Abstract
Background: Primary biliary cirrhosis (PBC), a chronic cholestatic liver disease, is frequently associated with severe hypercholesterolaemia but the clinical significance of this finding is unclear.
Aims: To characterise changes in serum lipid profile over time and to assess the risk of cardiovascular disease in PBC.
Subjects and methods: We studied a cohort of 400 PBC patients for 6.2 years (range 4 months to 24 years) by serial determinations of serum lipid levels and registration of all cardiovascular events. Subjects included in an Italian prospective population based study served as controls.
Results: At presentation, 76% of patients had serum cholesterol levels >5.2 mmol/l. Hyperbilirubinaemic patients had higher total cholesterol and lower high density lipoprotein (HDL) cholesterol levels (p<0.001). With time, disease progression was associated with a reduction in total (p<0.001) and HDL (p<0.05) cholesterol. The incidence of cardiovascular events was similar to that of the general population (cerebrovascular events: standardised ratio 1.4; 95% confidence interval 0.5–3.7; coronary events: 2.2; 0.9–4.3). Hypertension was associated with an increased risk of cardiovascular events (3.8; 1.6–8.9). Association with moderate hypercholesterolaemia was of borderline significance (3.8; 0.9–17) whereas severe hypercholesterolaemia was not associated with increased risk (2.4, 0.5–11).
Conclusions: In PBC, serum cholesterol levels markedly increase with worsening of cholestasis, and decrease in the late disease stages, despite a severe reduction in biliary secretion. Marked hypercholesterolaemia, typical of severe longstanding cholestasis, is not associated with an excess risk of cardiovascular disease while less advanced patients with moderate hypercholesterolaemia are exposed to an increased cardiovascular risk. Putative protective factors in PBC patients with severe hypercholesterolaemia should be assessed.
Keywords: primary biliary cirrhosis, hypercholesterolaemia, cholesterol, cardiovascular disease
Primary biliary cirrhosis (PBC) is a chronic cholestatic liver disease of unknown aetiology which may lead to death or liver transplantation for the complications of cirrhosis. The rate of disease progression is highly variable among patients.1 Most cases are being recognised with increasing frequency in the early stages of disease and may survive several decades after diagnosis.2 As in other cholestatic liver diseases, reduction in biliary lipid secretion leads to very high levels of serum cholesterol.3–6 Earlier cross sectional studies5,7 found higher levels of total cholesterol and lower levels of high density lipoprotein (HDL) cholesterol in patients with advanced disease compared with those in the earlier stages. Other studies8,9 suggested that reduced hepatic synthesis and intestinal absorption in the terminal stage of PBC may lead to decreased total cholesterol levels thus outweighing the cholesterol raising effect exerted by severely reduced biliary secretion. Lack of prospective evaluation of large cohorts of unselected patients means that this issue is unresolved.
In the general population, hypercholesterolaemia is associated with increased morbidity and mortality from cardiovascular disease,10 and a relationship between cholesterol levels and cardiovascular disease, with higher risks affecting subjects with severe hypercholesterolaemia (≥280 mg/dl), has been established.11 Middle aged and elderly individuals are particularly exposed to increased cardiovascular risk.11 As PBC is usually diagnosed in the fifth or sixth decade, hypercholesterolaemia may reasonably be expected to also involve a substantial risk of cardiovascular diseases in these patients. The risk of cardiovascular disease in PBC has been investigated in a few studies7,12 but an increase in related mortality was not demonstrated. Such data however were obtained retrospectively, and the incidence of non-fatal cardiovascular events in PBC was not addressed. Such information is important because studies restricting their scope to fatal cardiovascular events may be subject to bias due to selective removal of patients dying from liver disease.13
We have studied a large cohort of PBC patients who consecutively attended our clinic from 1974 to evaluate changes in serum total and HDL cholesterol concentrations over time, and to assess whether hyperlipidaemia is associated with an increased incidence of cardiovascular events. Follow up of patients, with clinical and laboratory assessments performed at the same centre, included serial determinations of lipid distribution and recording of all clinically relevant, both fatal and non-fatal, cardiovascular events.
METHODS
Patient population and study design
All patients who consecutively presented to the study centre at the San Paolo Hospital, Milan, from June 1974 to December 1997 were included in the study. Follow up was stopped in December 2000. Patients fulfilling the following widely accepted diagnostic criteria for PBC were included: presence of cholestatic liver disease for at least six months; liver biopsy compatible with PBC and a positive test for antimitochondrial antibodies; serum alkaline phosphatase levels at least 1.5 times the upper limit of normal values; and absence of biliary obstruction, as assessed by ultrasonography, computed tomography, or endoscopic cholangiography.
