Abstract
Background and aims: Cirrhosis with liver failure due to hepatitis C virus (HCV) infection is the most common indication for liver transplantation (LT). Reinfection of the transplanted liver by HCV is inevitable, and aggressive hepatitis with accelerated progression to graft cirrhosis may be observed. Of concern, recent reports suggest that the outcome of LT for HCV may have deteriorated in recent years. Determinants of rate of progression to cirrhosis in the immunocompetent non-transplant patient are well defined, and the most powerful determinant is patient age at the time of infection. Following LT for HCV, recipient age does not affect outcome of HCV reinfection. However, the impact of donor age on graft fibrosis progression rate following LT has not been examined.
Methods: We have examined post-transplant biopsies to assess histological activity, including fibrosis stage (scored 0–6 units, 6 representing established cirrhosis), and to calculate fibrosis progression rates in 101 post-transplant specimens from 56 HCV infected LT patients. Univariate and multivariate analyses examined the impact of parameters including recipient and donor age and sex on fibrosis progression rate, and on predicted time to cirrhosis.
Results: For the cohort, median fibrosis progression rate was 0.78 units/year, and median interval from transplantation to development of cirrhosis was 7.7 years. In multivariate analysis, donor age (not recipient age) was a powerful determinant (p=0.02) of fibrosis progression rate. When the liver donor was younger than 40 years, median progression rate was 0.6 units/year and interval to cirrhosis was 10 years. When the donor was aged 50 years or more, median progression rate was 2.7 units/year and interval to cirrhosis only 2.2 years. During the observation period there has been a significant increase in donor age (p=0.01) but date of transplantation per se is not a determinant of progression rate when included in multivariate analyses.
Conclusions: Donor age has a major influence on graft outcome following transplantation for HCV. The changing organ donor profile will affect the long term results of LT for HCV. These observations have important implications for donor liver allocation.
Keywords: hepatitis C virus, liver transplantation, donor age, fibrosis progression
End stage liver disease due to hepatitis C virus infection (HCV) is becoming the principal indication for liver transplantation (LT) in Europe and North America. Reinfection of the graft is inevitable but the impact of reinfection on short and long term liver function is highly variable.1,2 Clinical manifestations of HCV reinfection are seldom evident during the first three months post-transplant. Some patients then exhibit a severe acute hepatitis associated with histological features that are unique to the immunosuppressed state.3–6 This early hepatitis may be associated with graft failure and/or rapid progression to cirrhosis.7 For others, graft hepatitis more closely resembles chronic hepatitis observed in the immunocompetent state.8 In comparison with the liver of immunocompetent HCV infected patients,9 the HCV infected graft exhibits a greater rate of fibrosis progression with early development of cirrhosis.10,11 Also, the transplanted patient with cirrhosis decompensates at a greater rate than is observed for the immunocompetent HCV infected cirrhotic patient. As observed in a recently described cohort, approximately 50% of patients with HCV graft cirrhosis will experience an episode of decompensation during 12 months of follow up, and fewer than half of those survive a further 12 months.11 Thus recurrent infection of the graft is associated with relatively rapid progression to cirrhosis, and early decompensation and death.
Numerous clinical studies have examined the natural history of HCV infection in immunocompetent and immunosuppressed cohorts. Perhaps most informative have been those studies which have examined time dependent development of liver fibrosis, and have examined the impact of various clinical parameters on fibrosis progression rate.7,9 Those studies have established that liver fibrosis develops in a linear fashion whether or not there is suppressed patient immunity. For instance, Poynard et al observed that median time from infection to cirrhosis is 30 years for the immunocompetent,9 and Berenguer et al calculated a median time of approximately 10 years for the development of cirrhosis in the transplanted liver.7 In the immunocompetent, age at the time of infection, sex, and level of alcohol consumption affect fibrosis progression rate, with relatively faster rates observed for patients who are infected at an older age. In studies of post-transplant infection, the focus has been on viral and recipient factors as potential determinants of disease progression. In particular, those studies have established the adverse impact of early and repeated episodes of acute cellular rejection (and treatment of rejection) on the subsequent course and outcome of graft infection.7,12–16 However, despite the observation that sex and age related changes in liver function may affect the response to viral infection in the immunocompetent setting, few post-transplant studies have examined the impact of donor age and sex on outcome of graft reinfection.
Thus we have examined post-transplant liver biopsies of patients transplanted for HCV infection and have calculated the fibrosis progression rate for each patient. We then sought to identify an association of progression rate with established and putative determinants of graft outcome, and with donor age and sex.
