Abstract
Background: Carboxypeptidase B from the pancreatic gland may exist in three different molecular and immunoreactive forms: the proenzyme, the active enzyme, and the activation peptide.
Aims: To investigate levels of procarboxypeptidase B (proCAPB) and its activation peptide in serum in acute pancreatitis to test the accuracy of these two variables as markers for the diagnosis of acute pancreatitis and for prediction of pancreatic necrosis. To elucidate whether leakage of proenzymes and activation of proenzymes reflect two different pathophysiological events in acute pancreatitis.
Methods: Sera from patients with acute pancreatitis (n=85) and acute abdominal pain of non-pancreatic origin (n=53) were analysed for proCAPB and its activation peptide. Patients with pancreatitis were divided into necrotising (n=33) and oedematous attacks (n=52) using contrast enhanced computed tomography. Accuracy was determined using receiver operating characteristic curve analysis.
Results: Immunoreactive carboxypeptidase B activation peptide (ir-CAPAP) concentration in serum on admission was 0.7 nmol/l (0–18.1) in patients with oedematous pancreatitis compared with 5.8 nmol/l (1.9–34) in patients with later development of pancreatic necrosis. Elevated levels of the activation peptide on admission correlated with an accuracy of 92% to later development of pancreatic necrosis. Ir-proCAPB concentration in serum on admission was 16.0 nmol/l (1.4–50.5) in all patients with acute pancreatitis versus 0.3 nmol/l (0–3.6) in patients with non-pancreatic acute abdominal disorders. Cases with oedematous pancreatitis had ir-proCAPB levels of 15.4 nmol/l (1.4–50.5) versus 19.1 nmol/l (2.7–36.1) in cases with later development of pancreatic necrosis. Measurement of the proenzyme can thus be useful for the diagnosis of acute pancreatitis (accuracy 99%) but levels did not correlate with later development of pancreatic necrosis (accuracy 56%).
Conclusion: Leakage of proenzymes occurs in acute pancreatitis, irrespective of severity, while development of pancreatic necrosis occurs only when there is activation of the proenzymes.
Keywords: acute pancreatitis, carboxypeptidase B, amylase, activation peptide
Carboxypeptidase B (CAPB) is an exoprotease synthesised as an inactive proenzyme procarboxypeptidase B (proCAPB) by acinar cells together with other pancreatic (pro)enzymes. In the intestine this proenzyme is activated through the action of trypsin. During this activation a large N terminal activation peptide (81 amino acids; molecular weight 9398 Da), carboxypeptidase activation peptide (CAPAP), is released.1,2
Pancreas specific protein (PASP) in serum has previously been found to be a good marker of acute pancreatitis (AP).3,4 This protein was later found to be identical to proCAPB.5 Clinical studies using the PASP assay have shown that this variable is a good diagnostic as well as a prognostic marker in AP and after pancreas transplantation.6
Antibodies against proCAPB will cross react with both the active enzyme (CAPB) and the activation peptide (CAPAP) and the PASP method thus measures a combination of these three immunoreactivities in serum.7 The individual contribution of each of the three forms in the PASP assay has not been determined.
In the pathophysiology of AP, activation of trypsinogen and the subsequent activation of the other pancreatic proenzymes such as prophospholipase A2 are thought to be early and important events.8,9 Procarboxypeptidase A and proCAPB were shown to be activated very rapidly intracellularly in rat pancreatic acini in an in vitro pancreatitis model with cholecystokinin hyperstimulation.10
Clinically, this has been documented by studies on excretion of the trypsinogen activation peptide (TAP) in urine in AP11,12 and also by studies on the presence of trypsin-protease inhibitor complexes in serum.13,14 Previous studies in our laboratory in patients with AP have shown that excretion of the activation peptide from proCAPB (CAPAP) in urine is very high in severe forms of AP (>100 nmol/l) and that levels correlate with severity of the disease in a small number of patients with AP.2 It was also shown that proCAPB is not excreted in urine despite a relatively low molecular mass (45 kDa).2,15
This study describes the development of two sensitive immunoassays: one that measures immunoreactive proCAPB (ir-proCAPB) and the other the immunoreactivity of the activation peptide from the same proenzyme (ir-CAPAP) separately. The aim of the study was also to investigate levels of ir-proCAPB and ir-CAPAP in serum in AP to test the accuracy of these two variables as markers for the diagnosis of AP and for assessment of the severity of the disease. This would elucidate whether leakage and activation of a proenzyme occur in parallel or reflect different pathophysiological events in AP.
MATERIALS AND METHODS
Patients and controls
The study was approved by the ethics committee of the University of Bern. A total of 85 consecutive patients with AP were analysed, together with 53 consecutive patients with severe acute abdominal disorders of non-pancreatic origin (AAD). The pancreatitis patients were a consecutive series of patients admitted to the Department of Visceral and Transplantation Surgery, University Hospital, Bern, over a four year period between 1995 and 1999. Patients with acute abdominal disorders (disease controls) were also a consecutive series of patients admitted to the same hospital over a 16 month period between 1997 and 1998. In this group of disease controls, only patients with an APACHE-II score >3 were included. Patient characteristics are shown in table 1 ▶.