A total of 400 unselected patients were studied over a median follow up period of 6.2 years (range 4 months to 24 years), corresponding to an overall period of 10.4 years (range 4 months to 34 years) since clinical onset of disease. The study centre acted as a primary, secondary, and tertiary referral centre on a nationwide basis. Patients were visited at least every six months at the outpatient clinic, and a complete biochemical evaluation was performed in the hospital laboratory at each visit. When hospitalisation was required, either for liver related problems or for comorbid disease, it generally took place in one of the hospital divisions where patients could be followed by the same clinicians in charge of the PBC population throughout the study period. This was the case in 24 of 26 patients who were hospitalised for cardiovascular events.
The present study was based on analysis of observational data prospectively collected from each patient in a clinical database specifically characterising the clinical course of the disease, with special interest in all comorbid events. Such information was acquired with the aid of checklists and included history, symptoms, clinical findings, comorbid diseases, and any data from laboratory or other diagnostic investigations obtained at the time of the initial visit and during follow up. All data were reported on detailed clinical records and personal computer data base files, which were updated yearly.
Among the 400 patients included in this cohort study, 215 patients were administered ursodeoxycholic acid (UDCA) 10–12 mg/kg body weight for at least one year for the treatment of liver disease, and 34 patients received cholestyramine for the treatment of pruritus. Median duration of bile acid treatment was 3.7 years (range 1–11). As UDCA and cholestyramine may influence cholesterol metabolism,14–17 only determinations obtained before starting treatment were considered in the study of changes in serum cholesterol levels over time. To reduce individual variability and overcome regression to the mean effects, the average values of the first three determinations of total and HDL cholesterol obtained during the first year of follow up were considered as baseline levels. Serum lipid profile (total cholesterol, HDL cholesterol, and triglycerides levels) at presentation or during follow up was stratified according to Mayo risk score values calculated at the time of presentation.18 Risk score values were stratified according to probability of survival (low, intermediate, and high risk), as suggested by Grambsch and colleagues.19 Serum lipid profile was also stratified according to serum bilirubin levels, below and above the upper limit of the normal range (≤19 μmol/l or >19 μmol/l) as serum bilirubin is a specific biochemical marker of cholestasis in PBC.20 All blood samples were collected in fasting individuals and analysed by routine clinical methods at the hospital laboratory. Calculation of low density lipoprotein (LDL) levels was based on determination of total and HDL cholesterol and triglyceride values—that is, the sum of HDL cholesterol and triglyceride values divided by six was subtracted from total cholesterol values.
Both fatal and non-fatal coronary and cerebrovascular events were considered in the assessment of cardiovascular risk. Specific diagnoses, according to the WHO international codes, were the following: acute myocardial infarction and ischaemic heart disease (WHO ICD-9-CM codes 410–414) for coronary events, and ischaemic and haemorrhagic stroke and transitory ischaemic attack (codes 431–438) for cerebrovascular events. Coronary and cerebrovascular events observed in PBC patients during follow up were compared with data collected during the first three years (1983–1985) of the MONICA (Monitoring of Cardiovascular Diseases) project for Central Italy.21 In that study, 197 928 males and 206 338 females, aged 25–74 years, were observed for three years, and data for fatal and non-fatal cardiovascular events occurring among hospital patients and outpatients were collected. Incidence results are expressed as attack rates, which consider not only the first event but also relapses. In the study of the incidence of cardiovascular events in PBC, the time from clinical onset of disease to the last visit was considered. Comparison of the incidence of cardiovascular events between PBC patients and the control population focused on patients aged 25–74 years, corresponding to the ages of the latter population. All analyses addressing the incidence of cardiovascular events were initially performed including patients treated with cholestyramine and were repeated after exclusion of these patients from the data set.
Analysis of the data
Comparison of serum lipid values between PBC patients with serum bilirubin levels of ≤19 or >19 μmol/l at the time of presentation was performed using the Mann-Whitney test. Serum lipid values at the first and last follow up determinations were compared using the Wilcoxon signed rank test.