PATIENTS AND METHODS
The study group comprised all patients who underwent transplantation with a primary diagnosis of end stage liver disease caused by HCV infection. Organ allocation was determined by clinical need and by donor-recipient size compatibility, and blood group identical livers were used for all patients. Following transplantation, the immunosuppression regimen comprised cyclosporin or tacrolimus (dose adjusted according to blood levels) with azathioprine and corticosteroids (dose of prednisolone tapered and completely withdrawn during the first three post-transplant months). Episodes of significant acute cellular rejection were treated with prednisolone 200 mg daily for three consecutive days. No patient received antiviral therapy following transplantation. The Birmingham liver unit protocol required liver biopsy on day 7 post-transplant, and biopsy at times of unexplained liver dysfunction, and routine annual review biopsy (irrespective of biochemical liver function) was undertaken for most patients during the study period. Until 1996, annual review biopsy was scheduled every year. Since 1996, the protocol was amended to require review biopsies one, three, and five years post-transplant. Patients were followed from the time of transplantation until June 2000 or date of death.
For the purpose of this study, all post-transplant biopsies were reviewed by a single pathologist (RH) and scored according to the acknowledged system.17 The pathologist was unaware of organ donor details. Biopsies performed during the first three post-transplant months, when the dominant clinical problem is rejection and HCV reinfection is seldom manifest, were excluded from the study. A pathological diagnosis was established for all other biopsies. After three months, those biopsies that were normal or consistent with recurrent HCV infection were included in the study. Biopsies were excluded from study when the pathological diagnosis was other than HCV infection, for instance rejection or biliary obstruction. For each liver biopsy, a fibrosis progression rate was calculated. Simply, this rate is calculated by dividing the histological stage (represented numerically as 0–6, where 0=no fibrosis and 6=cirrhosis) by the interval from time of transplantation to time of biopsy (expressed in years). For each patient, a progression rate was calculated during the interval from transplantation until the time of the most recent liver biopsy. Also, for those patients who had more than one eligible biopsy performed during post-transplant follow up, a fibrosis progression rate between biopsies could be established.
Recipient data were retrieved from the medical records and from the Birmingham liver unit database. Data included date of transplantation, recipient age and sex, ethnic origin, primary immunosuppressive regimen (cyclosporin or tacrolimus), episodes of acute cellular rejection, hepatitis B virus (HBV) coinfection (defined as serum HBV surface antigen positivity), cold and warm organ ischaemic times, and donor age and sex. Cold ischaemic time was defined as the time between circulatory arrest in the donor and removal of the organ from ice at the time of transplantation. Warm ischaemic time was defined as the time between removal of the donor organ from the ice and release of clamps from the portal vein after implantation.
Statistical analysis
Continuous variables were summarised as median and range and compared using the Mann-Whitney test. Categorical data were expressed as numbers and percentages and compared using the χ2 test or Fisher's exact test. Logistic regression analysis was used to examine the association of fibrosis progression with putative risk factors. The dependent variable was the fibrosis progression rate, dichotomised about the median observed rate into >0.8 units per year versus ≤0.8 units per year. Date of transplantation was dichotomised into transplantation before and after November 1996. Since the cohort was ethnically heterogeneous, the ethnic background of the patients was also included in the multivariate analysis as a risk factor (non-Caucasians versus Caucasians). The expected time for the development of graft cirrhosis was estimated from the fibrosis progression rate. The odds ratio (relative risk) was used to examine the association of risk factors and rate of fibrosis.
All data were analysed using the statistical package SPSS version 6.0.
RESULTS
A total of 101 HCV positive patients who underwent 104 transplants between 1988 and December 1999 were included in the study. Three underwent early regrafting within one month of the first transplantation. Of 101 patients, 92 had at least one post-transplant liver biopsy (322 biopsies in total). Of 92 recipients, 70 had a total of 152 liver biopsies performed more than three months post-transplant. For 14/70 patients, pathological diagnoses other than normal or compatible with HCV recurrence were made, and 51 biopsies from those 14 patients were excluded from the study. Thus the final histological data included 101 liver biopsies from 56 patients. Table 1 ▶ compares the 56 included patients with 45 patients who did not have biopsies included in the final analysis. Predictably, the excluded fraction had significantly shorter follow up from the time of transplantation, and a significantly greater proportion died during follow up. None of the 45 received tacrolimus as primary immunosuppression but there were no other significant differences between the two groups. The study group included five patients who were serum HBV surface antigen (HBsAg) positive at the time of transplantation. These patients received lamivudine and HBV immunoglobulin after transplantation. All became and remain serum HBsAg negative during follow up. Also, immunohistology of post-transplant liver biopsy specimens was negative for HBV antigens.
