BECs). Thus the population was not pure. Fluorescein isothiocynate conjugated HEA-125 antibodies were used to label BECs. (B) However, BECs acquired expression of CD44 during in vitro culture basal expression (dotted curve). Treatment with the cytokines interferon γ and tumour necrosis factor α or IL-6 did not alter expression of CD44 on BECs (light and dark grey curves, respectively). (C) Increased expression of CD44 was observed on stimulated BECs after treatment with IgM fractions from sera of patients with primary sclerosing cholangitis with anti-BECs antibodies (thick grey curve). Slightly increased CD44 expression was observed after treatment with PBC IgM fraction (light grey curve), and remained unaltered after treatment with AIH (dotted curve) and normal control IgM fractions (dashed curve).
Table 3.
Induction of expression of various immune recognition molecules on biliary epithelial cells (BECs) by incubation with anti-BECs antibodies for 12 hours
| Antibodies to | Unstim BECs | TNF-α+IFN-γ stim BECs | Stim BECs+ IgM fraction from PSC sera | Stim BECs+ IgG fraction from PSC sera | Stim BECs+ IgM fraction from PBC sera | Stim BECs+ IgM fraction from AIH sera | Stim BECs+ IgM fraction from normal sera |
| HLA -DR | − | +++ (only after 3 days (with IFN-γ) | − | − | − | − | − |
| CD80 | − | − | − | − | − | − | − |
| CD44 | + | + | +++ | +++/++++ | +/++ | + | + |
| CD58 | − | − | − | − | − | − | − |
| CD40 | − | − | − | − | − | − | − |
| ICAM-1 | − | ++ | − | − | − | − | − |
+, >10–25; ++, >26–50; +++, >51–75 mean fluorescence channels compared with negative controls (only secondary antibodies).
PSC, primary sclerosing cholangitis; PBC, primary biliary cirrhosis; AIH, autoimmune hepatitis; TNF-α, tumour necrosis factor α; IFN-γ, interferon γ; stim, stimulated; unstim, unstimulated.
Detection of high levels of IL-6 produced by BECs on binding of anti-BECs antibodies from PSC and PBC patients
We found that of the cytokines tested, anti-BEC IgM/IgG fractions from PSC and PBC patients stimulated BECs to produce significantly high levels of IL-6 after 12 hours compared with baseline levels (p<0.001). Antibody negative sera from the same patient groups did not produce this cytokine. As seen in table 4 ▶, higher levels of IL-6 were produced when Ig fractions from PSC sera were used compared with PBC. Furthermore, no significant amounts of IL-6 were produced when IgM/IgG fractions from normal and AIH patients were used. (table 4 ▶).
Table 4.
Cytokine production in supernatants of stimulated biliary epithelial cells (BECs) incubated with anti-BECs antibodies
| Group | IL-1β (pg/ml) | IL-2 (pg/ml) | IL-4 (pg/ml) | IL-6 (pg/ml) | TNF-α (pg/ml) | TGF-β (pg/ml) | IFN-γ (pg/ml) |
| PSC | <10 | <30 | <10 | 2620 (461)*** | <50 | <30 | <30 |
| PBC | <10 | <30 | <10 | 1696 (349)*** | <50 | <30 | <30 |
| AIH | <10 | <30 | <10 | 577 (584) | <50 | <30 | <30 |
| Normals | <10 | <30 | <10 | 652 (132) | <50 | <30 | <30 |
| PSC w/o abs | <10 | <30 | <10 | 652 (257) | <50 | <30 | <30 |
| PBC w/o abs | <10 | <30 | <10 | 715 (208) | <50 | <30 | <30 |
Cytokines were determined after 12 hours of stimulation.
PSC, primary sclerosing cholangitis; PBC, primary biliary cirrhosis; AIH, autoimmune hepatitis; IL, interleukin; TNF-α, tumour necrosis factor α; TGF-β, transforming growth factor β; IFN-γ, interferon γ; w/0 abs, patients without anti-BEC antibodies.