To assess the presence of a gradient across more than two groups of patients arrayed in a prespecified order, the Kruskal-Wallis test, a non-parametric analysis of variance, was preliminarily performed on the variable of interest, followed, in the case of a significant result, by a non-parametric test for trend.22
Age and sex specific attack rates for coronary and cerebrovascular events observed in the control population were used to calculate the number of expected events in PBC patients using indirect standardisation procedures. The ratio between observed and expected events and 95% confidence interval was calculated assuming a Poisson distribution of cardiovascular events.23 Cumulative proportions of PBC patients developing a cardiovascular event were estimated by means of the Kaplan-Meier approach using the date of presentation as the starting point.
The log rank test was used to assess differences among groups of patients defined by levels of serum cholesterol at presentation (<5.2 mmol/l; 5.2–6.5 mmol/l; 6.6–7.8 mmol/l; >7.8 mmol/l). Univariate analyses of different risk factors for cardiovascular events were performed using the log rank test. The proportional hazards Cox model was used to assess within the framework of multivariate analysis the effects of the risk factors associated (p<0.20) with cardiovascular events at univariate analysis.
When incomplete data were available on variables conveying important prognostic information, a best case/worst case analysis was performed. According to this approach, the effects of missing information are estimated comparing results from two analyses: one assuming that lacking data were in the best prognostic class and the other assuming they were in the worst.
All statistical analyses were two sided and were made using Stata Statistical Software (Stata Corporation, College Station, Texas, USA).
RESULTS
Patient characteristics at the time of the first visit are reported in table 1 ▶. Patients with abnormal serum bilirubin concentrations showed significantly higher levels of serum total cholesterol (6.8, range 2.0–26.8 mmol/l v 6.2, range 3.0–11.1 mmol/l; p<0.001) and triglycerides (1.4, 0.4–5.5 mmol/l v 1.2, 0.3–5.3 mmol/l; p=0.004), and significantly lower levels of HDL cholesterol (1.3, 0.1–3.7 mmol/l v 1.5, 0.6–2.9 mmol/l; p<0.001) compared with patients with normal bilirubin values.
Table 1.
Patient characteristics at the time of the first visit
| Sex (M/F) | 40/360 |
| Age (y) | 54 (21–86) |
| No of symptomatic patients* | 188 (47%) |
| No with liver cirrhosis | 152 (38%) |
| Total bilirubin (μmol/l) (nv ≤19) | 14 (3–391) |
| Alkaline phosphatase (IU/l) (nv <279) | 729 (70–4615) |
| Albumin (g/l) | 41 (25–53) |
| Prothrombin time (INR) | 1.0 (0.4–1.8) |
| Total cholesterol (mmol/l) | 6.2 (2.0–26.8) |
| No with total cholesterol >5.2 mmol/l | 304 (76%) |
| HDL cholesterol (mmol/l) | 1.5 (0.1–3.7) |
| No with HDL cholesterol <1.0 mmol/l | 49 (17%) |
| Total/HDL cholesterol ratio | 4.1 (1.8–59.2) |
| *The following are considered specific symptoms of primary biliary cirrhosis: pruritus, jaundice, major complications of portal hypertension (ascites, variceal bleeding, portal-systemic encephalopathy). | |
| Laboratory data are presented as median (range). | |
| HDL, high density lipoprotein; INR, international normalised ratio; nv, normal value. | |
To convert SI units to mg/dl, total and HDL cholesterol values should be multiplied by 38.7 and bilirubin values should be divided by 17.1.
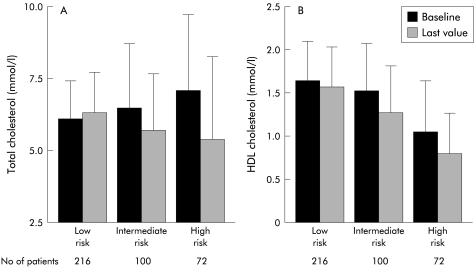
Decreasing levels of HDL cholesterol were observed among patients grouped according to disease progression, as assessed by the Mayo risk score (fig 1 ▶). Changes in total and HDL cholesterol over time showed a significant gradient across Mayo risk score groups, with patients characterised by more severe disease presenting greater reductions. Serum triglyceride levels did not change significantly during follow up.
Figure 1.