Table 1.
Clinical characteristic in 138 patients, 85 with acute pancreatitis and 53 with acute abdominal disorders of non-pancreatic origin
| AP | EP | NP | AAD | HC | |
| n | 85 | 52 | 33 | 53 | 20 |
| Age (y) | 60 (20–86) | 60 (20–86) | 61 (35–84) | 67 (25–95) | 39 (22–62) |
| Sex (M/F) | 57/28 | 32/20 | 24/9 | 27/26 | 10/10 |
| Aetiology | |||||
| Biliary | 55 (65%) | 36 (70%) | 19 (56%) | ||
| Alcohol | 21 (25%) | 11 (20%) | 10 (31%) | ||
| Other | 9 (10%) | 5 (5%) | 4 (13%) | ||
| APACHE II | 7 (1–19) | 6 (1–14) | 10 (3–19) | 8 (4–21) | — |
| Ranson | 2.7 (0–9) | 1.9 (0–5) | 4.0 (0–9) | — | — |
| Mortality | 3 (3.5%) | 0 | 3 (9%) | 8 (15%) | — |
AP, acute pancreatitis; EP, acute oedematous pancreatitis; NP, acute necrotising pancreatitis; AAD, acute abdominal disorders of non-pancreatic origin; HC, healthy controls.
Values are median (range) or number (%).
AP was diagnosed by a combination of acute upper abdominal pain and elevated serum amylase and/or serum lipase (more than three times the upper reference level). All patients with AP were referred for contrast enhanced computed tomography (CT).
CT diagnosis
Spiral CT was performed with 5 mm collimation and a 60–80 second scan delay after uniphasic injection of 120–140 ml of contrast medium at 3 ml/s (Obtiray 300 mg iodine/ ml). In patients with a serum creatinine concentration >180 μmol/l, prehydration with 100 ml NaCl 0.9%/h 12 hours before and 12 hours after the CT scan was given.
Oedematous pancreatitis was defined as diffuse swelling of the pancreas but without any peripancreatic reaction and with homogeneous contrast medium enhancement after intravenous injection of iodine contrast media. Pancreatic necrosis was defined as diffuse or focal areas of pancreatic parenchyma that did not enhance (<50 Hounsfield units) after intravenous contrast medium injection and that typically was associated with peripancreatic fat stranding. If the necrosis became infected, this was recognised on CT scans as bubbles of gas within areas of pancreatic gland necrosis.16
According to the CT findings, patients were grouped into 52 cases of acute interstitial oedematous pancreatitis and 33 cases of necrotising pancreatitis. According to the extent of necrosis, patients were divided into three groups: <30%, 30–50%, and >50% necrosis. Patients with oedematous pancreatitis were subjected to 1–2 CT examinations (mean 1.2) The first examination was performed 20–312 hours after the onset of pain (mean 92 hours). If the patient stayed for more than two weeks in hospital a second CT scan was performed. Patients with pancreatic necrosis were subjected to 2–9 CT examinations (mean 2.4). The first examination was done 24–216 hours after the onset of pain (mean 88 hours).
Among the disease controls, in 48 of 53 patients the diagnosis was verified by laparotomy.
Serum from 20 fasting healthy adults (laboratory technicians) was obtained as controls (see table 1 ▶).
Samples
Blood samples were drawn on admission and daily for four days. Samples were allowed to clot and serum was obtained after centrifugation and then immediately frozen and stored at −80°C until further analysis.
Number of samples
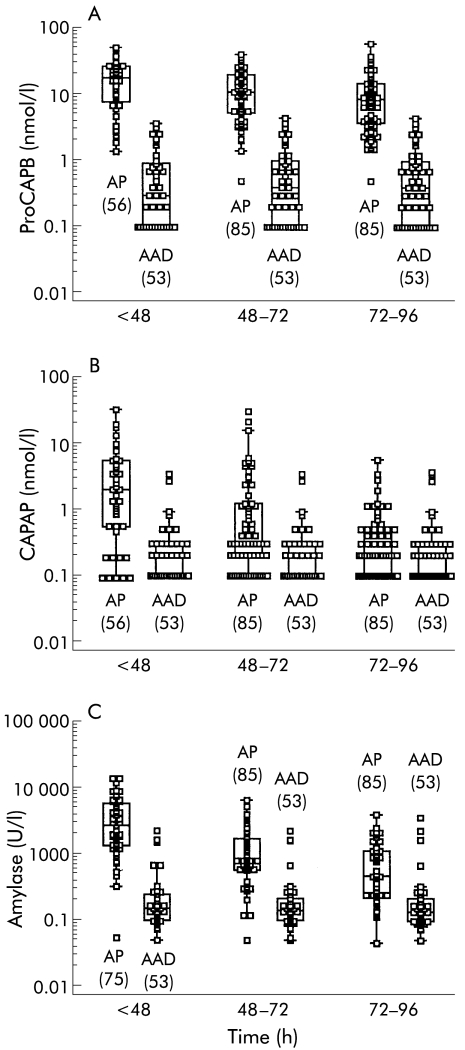
C reactive protein (CRP) and amylase were measured routinely in the emergency room at admission whereas CAPAP/proCAP were initially measured with some delay after study inclusion. The number of CRP/amylase samples was therefore larger than the number of CAPAP/proCAP samples in the <48 hour period (75 v 56 samples). If there were more than one measurement obtained within a given period, only one (the highest value) was used for calculations. The exact numbers of samples at each time interval are given in fig 1 ▶ and fig 3 ▶.