***PSC v normals, p<0.001; PBC v normals, p<0.001.
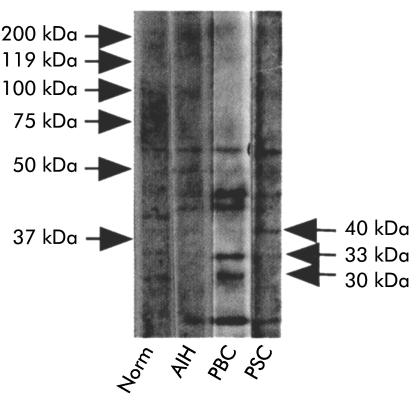
Detection of a 40 kDa protein, which is not tropomyosin using PSC sera
SDS-PAGE and western blot analysis using activated BEC membranes showed that sera from PSC patients immunoblotted a 40 kDa molecule while sera from PBC patients gave several bands with molecular weights of 45, 42, 31, and 33 kDa which were not detected in controls. No specific bands were detected with sera from controls and AIH (fig 4 ▶). In general, stronger bands were obtained with sera from PBC patients than PSC.
Figure 4.
Western blot analysis of stimulated biliary epithelial cell membranes (BECs) demonstrated the presence of several bands using sera from normal, primary sclerosing cholangitis (PSC), primary biliary cirrhosis (PBC), and autoimmune hepatitis (AIH) patients. However, PSC sera reacted with a specific protein of 40 kDa which was not detected with sera from other controls. Similarly, sera from PBC patients with anti-BECs antibodies detected two specific bands of 31 and 33 kDa, not observed in controls.
We initially tested for reactivity to tropomyosin but no cell surface expression of tropomyosin or blocking of patients' sera to BECs was observed with antitropomyosin monoclonal antibodies (data not shown).
Clinical and genetic correlation of the presence of anti-BECs antibodies
PSC patients with and without antibodies to BECs did not differ with respect to sex, age of onset of disease, or presence of inflammatory bowel disease. Moreover, only 50% of those with end stage disease had these antibodies. Of the two patients with cholangiocarcinoma, one patient had anti-BEC antibodies while the other did not. Altogether four patients had a history of colorectal cancer/high grade dysplasia and 2/4 had anti-BEC antibodies.
Anti-BEC antibodies were detected in 8/12 (66.6%) PBC patients with end stage cirrhosis and 4/8 (50%) of these patients had very high titre IgM+IgG antibodies >1:80. Anti-BEC antibodies were detected in only 3/17 (17.6%) PBC patients without end stage cirrhosis (p=0.018) (table 5 ▶)
Table 5.
Characterisation of biliary epithelial cell (BEC) antibodies in patients with primary biliary cirrhosis (PBC)
| Patient No | End stage disease fibrosis | MCF shift compared with negative controls* | Ig class | Titre |
| 1 | + | 73 | IgG+M | 1:120 |
| 2 | + | 33 | IgG+M | 1:30 |
| 3 | + | 57 | IgG+M | 1:140 |
| 4 | + | 21 | IgM | 1:20 |
| 5 | + | 52 | IgG+M | 1:100 |
| 6 | + | 23 | IgG+M | 1:40 |
| 7 | + | 55 | IgG+M | 1:200 |
| 8 | + | 25 | IgM | 1:20 |
| 9 | − | 19 | IgM | 1:10 |
| 10 | − | 18 | IgM | 1:10 |
| 11 | − | 21 | IgM | 1:20 |
| PBC (n=29) | ||||
| Anti-BECs ab− | Anti-BECs ab+ | |||
| MCF, mean channel fluorescence; ab, antibody. | ||||
| *MCF shift of >15 compared with the negative control was considered to be positive (see also materials and methods). | ||||
| End stage+ | 4 | 8 | ||
| p=0.018 | ||||
| End stage− | 14 | 3 | ||
In all, 10/30 PSC patients had the HLA-B8-DRB1*0301 haplotype while 10 had the −DR2 (15) allele. Interestingly, 9/10 (90%) PSC patients with the HLA-DR3 allele had anti-BEC antibodies compared with 10/20 (50%) without this haplotype (p<0.05). In addition, 7/10 with anti-BEC antibodies but without the HLA-B8, −DR3 haplotype had in fact the HLA DR2 (15) allele. Thus in total, 16/20 PSC patients with HLA-DR3 or the DR2 allele had anti-BEC antibodies compared with 3/10 without either of these two alleles (p<0.01) (table 6 ▶).