Table 1.
Comparison of 56 patients who had post-transplant histology suitable for inclusion in the study and 45 patients who did not
| Patients without histology | Patients with histology | p Value | |
| n | 45 | 56 | |
| Follow up (months) | 12 (0–100) | 42 (10–145) | <0.001 |
| Death | 16 (36%) | 4 (7%) | <0.001 |
| Death from liver failure | 1 | 2 | 0.4 |
| Age at transplant (y) | 49 (33–65) | 48 (28–67) | 0.4 |
| Males | 35 (78%) | 45 (80%) | 0.7 |
| Caucasian | 27 (60%) | 32 (57%) | 0.8 |
| Cyclosporin/tacrolimus | 45/0 | 49/7 | 0.01 |
| Treated rejection | 16 (36%) | 21 (37%) | 0.8 |
| More than 1 rejection episode | 2 | 3 | 0.8 |
| HBsAg positive | 1 | 5 | 0.15 |
| Cold ischaemic time (min) | 709 (221–1140) | 782 (201–1280) | 0.4 |
| Warm ischaemic time (min) | 50 (33–79) | 47 (25–76) | 0.1 |
| Donor age | 45 (14–64) | 42 (15–67) | 0.6 |
Data are median (range) or number (%).
HBsAg, hepatitis B virus surface antigen.
Of the 56 included patients, 29 had only one eligible biopsy, 13 had two, 10 had three, and four patients had four eligible biopsies.
Median interval from the time of transplantation to the most recent biopsy for the 56 patients was 27 months (range 3 months to 11 years 4 months). The histological grade and stage of the 56 biopsies is shown in table 2 ▶. Median fibrosis progression rate per post-transplant year was 0.78 fibrosis units (range 0–8.39). At that rate, 50% of patients will achieve cirrhosis (stage 6 fibrosis) within 7.7 years of infection.
Table 2.
Histological scoring of the most recently performed liver biopsy for 56 eligible patients (according to Ishak and colleagues17). For each biopsy, grade is the sum of scores for interface hepatitis, confluent necrosis, focal necrosis, and portal inflammation
| Histological feature | Score |
| Interface hepatitis (scored 0–4) | 1 (0–4) |
| Confluent necrosis (0–6) | 0 (0–6) |
| Focal necrosis (0–4) | 2 (0–3) |
| Portal inflammation (0–4) | 2 (0–4) |
| Grade (0–18) | 5 (0–15) |
| Stage (0–6) | 2 (0–6) |
| Duration post-transplant (y) | 2.25 (0.24–11.33) |
| Fibrosis rate per year (units) | 0.78 (0–8.4) |
Stage is scored 0–6.
Patient data are expressed as median (range).
To identify those factors which might affect the rate of development of fibrosis in the transplanted liver, the 56 recipients were divided about the median fibrosis progression rate (0.8 units/year) into two groups. Univariate and multivariate analyses were undertaken (table 3 ▶). Of the parameters examined, older donor age (p=0.02) and more prolonged liver warm ischaemic time (p=0.01) were independently associated in the multivariate analysis with more rapid fibrosis progression. Indeed, the presentation of data in table 4 ▶ highlights the impact of donor age and warm ischaemic time on the rate of progression to cirrhosis. Predicted median time to development of cirrhosis was 10 years (95% confidence interval 4.7–13.3 years) for those recipients whose donor age was less than 40 years and only 2.7 years (1.3–5.7 years) when donor age was 50 years or more. In the univariate, but not multivariate, analysis there was a significant association of fibrosis progression rate with date of transplantation (more rapid progression to cirrhosis observed for more recently transplanted patients). The association of transplantation date with donor age and with fibrosis progression rates is illustrated in table 5 ▶. The cohort of 56 was divided into two groups according to transplantation date before or after November 1996. Fibrosis progression rate, measured for the 56 patients from the time of transplantation (and for the 26 paired post-transplant biopsies described below), was greater in the more recently transplanted cohort. Recipient age did not differ between the two cohorts. Donor age was significantly older for the recently transplanted cohort (median 45, range 17–67) than for the earlier cohort (median age 32, range 15–59) (p=0.01).
Table 3.