Figure 1.
Median concentrations, interquartile ranges, 95% ranges, and outliers in the serum of patients with acute pancreatitis (AP) and in patients with acute abdominal disorders of non-pancreatic origin (AAD) at three different time periods after the onset of pain. (A) Immunoreactive procarboxypeptidase B (proCAPB), (B) immunoreactive carboxypeptidase B activation peptide (CAPAP), and (C) amylase. Values in parentheses indicate the number of samples.
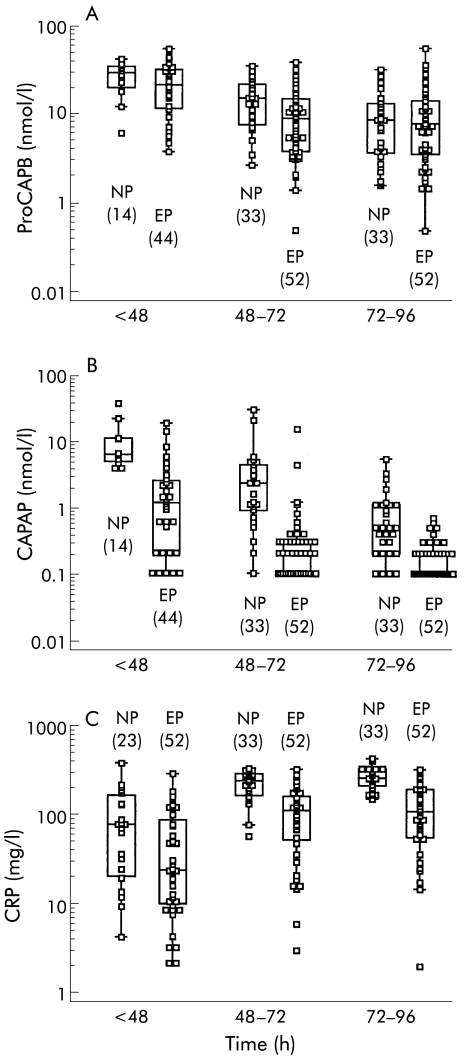
Figure 3.
Median concentrations, interquartile ranges, 95% ranges, and outliers in serum of patients with acute oedematous pancreatitis (ED) and necrotising pancreatitis (NP) at three different time periods after onset of pain. (A) Immunoreactive procarboxypeptidase B (proCAPB), (B) immunoreactive carboxypeptidase B activation peptide (CAPAP), and (C) C reactive protein (CRP). Values in parentheses indicate the number of samples.
Antisera
Human proCAPB, active CAPB, and the activation peptide (CAPAP) were purified from pancreatic juice, as described previously.2 Polyclonal antisera were raised after immunisation of the rabbits. Antisera against the active enzyme CAPB (9410 and 9411) cross react with the proenzyme but not with the activation peptide, as expected. Antisera against the activation peptide CAPAP (9415 and 9807) have been described previously and do not cross react with the active enzyme but do to a small extent with the proenzyme (10%).2 The immunoglobulin fraction of each antisera was purified using a protein A sepharose column.
Immunoassays
Two solid phase double antibody enzyme linked immunoassays were developed, principally as described previously for anionic and cationic trypsinogen.17 The first antibody (immunoglobulin fraction) was coated to Immunotech plates at a concentration of 10–20 mg/l in a bicarbonate buffer, pH 9.6. The second antibody was biotinylated and used at a dilution of 1/250–1/1000.
The different antibodies were combined in the following ways to obtain optimal specificity: proCAPB assay—first antibody against CAPB (9410), second antibody against CAPAP (9807); CAPAP assay—first antibody against CAPAP (9415), second antibody against CAPAP (9807).
The purified antigens were used as standards in serial dilutions from 5 to 0.02 nmol/l. Serum samples were diluted at least 1:5 before assay.
All dilutions were made with a Tris-HCl buffer 0.05 mol/l at pH 7.4 containing CaCl2 0.05 mol/l and bovine serum albumin 2 g/l.
The samples or standards (100 μl) were incubated in wells for two hours. Next, the wells were washed and incubated with the biotinylated second antibody for 1.5 hours and then washed and incubated with avidin conjugated alkaline phosphatase (ALP) (Dako A/S, Denmark) in a dilution of 1/1000 for one hour. After another wash, ALP activity in each well was detected using disodium p-nitrophenyl phosphate (Sigma 104 phosphate substrate tablets; Sigma Diagnostics, USA) as substrate in a concentration of 1 g/l in a 10% ethanolamine buffer at pH 9.8. The substrate solution (100 μl) was incubated in each well for approximately 45 minutes after which the colour of each well was determined in a Titertek Multiscan photometer at 410 nm.