Table 6.
Correlation of presence of biliary epithelial cell (BEC) antibodies with HLA type in patients with primary sclerosing cholangitis (PSC)
| Patient No | MCF shift compared with negative controls* | Ig class | Titre | HLA type |
| 1 | 59 | IgG+M | 1:20 | A1, 2 B8 DRB1*0301 |
| 2 | 71 | IgG+M | 1:50 | A1, 24 B8, 16 DRB1*0301, 8 |
| 3 | 31 | IgG+M | 1:20 | A1 B8 DRB1*0301 |
| 4 | 24 | IgM | 1:10 | A2, 68 B7 DR15(2) |
| 5 | 47 | IgG+M | 1:10 | A1, 2 B7, 8 DRB1*0301, 15(2) |
| 6 | 19 | IgG+M | 1:4 | A1, 2 B50, 57 DR7 |
| 7 | 27 | IgG+M | 1:5 | A2, 3 B7, DR15(2) |
| 8 | 66 | IgM | 1:50 | A2 B8 DRB1*0301 |
| 9 | 25 | IgM | 1:5 | A2, 68 B51, 71 DR13 |
| 10 | 25 | IgM | 1:4 | A1 B8 DRB1*0301, 4 |
| 11 | 21 | IgM | 1:10 | A1 B8 DRB1*0301 |
| 12 | 57 | IgM+G | 1:50 | A1, 2 B7, 8 DRB1*0301, 13 (6) |
| 13 | 30 | IgM | 1:4 | A1, 24 B8, 65 DRB1*0301, 13 (6) |
| 14 | 40 | IgM+G | 1:5 | A2 B7, 51 DR13 (6), 15(2) |
| 15 | 42 | IgM+G | 1:5 | A3 B7 DR13(6), 15(2) |
| 16 | 27 | IgM | 1:10 | A2, 3 B15, 35 DR 13, 15 (2) |
| 17 | 23 | IgM | 1:4 | A25, 26 B7, 35 DR11, 15(2) |
| 18 | 28 | IgM | 1:10 | A2, 68 B7, DR15(2) |
| 19 | 20 | IgM | 1:4 | A2 B44, 62 DR4, 13 |
| PSC (n=30) | ||
| Anti-BECs ab− | Anti-BECs ab+ | |
| MCF, mean channel fluorescence; ab, antibody; DRB1*0301, DR3. | ||
| *MCF shift of >15 compared with the negative control was considered to be positive (see also materials and methods). | ||
| DR3/DR2+ | 4 | 16 |
| p<0.01 | ||
| DR3/DR2− | 7 | 3 |
DISCUSSION
In the present study, we have demonstrated that a significantly higher percentage of PSC patients have autoantibodies to surface antigens expressed on BECs compared with PBC, AIH, and normal individuals. In addition, we have shown that these autoantibodies induced increased expression and production of CD44 and IL-6, respectively, on cultured BEC. Thus for the first time we report the presence and functional capacity of autoantibodies in sera of PSC patients to surface antigens expressed on the clinically relevant target cells of destruction, namely BECs.
Initially, the main immunoglobulin class of anti-BEC antibodies detected in PSC sera was IgM. The presence of IgG anti-BEC autoantibodies however was detected only after isolation of the IgG fraction from the sera of these patients. It is likely that higher levels of the IgM pentamer immunoglobulin may sterically hinder or mask the binding of divalent IgG antibodies. Such findings have also been reported in other studies.19 In addition, as stated in the introduction, approximately half of all PSC patients have an abnormal increase in IgM levels thus adding to the masking of IgG antibody binding. Thus anti-BEC autoantibodies may be both IgM and IgG. Interestingly, in some PSC patients with end stage disease, predominantly IgG anti-BECs antibodies were found. In addition, the presence of IgG antibodies reflects the presence of cellular sensitisation.