Comparison of recipients who experienced graft fibrosis at a fast (greater than 0.8 fibrosis units per year) or slow (less than 0.8 units per year) rate
| Slow fibrosis (<0.8 units/year) | Fast fibrosis (>0.8 units/year) | Univariate analysis | Multivariate analysis | |
| n | 29 | 27 | ||
| Recipient age (y) | 50 (34–67) | 46 (28–65) | 0.3 | 0.7 |
| Recipient sex (M/F) | 24/5 | 21/6 | 0.6 | 0.8 |
| Caucasian | 15 | 14 | 0.4 | 0.5 |
| Cyclosporin/tacrolimus | 26/3 | 23/4 | 0.6 | 0.4 |
| Treated rejection | 10 | 11 | 0.6 | 0.12 |
| >1 episode rejection | 3 | 0 | 0.2 | 0.8 |
| HBsAg positive* | 3 | 2 | 0.7 | 0.5 |
| Cold ischaemic time (min) | 787 (261–1280) | 780 (353–1055) | 0.7 | 0.4 |
| Warm ischaemic time (min) | 44 (25–58) | 51 (33–76) | 0.02 | 0.01 |
| Donor sex (M/F) | 18/11 | 20/7 | 0.34 | 0.34 |
| Donor age (y) | 38 (15–64) | 45 (17–67) | 0.09 | 0.02 |
| Date of transplantation (pre/post 11/96) | 16/13 | 12/15 | 0.42 | 0.96 |
HBsAg, hepatitis B virus surface antigen.
*Serum status pre-transplant—all were serum HBsAg negative post-transplant.
Data are median (range).
Table 4.
Rate of fibrosis progression and predicted interval from time of liver transplantation until established cirrhosis according to donor age and warm ischaemic time (WIT)
| Parameter | No of patients | Fibrosis rate per year (median) | Predicted interval from transplant to cirrhosis* |
| Donor age <40 y | 25 | 0.6 | 10 (4.7–13.3) |
| Donor age 40–49 y | 19 | 0.9 | 6.7 (3.3–11.3) |
| Donor age >49 y | 12 | 2.2 | 2.7 (1.3–5.7) |
| WIT <40 min | 14 | 0.5 | 12 (3.3–21.4) |
| WIT 40–49 min | 19 | 0.6 | 10 (2.5–21.4) |
| WIT 50–59 min | 17 | 1.0 | 6 (2.9–9.4) |
| WIT >59 min | 6 | 1.3 | 4.6 (1.1–33) |
*Values are median (95% confidence interval).
Table 5.
Impact of date of transplantation on fibrosis progression rate
| Transplanted pre 11/96 | Transplanted post 11/96 | p Value | |
| No (total) | 28 | 28 | |
| Recipient age (y) | 47 (28–66) | 48 (39–67) | 0.5 |
| Donor age (y) | 32 (15–59) | 45 (17–67) | 0.01 |
| Cold ischaemic time (min) | 787 (201–1280) | 767 (324–1055) | 0.35 |
| Warm ischaemic time (min) | 48 (25–76) | 45 (25–64) | 0.78 |
| Fibrosis progression rate (transplant to most recent biopsy) | 0.65 (0–2.6) | 1.1 (0–8.4) | 0.17 |
| Number (with paired biopsies) | 17 | 9 | |
| Fibrosis progression rate (between biopsies) | 0.2 (0–1.7) | 0.9 (−0.5–6) | 0.03 |
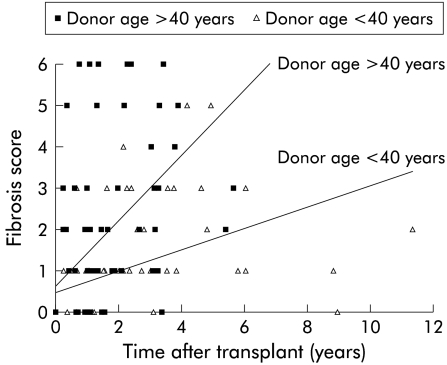
We have assumed in the analysis above that fibrosis was absent from the donor liver at the time of transplantation (fig 1 ▶). That assumption may be incorrect, and older donors may have had more fibrosis than younger donors. Under that circumstance, the observation that more rapid progression from time of transplantation until most recent post-transplant biopsy could be explained entirely by age associated differences in the donor liver at the time of transplantation. To examine and exclude this possibility, we have calculated fibrosis progression rates between pairs of post-transplant biopsies. Twenty seven patients had more than one eligible liver biopsy. One of the 27 had established cirrhosis in the first biopsy and thus paired biopsies were examined for 26 patients. For those patients, fibrosis progression rate between the first and most recent liver biopsies was calculated. Twenty six pairs separated by a median interval of 1.8 years were examined (table 6 ▶). Concerning histological grade and total activity index, there were no significant differences between the first and most recent biopsies. However, during follow up there was a significant increase in liver fibrosis, and the calculated median fibrosis progression rate between biopsies was 0.48 fibrosis units per year. Comparison of paired specimens confirmed that histological stage deteriorated for 18, remained unchanged for seven, and apparently improved for one patient. Multivariate analysis, as previously undertaken to identify risk factors for fibrosis progression in the entire cohort of 56 patients, was repeated to identify factors associated with progression between the 26 paired biopsies. Of the parameters examined, donor age was again identified as a potentially important determinant of fibrosis progression. For those with fibrosis rate greater than the median, median donor age was 46.5 years (range 23–57). For those with slower fibrosis progression, median donor age was only 32 years (range 17–59).