Precision
Interassay variation was given as the coefficient of variation (1 SD/mean×100) for one sample run 20 consecutive times in the assay.
Other analyses
CRP was measured in mg/l using a turbidimetric immunoassay (Autokit CRP) from Wako (Osaka, Japan). Total alpha amylase activity in serum was determined with the Amyl enzymatic assay (Roche, Basel CH, Switzerland) (normal range 36–228 U/l).
Statistics
Medians and ranges of the parameters were calculated. The cut off levels, sensitivities, and specificities were evaluated by receiver operating characteristic (ROC) curve analysis.18 The area under the curve describes the accuracy of the test either for diagnosis of AP or for severity determination (prognosis), with 1.0 indicating 100% sensitivity and 100% specificity and 0.5 indicating no discriminatory value. Box and whisker plots combined with dot plots were used to present medians, ranges, and distributions of concentrations. Comparisons between groups were done using Wilcoxon's non-parametric rank test. Correlations were studied according to Spearman. Data processing and presentation were facilitated by MedCalc 4.16 for Windows 95.
RESULTS
Patient material
Clinical data for patients with AP are shown in table 1 ▶. In patients with AP the extent of pancreatic necrosis according to contrast enhanced CT was: no necrosis in 52 patients (oedematous pancreatitis), <30% necrosis in 19, 30–50% necrosis in nine, and >50% necrosis in five patients.
The diagnosis in patients with non-pancreatic disorders were: intestinal obstruction in 11 cases, perforated ulcers in eight, appendicitis in seven (four with perforation), diverticulitis in six (five with perforations), mesenteric infarction in six, acute cholecystitis in five, iatrogen endoscopic bowel perforations in four, gastritis in two, renal colic in two, intoxication in one, and intestinal bleeding in one case.
ProCAPB assay
The proCAPB assay showed no cross reactivity with either CAPB or CAPAP. Maximal sensitivity, as judged from the standard curve, was 0.04 nmol/l (≈1 μg/l). As serum samples have to be diluted at least five times, the lowest detectable concentration in the circulation was 0.1–0.2 nmol/l (≈5–10 μg/l). Interassay variation (precision) was 5.1% at approximately 1.3 nmol/l and 15% at approximately 0.25 nmol/l.
Median serum level of ir-proCAPB in the 20 healthy controls was 0.45 nmol/l (range 0.15–1.75). According to our small sample, the upper reference value for ir-proCAPB in normal serum would be 1.6 nmol/l (mean+2 SD).
Assay for the activation peptide from proCAPB (CAPAP assay)
The CAPAP assay showed no cross reactivity with active CAPB but 5 nmol of proCAPB was measured as 0.25 nmol of CAPAP, giving a cross reaction of approximately 5%. Sensitivity was 0.02 nmol/l. As serum samples have to be diluted at least five times, the lowest detectable concentration in the circulation was 0.1 nmol/l (≈1 μg/l). Interassay variation (precision) was 7.7% at approximately 1.4 nmol/l and 11.2% at approximately 0.35 nmol/l. Ir-CAPAP in serum of 20 healthy controls was undetectable in 18 samples, giving a median concentration of 0 nmol/l (range 0–0.19).
Accuracy for the diagnosis of AP
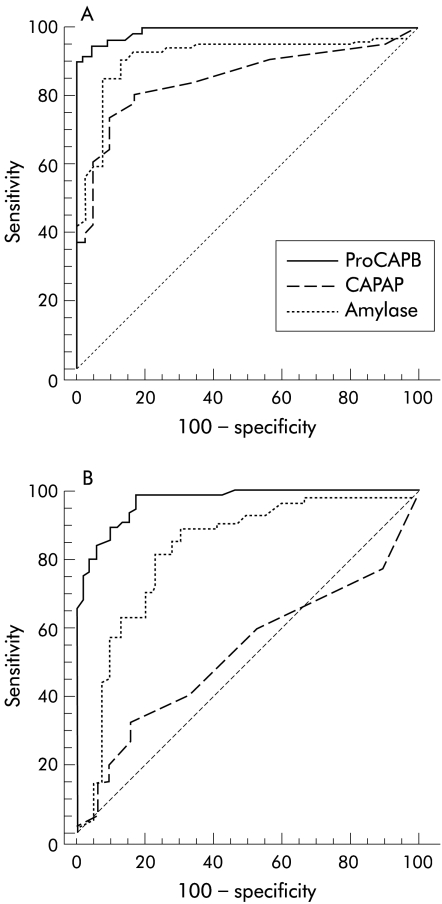
Levels of ir-proCAPB and amylase were significantly more elevated in patients with AP compared with patients with AAD for all four days studied (fig 1A ▶, table 2 ▶). The accuracy of the diagnosis of AP, calculated from the ROC curves, was higher for proCAPB than for total amylase, especially 72 hours after the onset of pain (fig 2 ▶). Ir-CAPAP levels were also elevated in patients with AP (fig 1B ▶) but the accuracy of the diagnosis of AP was not as high as that for ir-proCAPB and amylase (table 2 ▶). This was due to low levels of ir-CAPAP in several patients with oedematous pancreatitis (fig 3B ▶). The optimal cut off level for ir-proCAPB for the diagnosis of AP within 48 hours of onset of pain, according to the ROC curve analysis, was 2.5 nmol/l.