Another interesting finding was the significant association of production of these autoantibodies with the HLA haplotype −B8, −DR3, or −DR2 found in PSC patients. Classic (type 1) AIH is also associated with the −DR3 allele but in this study only one AIH patient with this allele had anti-BEC antibodies. Neither sex nor age could account for this difference. Thus a heterogenous population of PSC patients may exist in whom different mechanisms may be involved in the pathogenesis of PSC depending on the presence or absence of anti-BEC antibodies.
Most PSC sera reacted with only cytokine stimulated BECs. This implies that a clinically important target(s) for antibody binding is probably induced or alternatively requires modification resulting in binding of antibodies.
Studies on the functional capacity of anti-BEC antibodies showed that not all anti-BEC antibodies could lyse BECs. A few patients with high titre anti-BEC antibodies had complement fixing capacity and were found to cause low level lysis of BECs. However, this did not correlate with any clinical parameter. An important effect of anti-BEC antibodies was induction of increased expression of CD44 on BECs by the Ig fractions isolated from PSC patients. Cruickshank et al have reported that normal bile ducts do not express CD44 but increased expression of CD44 on BECs of PSC and some PBC livers was observed.20 In our study, expression of CD44 was not observed in freshly isolated BECs but purified cultured BECs (after passage 1) expressed CD44. This expression was not altered by treatment with TNF-α or IFN-γ, a finding similar to that reported by Cruickshank and colleagues.20 Thus actively proliferating cells express CD44. Induction of CD44 expression on BECs by anti-BEC autoantibodies has important implications—for example, these antibodies may facilitate the recruitment of memory T cells (via CD44) and activate other cellular mechanisms leading to the destruction of bile ducts in these patients. The multifunctional properties of CD4421–24 may in addition have other important clinical implications in the inflammatory process involved in PSC. Some of the possible functions for CD44 in diseased livers have been discussed by Cruickshank and colleagues.20
Induction of high levels of the proinflammatory cytokine IL-6 by anti-BECs autoantibodies further indicates their importance in the pathogenesis of these diseases. It has been reported that under conditions of inflammation or stress in vivo, BECs produce, secrete, and respond to IL-6.25 IL-6 induces BEC proliferation in vitro26, 27 and suppresses BEC apoptosis.28 This cytokine is markedly elevated in the bile of patients with cholangitis29 and serum of patients with cholangiocarcinoma.30 IL-6 has been suggested to exert a profibrogenic and mitoinhibitory effect on the development of cirrhosis.31–35 Thus the fibro-obliterative inflammation of both the intra- and extrahepatic biliary tree seen in PSC may be a consequence of persistent IL-6 production contributing to uncontrolled proliferation and fibrosis.
One of the primary roles of IL-6 in vivo is as the major cytokine that initiates the hepatic acute phase response.36 In addition, IL-6 is well established as a late stage differentiation factor for B cell to plasma cell transition,37 enhancing immunoglobulin production and augmenting secondary antibody responses to antigens in vivo.38 In PSC, a significant increase in the number of B lymphocytes which correlates with histological stage and serum levels of immunoglobulin and bilirubin has been reported.39
Our data suggest that anti-BEC autoantibodies transform BECs into an inflammatory phenotype. It is important to mention that recombinant IL-6 alone did not induce expression of CD44, a finding also shown by others,40 indicating that it is the anti-BEC antibodies that are responsible for induction of CD44 expression.