Serum levels of total (A) and high density lipoprotein (HDL) (B) cholesterol at the initial and last determinations, according to the baseline Mayo risk score. At the time of initial evaluation, decreasing concentrations of HDL cholesterol (p<0.001) were observed among patients with increasing severity of disease, as assessed by the Mayo risk score (low, intermediate, or high risk).19 The trend towards increasing levels of total cholesterol was not significant (p=0.260). During follow up, greater reductions in serum lipids tended to occur among patients with more severe disease (significant trends for reduction of total cholesterol, p<0.001, and of HDL cholesterol, p=0.010). The last serum lipid determination was obtained before starting ursodeoxycholic acid or cholestyramine treatment. Average values of the first three lipid determinations, obtained during the first year of follow up, were considered baseline levels. Values are mean (SD).
At the end of the study, 197 patients were alive and attending regular follow up, 105 had died (80 from complications of end stage liver disease, 25 from other causes), and 28 had undergone orthotopic liver transplantation. In the remaining 70 patients, who at any time had discontinued regular follow up, information on clinically relevant events were obtained by telephone interview and examination of hospital records. During the overall follow up period, 28 cardiovascular events (16 coronary and 12 cerebrovascular events) occurred in 26 PBC patients with an overall incidence of 5.2 events per 1000 patient years. Three of the above cardiovascular events were fatal (3% of all deaths). The main characteristics of patients who died from a cardiovascular event are reported in table 2 ▶. The number of expected fatal and non-fatal events and the standardised event ratio are reported in table 3 ▶.
Table 2.
Main characteristics of the patients who died of cardiovascular disease
| Patient BG | Patient GG | Patient TC | |
| Sex | Male | Female | Male |
| Age at death (y) | 68 | 63 | 58 |
| Cause of death | Ischaemic stroke | Ischaemic stroke | Acute myocardial infarction |
| Total bilirubin (μmol/l) | 14 | 12 | 176 |
| Total cholesterol (mmol/l) | 3.5 | 5.9 | 19.1 |
| HDL cholesterol (mmol/l) | 1.1 | 1.8 | 1.4 |
| Total /HDL cholesterol ratio | 3.2 | 3.3 | 13.6 |
| Other risk factors | Diabetes | Cigarette smoking, obesity | Diabetes |
Laboratory data were obtained within the six months preceding death.
HDL, high density lipoprotein.
Table 3.
Standardised cardiovascular event ratio in 350 patients with primary biliary cirrhosis, aged 25–74 years
| Cardiovascular event | Observed (n) | Expected (n) | Standardised ratio (95% CI) |
| Coronary events | 8 | 3.66 | 2.19 (0.94–4.31) |
| Cerebrovascular events | 7 | 4.86 | 1.44 (0.47–3.66) |
CI, confidence interval.
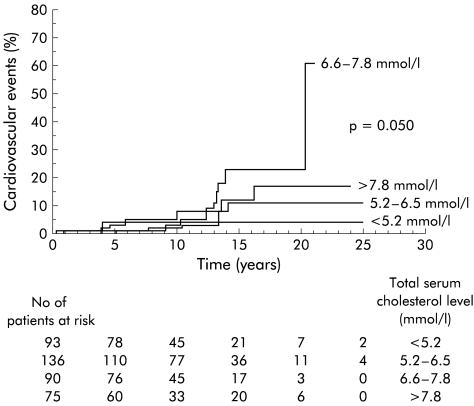
The cumulative incidence of cardiovascular events differed significantly (p=0.050) among patients showing different levels of serum total cholesterol at the time of the first visit (fig 2 ▶). The incidence was higher in patients, with values ranging from 6.5 to 7.8 mmol/l, while patients with levels higher than 7.8 mmol/l did not differ substantially from normocholesterolaemic patients. Among the total serum cholesterol groups, the proportion of patients with more severe cholestasis, as reflected by serum bilirubin values above 34 μmol/l, was significantly different (serum cholesterol <5.2 mmol/l, 26%; 5.2–6.5 mmol/l, 12%; 6.6–7.8 mmol/l, 22%; >7.8 mmol/l, 39%; p<0.001). HDL and LDL cholesterol levels, total to HDL cholesterol ratio, and HDL to LDL cholesterol ratio were not associated with an increased incidence of cardiovascular events.
Figure 2.
Kaplan-Meier estimates of the cumulative proportion of cardiovascular events in patients with different serum cholesterol levels at entry.
Apart from serum total cholesterol levels, hypertension (p<0.001) showed a significant association with incidence of cardiovascular events on univariate analysis. Cigarette smoking (p=0.135) and diabetes (p=0.191) were also weakly associated with cardiovascular events. The results of the multivariate assessment of risk factors for cardiovascular events are reported in table 4 ▶.