Table 2.
Accuracy for diagnosis and prediction of development of pancreatic necrosis in acute pancreatitis. Receiver operating curve (ROC) analysis in patients with acute pancreatitis (n=85) and acute abdominal pain of non-pancreatic origin (n=53)
| Diagnosis | Necrosis | |||||||
| Analysis time (h)* | Cut off (nmol/l) | Sens (%) | Spec (%) | Accuracy (AUC*) | Cut off (nmol/l) | Sens (%) | Spec (%) | Accuracy (AUC*) |
| ProCAPB <48 h | 2.5 | 95 | 95 | 0.990 | 13.2 | 71 | 49 | 0.565 |
| ProCAPB 48–72 h | 1.2 | 94 | 94 | 0.982 | 6.5 | 88 | 44 | 0.663 |
| ProCAPB 72–96 h | 2.5 | 99 | 82 | 0.968 | 27.4 | 97 | 11 | 0.511 |
| CAPAP <48 h | 0.3 | 81 | 83 | 0.850 | 3.2 | 95 | 87 | 0.920 |
| CAPAP 48–72 h | 0.5 | 40 | 90 | 0.675 | 0.4 | 89 | 84 | 0.882 |
| CAPAP 72–96 h | 0.3 | 33 | 84 | 0.527 | 0.3 | 67 | 89 | 0.848 |
| Amylase†/CRP‡ <48 h | 304† U/l | 90 | 87 | 0.902 | 28‡ mg/l | 60 | 68 | 0.635 |
| Amylase† /CRP‡ 48–72 h | 262† U/l | 92 | 87 | 0.912 | 129‡ mg/l | 93 | 66 | 0.845 |
| Amylase† /CRP‡ 72–96 h | 201† U/l | 82 | 77 | 0.819 | 148‡ mg/l | 100 | 65 | 0.878 |
AUC, area under the curve in the ROC plot; CAPAP, carboxypeptidase B activation peptide; proCAPB, procarboxypeptidase B.
*Time after onset of symptoms to blood sampling (hours).
†Amylase for diagnosis; ‡CRP for prediction of necrosis.
Figure 2.
Receiver operating characteristic curve for diagnosis of acute pancreatitis with samples obtained within 48 hours (A) and 72–96 hours (B) after the onset of pain. ProCAPB, immunoreactive procarboxypeptidase B; CAPAP, immunoreactive carboxypeptidase B activation peptide.
Accuracy for the prediction of pancreatic necrosis
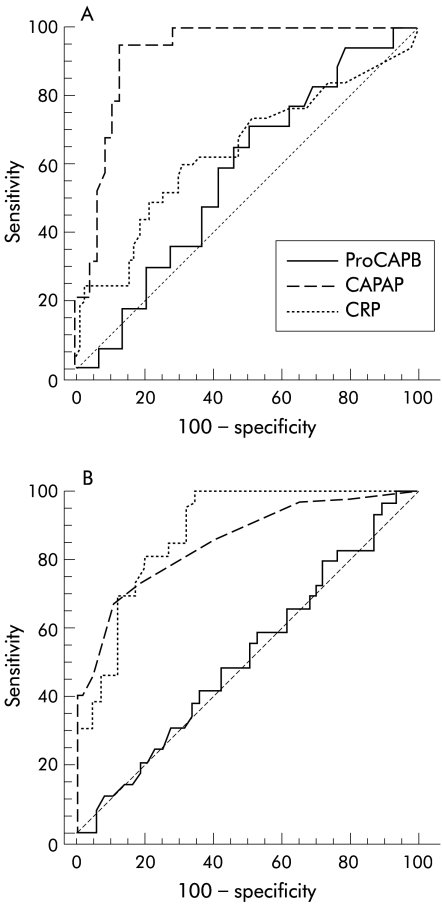
Levels of ir-proCAPB in serum were similar in patients with oedematous pancreatitis and pancreatic necrosis at all times (fig 3A ▶) Ir-CAPAP levels however were significantly more elevated in sera from patients with pancreatic necrosis compared with patients with oedematous pancreatitis (fig 3B ▶, table 2 ▶). This was especially so within 48 hours of the onset of pain. In contrast, CRP levels did not differ between oedematous pancreatitis and pancreatic necrosis until after 48 hours (table 2 ▶). In samples collected within 48 hours of the onset of pain, the accuracy for prediction of pancreatic necrosis was very good with an AUC of 0.920 compared with CRP (0.635) and ir-proCAPB (0.565). If samples were obtained more than 48 hours after onset, ir-CAPAP and CRP levels were equally good for prediction of pancreatic necrosis but ir-proCAPB levels still did not correlate (AUC values of 0.848, 0.878, and 0.511 respectively) (fig 4 ▶, table 2 ▶).