Taken together, the in vitro data suggest that a proinflammatory loop exists between the binding of autoantibodies to BECs and possible recruitment of mononuclear leucocytes via CD44 as well as sustained production of autoantibodies by B cells via IL-6. This may represent a coordinated inflammatory response that implicates both the cellular and humoral arm of the immune response in the pathogenesis of PSC. As a significantly high proportion of PSC patients have anti-BECs antibodies compared with PBC and AIH patients, it is tempting to speculate that probably antibodies play a more significant role in the pathogenesis of PSC.
The anti-BEC antibodies from PSC patients detected a specific band of 40 kDa not detected in the other patient groups. Similarly, 31 and 33 kDa bands were detected using sera from PBC patients not detected in the other patient groups. Several reasons may account for the increased intensity of bands obtained in western blots using PBC sera: (a) abundance of protein(s) expressed on a cell surface; (b) titre of antibodies; (c) affinity of antibodies (low titre antibodies may have high affinity); and (d) nature of the antigen(s) detected (if expression of certain antigens is dependent on structural/chemical conformation, the use of SDS may affect binding). However, further work is needed to identify the proteins (using the technology of proteomics) detected by anti-BEC antibodies in the sera of PSC and PBC patients. Such studies are currently being performed at our centre. Results from such studies will add to the knowledge of possible therapeutic interventions that may be used in the treatment of these patients.
No correlation between the presence of these antibodies and any clinical parameter was found in PSC patients. However, a significantly high proportion of PBC patients with end stage disease had anti-BEC antibodies. Thus these antibodies may be associated with progression of disease in these patients.
Even though in vivo expression of CD44 and IL-6 has been demonstrated on bile ducts of PSC and PBC patients, the mechanism for this increased expression is not known. In this study, we showed that one mechanism may be due to cross linking/binding of cell surface expressed specific protein/proteins by anti-BEC autoantibodies which may induce an intracellular signal(s) that upregulates expression of several molecules, among them CD44 and IL-6. In the past, the importance and contribution of autoantibodies in inflammatory processes involved in autoimmune liver diseases has been underestimated. However, using the clinically relevant target cells of destruction in PSC and PBC, we have shown that anti-BEC autoantibodies in these patients may have an important role in the pathogenesis of these diseases.
Acknowledgments
The present study was financed by grants from the Medical Research Council No K2002-0bx-14004-02B, the Magnus Bergvall Foundation and the Ruth and Richard Julins Foundation, Clas Grotchinsky Foundation, the Swedish Medical Society, Nanna Svartz Foundation, and the Karolinska Institutet. We wish to thank Dr Johan Bratt for supplying serum samples from patients with RA, SLE, and Wegeners granulomatosis.
Abbreviations
AIH, autoimmune hepatitis
PBC, primary biliary cirrhosis
PSC, primary sclerosing cholangitis
ALDs, autoimmune liver diseases
BECs, biliary epithelial cells
LECs;lung epithelial cells
FITC, fluorescein isothiocynate
TNF-α, tumour necrosis factor α
IFN-γ, interferon γ
IL, interleukin
SDS-PAGE, sodium dodecyl sulphate-polyacrylamide gel electrophoresis
PBS, phosphate buffered saline
REFERENCES
- 1.Manns MP. The concept of autoimmune liver diseases. Autoimmune liver diseases, 2nd Edn. Elsevier: Amsterdam, 1998.