Table 4.
Impact of different risk factors on the development of cardiovascular events in primary biliary cirrhosis, as assessed by the proportional hazards Cox model
| Risk factor* | No of patients | Hazard ratio | 95% CI | p Value |
| Total cholesterol levels (mmol/l) | ||||
| <5.2 | 94 | 1 | – | |
| 5.2–6.5 | 137 | 1.14 | (0.23–5.78) | 0.873 |
| 6.6–7.8 | 91 | 3.85 | (0.88–16.76) | 0.073 |
| >7.8 | 76 | 2.41 | (0.51–11.28) | 0.262 |
| Hypertension | 77 | 3.80 | (1.61–8.95) | 0.002 |
| Diabetes | 15 | 4.81 | (0.91–25.37) | 0.064 |
| Cigarette smoking | 55 | 2.29 | (0.80–6.55) | 0.121 |
CI, confidence interval.
Total cholesterol values of 5.2 mmol/l correspond to 200 mg/dl, 6.5 mmol/l to 250 mg/dl, and 7.8 mmol/l to 300 mg/dl.
*Factors associated with the incidence of cardiovascular events beyond the p=0.20 level at univariate analysis were tested. Hypertension was defined as the presence of a blood diastolic pressure above 90 mm Hg and systolic pressure above 140 mm Hg on at least two measurements taken at each of two consecutive visits, according to the classification of blood pressure elaborated by the Joint Committee on Detection, Evaluation, and Treatment of High Blood Pressure. Presence of diabetes was defined according to the 1979 diagnostic criteria of the National Diabetes Data Group of the National Institutes of Health, USA. Cigarette smoking was defined as a daily smoking habit of more than 10 cigarettes.
When patients treated with cholestyramine (n =34) were excluded from analysis of cardiovascular events due to a possible protective effect of the resin, the results did not change.
DISCUSSION
In the present study, we have provided data on changes in serum lipid levels and their clinical significance in a wide cohort of patients with PBC who were followed up at the same centre for up to 20 years. At time of the first visit, more than 75% of patients with this chronic cholestatic disease showed serum total cholesterol levels above 5.2 mmol/l (200 mg/dl). More marked alterations in serum lipid profile were detected in patients with more advanced disease, as assessed by serum bilirubin values, mainly reflecting the severity of cholestasis, and by the Mayo risk score, a validated prognostic index in PBC. At time of the first visit, HDL cholesterol displayed a different pattern from total cholesterol, which was characterised by decreased, rather than increased, values in patients with more advanced disease. Similar results were also reported in previous studies based on cross sectional evaluation of PBC patients.5,7 In the present study, we have shown that a progressive decrease in both total and HDL cholesterol occurs over time, and that such changes are more prominent in patients with more advanced disease. Overall, these findings indicate that, paralleling the development of cholestasis, an increase in serum cholesterol initially occurs, which is followed by a trend towards decreasing values with further worsening of liver disease. This suggests that progressive loss of liver synthetic activity and impaired intestinal lipid absorption, which characterise PBC during its late stages, may overcome the lipid raising effect of chronic cholestasis. Compared with total cholesterol, the decrease in HDL cholesterol levels occurs earlier in the clinical course of PBC, and further studies addressing the use of this determination as a marker of disease progression are warranted.
Despite marked hypercholesterolaemia, excess mortality from cardiovascular diseases was not found in our PBC population. This finding is in agreement with other studies, and led some investigators to surmise that PBC patients may even be protected from cardiovascular disease.7,12,24 It should be highlighted however that relying solely on mortality figures in the assessment of risk from a comorbid condition may be misleading in patients affected by a chronic, ultimately fatal disease.13 In fact, patients at risk of cardiovascular disease may be progressively removed from subsequent follow up due to the competing risk of the underlying liver disease. Such selection artefacts may have been limited in our population by accurate detection of non-fatal earlier comorbid events. Close patient follow up at the same centre, with immediate recording of any clinically relevant event, allowed us to reliably estimate for the first time the incidence of non-fatal cardiovascular events in PBC. The present data suggest that despite the high prevalence of hypercholesterolaemia, patients with PBC are not exposed to a higher risk of cardiovascular events than the general population. Active patient observation that may condition differential detection rates of clinical events in diseased patients and in the general population25 may be the most likely explanation for the incidences of coronary events that appeared to be slightly higher and of borderline significance in PBC. However, it should be noted that, as indicated by the confidence intervals, the number of cardiovascular events that occurred in the PBC cohort may not be large enough to definitely exclude an association between PBC and cardiovascular morbidity. Finally, the possibility remains that active follow up of our patient cohort favoured more prompt recognition and treatment of risk factors other than hypercholesterolaemia, resulting in more effective prevention of cardiovascular events in PBC than in the general population.