Figure 4.
Receiver operating characteristic curve for prediction of pancreatic necrosis in acute pancreatitis with samples obtained within 48 hours (A) and 72–96 hours (B) after the onset of pain. ProCAPB, immunoreactive procarboxypeptidase B; CAPAP, immunoreactive carboxypeptidase B activation peptide; CRP, C reactive protein.
Correlation between ir-CAPAP and CRP
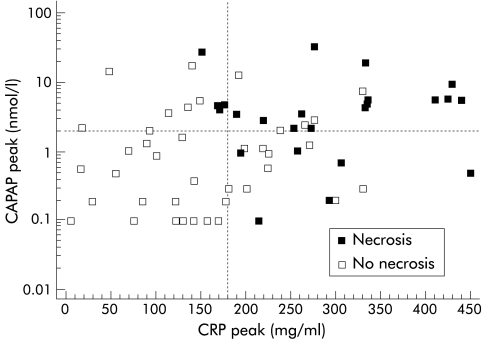
The correlation between peak values for ir-CAPAP and CRP obtained within 96 hours after the onset of pain is shown in fig 5 ▶. There was a statistically significant correlation (Spearman's rho 0.525, p=0.001) although a substantial number of cases of oedematous pancreatitis had CRP levels >150 mg/l but still low ir-CAPAP levels (< 2 nmol/l).
Figure 5.
Correlation between peak levels of immunoreactive carboxypeptidase B activation peptide (CAPAP) in serum and peak levels of C reactive protein (CRP) in serum during the first four days after the onset of pain in patients with acute pancreatitis (n=85).
Correlation with extent of necrosis
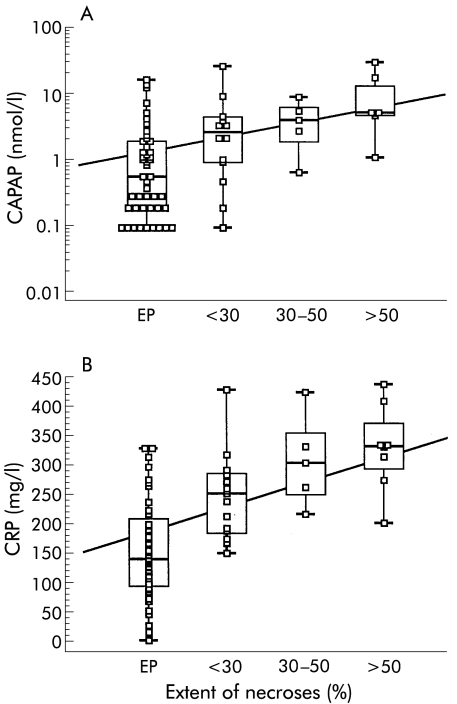
There was no correlation between serum levels of ir-proCAPB on admission and the extent of necrosis. The correlation between peak serum levels of ir-CAPAP and extent of necrosis is shown in fig 6A ▶ (Spearman's rho 0.559, p<0.0001). A similar correlation with extent of necrosis was seen for peak serum levels of CRP, as shown in fig 6B ▶ (Spearmans's rho 0.680, p<0.0001).
Figure 6.
Correlation between peak levels of serum immunoreactive carboxypeptidase B activation peptide (CAPAP) (A) and C reactive protein (CRP) (B) during the first four days after the onset of pain and extent of pancreatic necrosis in patients with acute pancreatitis.
DISCUSSION
The PASP method measures a mixture of three different molecular forms of immunoreactive proCAPB—that is, the proenzyme, the active enzyme, and the activation peptide (CAPAP).7 We measured, for the first time, two of these variables, the proenzyme and the activation peptide, separately. An early study on PASP/ProCAPB showed a high sensitivity for the diagnosis of AP but no correlation with severity or aetiology of the disease.4 In a more recent study, the overall accuracy of the PASP assay to differentiate patients with pancreatic necrosis from patients with oedematous pancreatitis within the first four days after onset of pain was reported to be 79% (AUC 0.790).19 The specific CAPAP assay reported here gives an accuracy of 92% (AUC 0.920) in this respect. The specific proCAPB assay however cannot differentiate pancreatic necrosis from oedematous pancreatitis (AUC 0.565).
In contrast, the capacity of the specific proCAPB assay to diagnose AP, irrespective of severity grade, was higher than that of the specific CAPAP assay (AUC 0.990 for the proCAPB assay and 0.850 for the CAPAP assay within 48 hours of onset).
These results show that by measuring a specific marker for proenzyme activation (ir-CAPAP) alone, an assay with a very high accuracy for prediction of pancreatic necrosis can be obtained although the diagnostic accuracy for AP is not very high. By measuring a specific marker for proenzyme leakage such as ir-proCAPB, an assay that is more specific for the diagnosis of AP but without any discriminatory capacity concerning prediction of pancreatic necrosis can be obtained, in common with assays such as those for amylase and lipase in serum used in clinical practice.