- 2.Wen L, Peakman M, Lobo-Yeo A, et al. T cell directed hepatocyte damage in autoimmune chronic active hepatitis. Lancet 1990;336:1527–30. [DOI] [PubMed] [Google Scholar]
- 3.Gershwin ME, Mackay IR. Primary biliary cirrhosis: paradigm or paradox for autoimmunity. Gastroenterology 1991;100:822–33. [DOI] [PubMed] [Google Scholar]
- 4.Aadland E, Schrumpf E, Fausa O, et al. Primary sclerosing cholangitis: a long-term follow-up study. Scand J Gastroenterology 1987;22:655–64. [DOI] [PubMed] [Google Scholar]
- 5.Broome UR, Olsson,L, Loof,G, et al. Natural history and prognostic factors in 305 Swedish patients with primary sclerosing cholangitis. Gut 1996;38:610–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manns M, Johnson EF, Griffen KJ, et al. Major antigen of liver kidney microsomal autoantibodies in idiopathic autoimmune hepatitis is cytochrome P450. J Clin Invest 1989;83:1066–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manns M, Gerken G, Kyriatsoules A, et al. Characterization of a new subgroup of autoimmune chronic active hepatitis by autoantibodies against a soluble liver antigen. Lancet 1987;1:292–94. [DOI] [PubMed] [Google Scholar]
- 8.Metcalf JV, Mitchinson HC, Palmer JM, et al. Natural history of early primary biliary cirrhosis. Lancet 1996;348:1399–402. [DOI] [PubMed] [Google Scholar]
- 9.Zauli D, Schrumpf E, Crespi C, et al. An autoantibody profile in primary sclerosing cholangitis. J Hepatol 1987;5:14–18. [DOI] [PubMed] [Google Scholar]
- 10.Duerr RH, Targan SR, Landers CJ, et al. Neutrophil cytoplasmic antibodies: a link between primary sclerosing cholangitis and ulcerative colitis. Gastroenterology 1991;100:1385–91. [PubMed] [Google Scholar]
- 11.Orth T, Kellner R, Diekmann O, et al. Identification and characterization of autoantibodies against catalase and α-enolase in patients with primary sclerosing cholangitis. Clin Exp Immunol 1998;112:507–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mandal A, Dasgupta A, Jeffers L, et al. Autoantibodies in sclerosing cholangitis against a shared peptide in biliary and colon epithelium. Gastroenterology 1994;106:185–92. [DOI] [PubMed] [Google Scholar]
- 13.Joplin R, Strain AJ, Neuberger JM. Immuno-isolation and culture of biliary epithelial cells from normal human liver. In Vitro Cell Dev Bio 1989;25:1189–92. [DOI] [PubMed] [Google Scholar]
- 14.Bo X, Broome U, Remberger M, Sumitran-Holgersson S. Chronic exposure to tumor necrosis factor in vivo alters the function of liver T lymphocytes in patients with primary sclerosing cholangitis. Gut 2001;48:131–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sumitran S. The clinical importance of choosing the right assay for detection of relevant panel and donor reactive antibodies. Transplantation 1999;68: 502–10. [DOI] [PubMed] [Google Scholar]
- 16.Terasaki PI, McElleland JD. Microdroplet assay of human serum cytotoxins. Nature 1964;204:998–1002. [DOI] [PubMed] [Google Scholar]
- 17.Sumitran S, Liu J, Kimberly A, et al. Human natural antibodies cytotoxic to pig embryonic brain cells recognize novel non-Galα1,3Gal-based xenoantigens. Exp Neurol 1999:159:347–61. [DOI] [PubMed] [Google Scholar]
- 18.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680–85. [DOI] [PubMed] [Google Scholar]
- 19.Mach PS, Piatier D, Le Go A, et al. Interactions between IgM antiglobulins and IgG antinuclear antibodies. Some aspects of D-penicillamine activities. Clin Exp Immunol 1979;36:311–16. [PMC free article] [PubMed] [Google Scholar]
- 20.Cruickshank SM, Southgate J, Wyatt JI, et al. Expression of CD44 on bile ducts in primary sclerosing cholangitis and primary biliary cirrhosis. J Clin Pathol 1999;52:730–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stauder R, Gunthert U. CD44 isoforms. Impact on lymphocyte activation and differentiation. Immunologist 1995;3:78–83. [Google Scholar]