Analysis of risk factors for cardiovascular events shows that, as it occurs in the general population, hypertension retains a significant role in PBC. Interestingly, the effect of hypercholesterolaemia as a risk factor in the disease seems to be restricted to patients with moderate elevations in serum cholesterol levels—that is, between 5.2 and 6.5 mmol/l (200 and 250 mg/dl)—while patients with higher levels have a lower risk. Lack of a continuous relationship between serum cholesterol levels and risk of cardiovascular disease11 in patients with a high prevalence of severe hypercholesterolaemia suggests that two groups of hypercholesterolaemic patients coexist in the PBC population: a group of patients with hypercholesterolaemia related to cholestasis, and a group with less severe hypercholesterolaemia of familial and nutritional origin. These latter patients, also characterised by less severe cholestasis, are affected by hypercholesterolaemia of the same origin as their counterparts in the general population, and are exposed to the same increased risk of cardiovascular events. More severe hypercholesterolaemia related to cholestasis instead is characterised by a particular lipoprotein pattern, with elevated HDL levels26 and modified LDL composition (lipoprotein-X),4 which may be protective against atherogenesis. It is likely that chronic cholestasis and declining liver synthetic activity, with their opposite effects on cholesterol metabolism, offset the predictive value of cholesterol lipoprotein fractions in PBC.
In a recent pilot study, HMG CoA inhibitors proved safe and effective in reducing serum cholesterol levels in patients with PBC.27 Therefore, from a clinical point of view, differentiating between patients with hypercholesterolaemia of familial and nutritional origin may be important in selecting specific lipid lowering treatments. Such a goal may not be easily achieved in patients with initial cholestasis in whom it may be many years before a typical cholestatic profile of liver function tests develops, thus clarifying the origin of hypercholesterolaemia. Further studies, aimed at identifying some biochemical marker useful to differentiate PBC patients with familial hypercholesterolaemia from those with hypercholesterolaemia associated with cholestasis, are therefore warranted. On the other hand, in PBC patients with severe hypercholesterolaemia, greater insight into the possible antiatherogenic properties of lipoprotein patterns or of other factors that may result in protection at the endothelial surface is needed.
Abbreviations
PBC, primary biliary cirrhosis
HDL, high density lipoprotein
LDL, low density lipoprotein
UDCA, ursodeoxycholic acid
REFERENCES
- 1.Kaplan MM. Primary biliary cirrhosis. N Engl J Med 1996;335:1570–80. [DOI] [PubMed] [Google Scholar]
- 2.Metcalf JV, Mitchison HC, Palmer JM, et al. Natural history of early primary biliary cirrhosis. Lancet 1996;348:1399–402. [DOI] [PubMed] [Google Scholar]
- 3.McIntyre N, Harry DS, Pearson AJG. Progress report: The hypercholesterolemia of obstructive jaundice. Gut 1975;16:379–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agorastos J, Fox C, Harry DS, et al. Lecitin-cholesterol acyltransferase and the lipoprotein abnormalities of obstructive jaundice. Clin Sci Mol Med 1978;54:369–79. [DOI] [PubMed] [Google Scholar]
- 5.Jahn CE, Schaefer EJ, Taam LA, et al. Lipoprotein abnormalities in primary biliary cirrhosis. Gastroenterology 1985;89:1266–78. [PubMed] [Google Scholar]
- 6.Gylling H, Farkkila M, Vuoristo M, et al. Metabolism of cholesterol and low- and high-density lipoproteins in primary biliary cirrhosis: cholesterol absorption and synthesis related to lipoprotein levels and their kinetics. Hepatology 1995;21:89–95. [PubMed] [Google Scholar]
- 7.Crippin JS, Lindor KD, Jorgensen R, et al. Hypercholesterolemia and atherosclerosis in primary biliary cirrhosis: what is the risk? Hepatology 1992;15:858–62. [DOI] [PubMed] [Google Scholar]