Trypsin may be regarded as an early mediator of inflammation in AP, primarily due to its capability to activate different cascade systems and also other pancreatic proenzymes.8 Trypsin activity in biological fluids may be a function of at least three processes: firstly, activation of trypsinogen, secondly, development of an imbalance between trypsin and trypsin inhibitors locally in and around the pancreatic gland, and thirdly, there may also be an inhibitory trypsin autoinactivation mechanism, as suggested by studies in hereditary pancreatitis.20 Activation of other pancreatic proenzymes, more downstream in the activation cascade, such as proCAPB, are trypsin induced and can only occur when there is a local imbalance between trypsin and trypsin inhibiting mechanisms. It is thought that active pancreatic enzymes such as phospholipase A2, pancreatic elastase, and carboxypeptidases contribute to the development of necrosis in AP (“autodigestion concept”). This could be the explanation for why markers for proenzyme activation, such as ir-CAPAP levels, correlate with the degree of necrosis in AP.
The correlation between peak ir-CAPAP values and maximum CRP values during the course of the disease (fig 5 ▶) show that many attacks of mild pancreatitis are accompanied by CRP levels in the range 100–200 mg/l but low levels of ir-CAPAP (<2 nmol/l). Ir-CAPAP correlates better with necrosis than inflammation.
Measuring ir-CAPAP is not an optimal method of diagnosing AP, primarily as a substantial number of patients with mild AP do not have elevated levels. This is in accordance with results from studies on TAP in ERCP induced mild AP where 50% of cases had undetectable levels of TAP in urine.21 Thus proenzyme activation may not be prominent in mild AP.
Levels of ir-proCAPB in the circulation of healthy controls were approximately 0.45 nmol/l (10 μg/l). This is in the same range as levels of other pancreatic proenzymes such as anionic and cationic trypsinogens which are present in normal serum in concentrations of 20–25 μg/l17 and are also close to levels in serum reported for human PASP (15 μg/l).19
Normal serum levels of ir-CAPAP are probably very low. Most samples from healthy controls showed zero levels and only two had measurable levels (0.10 and 0.19 nmol/l). These were subjects with ir-proCAPB levels in the upper range (1.55 and 1.75 nmol/l, respectively). Thus these CAPAP levels may still represent cross reacting proCAPB. Zero levels of ir-CAPAP in serum is what you expect when there is no premature activation of proCAPB within the pancreatic gland and no reabsorption of the CAPAP from the intestine.
We excluded patients with an APACHE II score ≤3 in the disease control group with acute abdominal pain of non-pancreatic origin. This was because in a group of patients with mild abdominal disorders, even without increased amylase levels, it is difficult to completely rule out a diagnosis of AP as these patients have usually not undergone CT or surgery. The question would then arise of whether or not there were any patients with acute pancreatitis in the control group. In our control group, the diagnosis was verified in 48 of 53 cases by laparotomy. Only two patients were diagnosed without laparotomy or CT. We do not believe that this overestimates the diagnostic accuracy of proCAPB because of our true control group but it may hamper comparisons with other studies.
It is well known that amylase levels may decrease rapidly after the onset of pain in AP. Lipase activity decreases more slowly than amylase and this is why some authors prefer lipase to amylase in establishing a diagnosis of AP.22 The same appears to be true for ir-proCAPB levels as in this study we found a slightly higher accuracy for proCAPB levels compared with amylase levels. This may be due to the longer half life of proCAPB in the circulation as differences are seen, especially between 48 and 96 hours after the onset of disease. The same conclusion was also drawn in an earlier study on PASP/ProCAPB in AP.4
Measuring the active enzyme CAPB specifically is not yet possible due to cross reactions with the proenzyme but such an assay could be fruitful in the future. One advantage over CAPAP could be that CAPB levels would probably stay elevated for longer in the circulation after the onset of disease as CAPB is eliminated more slowly than CAPAP from the circulation.15
The optimum method for early determination of the severity of AP at admission could be a fast method for measurement of active CAPB or CAPAP in serum or urine. The technology for rapid determination of peptides in urine using “immunosticks” is available today23 and can probably be applied to the measurement of active CAPB or CAPAP in the near future.
Acknowledgments
This study was supported by grants from the Swedish Medical Research Council, projects No 17-X-8305–09A, and the Foundations for Research at the University Hospital, Malmö, Sweden.