- 22.Gunthert U, Stauder R, Mayer B, et al. Are CD44 variant isoforms involved in human tumor progression? Cancer Surv 1995;24:19–42. [PubMed] [Google Scholar]
- 23.Ruiz P, Schwrzler C, Gunthert U. CD44 isoforms during differentiation and development. Bio Essays 1995;17:17–24. [DOI] [PubMed] [Google Scholar]
- 24.Herrlich P, Zoller M, Pals T, et al. CD44 splice variants: Metastases meet lymphocytes. Immunol Today 1993;14:395–9. [DOI] [PubMed] [Google Scholar]
- 25.Yokomuro S, Lunz III JG, Sakamoto T, et al. The effect of interleukin-6 (IL-6)/gp130 signalling on biliary epithelail cell growth, in vitro. Cytokine 2000;12:727–30. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto K, Fujii H, Michalopoulos G, et al. Human biliary epithelial cells secrete and respond to cytokines and hepatocyte growth factors in vitro: interleukin-6, hepatocyte growth factor and epidermal growth factor promote DNA synthesis in vitro. Hepatology 1994;20:376–82. [PubMed] [Google Scholar]
- 27.OkadaK, Shimizu Y, Nambe S, et al. Interleukin-6 functions as an autocrine growth factor in a cholangiocarcinoma cell line. J Gastroenterol Hepatol 1994;9:462–67. [DOI] [PubMed] [Google Scholar]
- 28.Patel T, LaRusso NF, Gores GJ. Interleukin-6 suppresses cholangiocyte apoptosis by down-regulation of Bax. Hepatology 1997;26:226A.9214474 [Google Scholar]
- 29.Rosen HR, Winkle PJ, Kendall BJ, et al. Biliary interleukin-6 and tumor necrosis factor-alpha in patients undergoing endoscopic retrograde cholangiopancreatography. Dig Dis Sci 1997;42:1290–94. [DOI] [PubMed] [Google Scholar]
- 30.Goydos JS, Brumfield AM, Frezza E, et al. Marked elevation of serum interleukin-6 in patients with cholangiocarcinoma: validation of utility as a clinical marker. Ann Surg 1998;227:398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malaguarnera M, Trovato BA, Laurino A, et al. Interleukin-6 in hepatitis C cirrhosis. Panminerva Med 1996;38:207–10. [PubMed] [Google Scholar]
- 32.Kakumu S, Fukatsu A, Shinagawa T, et al. Localisation of intrahepatic interleukin 6 in patients with acute and chronic liver diseases. J Clin Pathol 1992;45:408–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu J, Kuncio GS, Zern MA. Human liver growth in fibrosis and cirrhosis. In: Strain AJ, Diehl AM, editors. Liver growth and repair. London: Chapman and Hall, 1998;558–76.
- 34.Tiggelman AM, Boers W, Linthorst C, et al. Interleukin-6 production by human liver (myo)fibroblasts in culture. Evidence for a regulatory role of LPS, IL-1 beta and TNF alpha. J Hepatol 1995;23:295–306. [PubMed] [Google Scholar]
- 35.Choi I, Kang HS, Yang Y, et al. Il-6 induces hepatic inflammation and collagen synthesis in vivo. Clin Exp Immunol 1994;95:530–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koy A, Gordon AH. The acute-phase response to injury and infection, Introduction. In: Gordon AH, Koj A, eds. Research monographs in cell and tissue physiology, vol 10. Amsterdam: Elsevier, 1985:xxi-xxix.
- 37.Muraguchi A, Hirano T, Tang B, et al. The essential role of B cell stimulatory factor 2 (BSF-2/IL-6) for the terminal differentiation of B cells. J Exp Med 1988;167:332–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takatsuki F, Okana A, Suzuki C et al. Human recombinant IL-6/B cell stimulatory factor 2 augments murine antigen-specific antibody responses in vitro and in vivo. J Immunol 1988;141:3072–7. [PubMed] [Google Scholar]
- 39.Boberg KM, Lundin KEA, Schrumpf E. Etiology and pathogenesis in primary sclerosing cholangitis. Scand J Gastroenterol 1994;204:47–58. [DOI] [PubMed] [Google Scholar]
- 40.Trejdosiewicz LK, Morton R, Yang Y, et al. Interleukins 4 and 13 upregulate expression of CD44 in human colonic epithelial cell lines. Cytokine 1998;10:756–65. [DOI] [PubMed] [Google Scholar]