- 8.Miettinen TA. Lipid absorption, bile acid and cholesterol metabolism in patients with chronic liver disease. Gut 1972;13:682–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nikkila K, Hockerstedt K, Miettinen TA. High cholestanol and low campesterol-to-sitosterol ratio in serum of patients with primary biliary cirrhosis before liver transplantation. Hepatology 1991;13:663–9. [PubMed] [Google Scholar]
- 10.Pekkanen J, Linn S, Heiss G, et al. Ten-year mortality from cardiovascular disease in relation to cholesterol level among men with and without preexisting cardiovascular disease. N Engl J Med 1990;322:1700–7. [DOI] [PubMed] [Google Scholar]
- 11.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatement Panel III). JAMA 2001;285:2486–96. [DOI] [PubMed] [Google Scholar]
- 12.Van Dam GM, Gips CH. Primary biliary cirrhosis in the Netherlands. An analysis of associated diseases, cardiovascular risk, and malignancies on the basis of mortality figures. Scand J Gastroenterol 1997;32:77–83. [DOI] [PubMed] [Google Scholar]
- 13.Feinstein AR, Pritchett JA, Scimpff CR. The epidemiology of cancer therapy. II. The clinical course: data, decisions, and temporal demarcations. Arch Intern Med 1969;123:323–44. [DOI] [PubMed] [Google Scholar]
- 14.Poupon RE, Oguerram K, Chrétien Y, et al. Cholesterol-lowering effect of ursodeoxycholic acid in patients with primary biliary cirrhosis. Hepatology 1993;17:577–82. [DOI] [PubMed] [Google Scholar]
- 15.Miettinen TA, Färkkilä M, Vuoristo M, et al. Serum cholestanol, cholesterol precursors, and plant sterols during placebo-controlled treatment of primary biliary cirrhosis with ursodeoxycholic acid or colchicine. Hepatology 1995;21:1261–8. [PubMed] [Google Scholar]
- 16.Nazir DJ, Horlick L, Kudchodkar BJ, et al. Mechanism of action of cholestyramine in the treatment of hypercholesterolemia. Circulation 1972;46:95–102. [DOI] [PubMed] [Google Scholar]
- 17.La Rosa J. Review of clinical studies of bile acid sequestrants for lowering plasma lipid levels. Cardiology 1989;76:55–61. [DOI] [PubMed] [Google Scholar]
- 18.Dickson RE, Grambsch PM, Fleming TR, et al. Prognosis in primary biliary cirrhosis: model for decision making. Hepatology 1989;10:1–7. [DOI] [PubMed] [Google Scholar]
- 19.Grambsch PM, Dickson ER, Kaplan M, et al. Extramural cross-validation of the Mayo primary biliary cirrhosis survival model establishes its generalizability. Hepatology 1989;10:846–50. [DOI] [PubMed] [Google Scholar]
- 20.Shapiro JM, Smith H, Schaffner F. Serum bilirubin: a prognostic factor in primary biliary cirrhosis. Gut 1979;20:137–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giampaoli S, Menotti A, Righetti G, et al. Sorveglianza degli eventi coronarici e cerebrovascolari. L'esperienza e gli insegnamenti dell'area Latina del Progetto MONICA. G Ital Cardiol 1990;20:267–71. [PubMed] [Google Scholar]
- 22.Altman DG. Practical statistics for medical research. London: Chapman and Hall, 1991.
- 23.Bailar III JC. Significance factors for the ratio of a Poisson variable to its expectation. Queries and notes. Biometrics 1964;202:639–43. [Google Scholar]
- 24.Propst A, Propst T, Lechleitner M, et al. Hypercholesterolemia in primary biliary cirrhosis is no risk factor for atherosclerosis. Dig Dis Sci 1993;38:379–80. [DOI] [PubMed] [Google Scholar]
- 25.Feistein AR. Clinical biostatistics. XLVIII. Efficacy of different research structures in preventing bias in the analysis of causation. Clin Pharmacol Ther 1979;26:129–41. [DOI] [PubMed] [Google Scholar]
- 26.Rössner ST, Befrits R, Carlson K, et al. Serum lipoproteins and lipase activities in primary biliary cirrhosis. Eur J Gastroenterol 1991;3:271–6. [Google Scholar]
- 27.Del Puppo M, Galli-Kienle M, Crosignani A, et. al. Cholesterol metabolism in primary biliary cirrhosis during simvastatin and UDCA administration. J Lipid Res 2001;42:437–41. [PubMed] [Google Scholar]