Abbreviations
AAD, acute abdominal disorders of non-pancreatic origin
AP, acute pancreatitis
ALP, alkaline phosphatase
CAPAP, carboxypeptidase B activation peptide
CAPB, carboxypeptidase B
CRP, C reactive protein
CT, computed tomography
ir, immunoreactive
PASP, pancreas specific protein
proCAPB, procarboxypeptidase B
ROC, receiver operating characteristic
TAP, trypsinogen activation peptide
REFERENCES
- 1.Burgos FJ, Salva M, Villegas V, et al. Analysis of the activation process of porcine procarboxypeptidase B and determination of the sequence of its activation segment. Biochemistry 1991;30:4082–9. [DOI] [PubMed] [Google Scholar]
- 2.Appelros S, Thim L and Borgström A. Activation peptide of carboxypeptidase B in serum and urine in acute pancreatitis. Gut 1998;42:97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernstad R, Pousette A, Carlström K, et al. A novel assay for pancreatic cellular damage. IV. Serum concentrations of pancreas-specific protein (PASP) in acute pancreatitis and other abdominal diseases. Pancreas 1990;5:42–9. [PubMed] [Google Scholar]
- 4.Pezzilli R, Billi P, Platé L, et al. Human pancreas-specific protein/procarboxypeptidase B: a useful serum marker of acute pancreatitis. Digestion 1994;55:73–7. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto KK, Pousette Å, Chow P, et al. Isolation of a cDNA encoding a human serum marker for acute pancreatitis. Identification of pancreas-specific protein as pancreatic probarboxypeptidase B. J Biol Chem 1992;267:2575–81. [PubMed] [Google Scholar]
- 6.Schmid SW, Uhl W, Steinle A, et al. Human pancreas-specific protein. A diagnostic and prognostic marker in acute pancreatitis and pancreas transplantation. Int J Pancreatol 1996;19:165–70. [DOI] [PubMed] [Google Scholar]
- 7.Fernstad R, Kylander C, Tsai L, et al. Isoforms of procarboxypeptidase B (pancreas-specific protein, PASP) in human serum, pancreatic tissue and juice. Scand J Clin Lab Invest Suppl 1993;213:9–17. [DOI] [PubMed] [Google Scholar]
- 8.Ohlsson K, Balldin G, Bohe M, et al. Pancreatic proteases and antiproteases in pancreatic disease; biochemical, pathophysiological and clinical aspects. Int J Pancreatol 1988;3(suppl 1):S67–78. [PubMed] [Google Scholar]
- 9.Heath DI, Cruickshank A, Gudgeon AM, et al. The relationship between the pancreatic enzyme release and activation and the acute-phase protein response in patients with acute pancreatitis. Pancreas 1995;10:347–53. [DOI] [PubMed] [Google Scholar]
- 10.Leach SD, Modulin IM, Scheele GA, et al. Intracellular activation of digestive zymogens in rat pancreatic acini. Stimulation by high doses of cholecystokinin. J Clin Invest 1991;87:362–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gudgeon AM, Heath DI, Hurley P, et al. Trypsinogen activation peptides assay in the early prediction of severity of acute pancreatitis. Lancet 1990;335:4–8. [DOI] [PubMed] [Google Scholar]
- 12.Neoptolemos JP, Kemppainen EA, Mayer JM, et al. Early prediction of severity in acute pancreatitis by urinary trypsinogen activation peptide: a multicentre study. Lancet 2000;355:1955–60. [DOI] [PubMed] [Google Scholar]
- 13.Borgström A, Lasson Å. Trypsin-alpha 1-protease inhibitor complexes in serum and clinical course of acute pancreatitis. Scand J Gastroenterol 1984;19:1119–22. [PubMed] [Google Scholar]
- 14.Hedström J, Sainio V, Kemppainen E, et al. Serum complex of trypsin 2 and alpha 1 antitrypsin as diagnostic and prognostic marker of acute pancreatitis: clinical study in consecutive patients. BMJ 1996;313:333–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Appelros S, Borgström A. Studies on the turnover of procarboxypeptidase B, its active enzyme and the activation peptide in the pig. Biol Chem 1998;379:893–8. [DOI] [PubMed] [Google Scholar]
- 16.Balthazar EJ, Freeny CF, von Sonnenberg E. Imaging and intervention in acute pancreatitis. Radiology 1994;193:297–306. [DOI] [PubMed] [Google Scholar]
- 17.Kimland M, Russik C, Marks WH, et al. Immunoreactive anionic and cationic trypsin in human serum. Clin Chim Acta 1989;184:31–46. [DOI] [PubMed] [Google Scholar]
- 18.Zweig MH, Campbell G. Receiver-operating characteristics (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem 1993;39:561–77. [PubMed] [Google Scholar]
- 19.Rau B, Cebulla M, Uhl W, et al. The clinical value of human pancreas-specific protein procarboxypeptidase B as an indicator of necrosis in acute pancreatitis: comparison to CRP and LDH. Pancreas 1998;17:134–9. [DOI] [PubMed] [Google Scholar]
- 20.Whitcomb DC, Gorry MC, Preston RA, et al. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet 1996;14:141–5. [DOI] [PubMed] [Google Scholar]
- 21.Banks PA, Carr-Locke DL, Slivka A, et al. Urinary trypsinogen activation peptides (TAP) are not increased in mild ERCP-induced pancreatitis. Pancreas 1996;12:294–7. [DOI] [PubMed] [Google Scholar]
- 22.Chase CW, Barker DE, Russell WL, et al. Serum amylase and lipase in the evaluation of acute abdominal pain. Am Surg 1996;62:1028–33. [PubMed] [Google Scholar]
- 23.Hedstrom J, Korvuo A, Kenkimaki P, et al. Urinary trypsinogen-2 test strip for acute pancreatitis. Lancet 1996;347:729–30. [DOI] [PubMed] [Google Scholar]