Figure 1.
Association of fibrosis score with post-transplant interval by linear regression analysis. For calculation of the regression lines, it was assumed that fibrosis was absent at the time of transplantation (time zero biopsies were available for a minority). The gradient of the regression line for liver biopsies performed for recipients with a donor age greater than 40 was 0.79 fibrosis units per year and for those less than 40 years 0.26.
Table 6.
Twenty six patients had paired biopsies for examination, performed a median of 1.8 (range 0.1–8) years apart. The fibrosis progression rate between biopsies was median 0.48 (range –0.58–3.75) fibrosis units per year
| 1st biopsy | 2nd biopsy | p Value | |
| Histology grade | 5 (1–15) | 2 (2–13) | 0.83 |
| Histology stage (fibrosis) | 1 (0–6) | 2 (0–6) | <0.001 |
| Total HAI | 6 (1–21) | 8 (2–17) | 0.34 |
HAI, histological activity index.
DISCUSSION
The results of this analysis may provide an important insight into previously made but unexplained observations of the natural history of HCV infection in immunocompetent and immunosuppressed patients. It is clear that age at the time of infection is an important and powerful determinant of fibrosis development in immunocompetent HCV patients. Poynard et al, examining the determinants of fibrosis progression in more than 2000 HCV infected patients, found that age at infection, sex, and level of alcohol consumption were important determinants of fibrosis progression.9 From their observations, it could be calculated that the median duration from time of infection to cirrhosis was 30 years (for the entire cohort), and that the rates for those aged 31–40, 41–50, and >50 years were 30, 20, and 12 years, respectively. In our post-transplant cohort, it appears that liver age at the time of infection has an equally important predictive value. For our entire cohort, median duration to development of graft cirrhosis was 7.7 years, and rates for those with a donor age <40, 41–50, and >50 were 10, 6.7, and 2.7 years, respectively. Thus we observed more rapid progression to cirrhosis for the liver transplant recipient, a rate which was affected to the same extent as observed in the immunocompetent patient by liver age at time of infection. Poynard et al could not distinguish age related changes in liver response from age related changes in immune function as an explanation for their observations. Our observations, made in the context of LT, suggest that age related changes in liver response may be the key factor that determines the increased susceptibility of the older liver to HCV induced fibrosis.
Patient sex influences fibrosis progression rate in the immunocompetent but the impact of sex is significantly less than the impact of age at the time of infection.9 In our post-transplant cohort, we also observed more rapid development in the recipients of male donor than of female donor livers (median fibrosis progression rate 0.92 for males, 0.45 for females; p=0.17).
Few other studies have examined the impact of donor liver age on outcome of HCV graft infection,18–20 and none has examined the effect of donor age on fibrosis progression rate. However, our observations may have been anticipated by the study of Berenguer and colleagues7 who reported a powerful association of graft fibrosis progression in Spanish and American HCV infected transplant patients with date of transplantation. In that study, more recently transplanted patients suffered rapid development of liver fibrosis. A European collaborative study also found that year of transplantation was associated with graft outcome, and inferior outcome was observed for more recently transplanted patients.2 We speculate that the inferior outcome of recently transplanted patients described in these reports may be a direct consequence of changes in organ donor profile observed during the same period. In most countries, donor organ numbers have plateaued or are declining. Accidental death is less common, and an increasing proportion of donors are victims of cerebrovascular disease. Thus the age of donors is increasing, and in the UK median donor age has increased by approximately 10 years over the last decade. In the cohort of 56 patients who are the subject of this report, there was a highly significant difference in donor age between those patients transplanted before or after November 1996. There was an associated impact of transplantation date on fibrosis progression during follow up of 56 patients, and in progression rate between paired post-transplant biopsies for 26 patients.
Although fibrosis progression rate may be modulated by liver age at the time of infection, accelerated progression to cirrhosis remains a key observation of this and other studies of HCV infected liver transplant recipients. Observations made in other groups of immunosuppressed patients, including human immunodeficiency virus positives21 and other (non-liver) solid organ transplant recipients,22–25 suggest that rapid progression may be related to immunosuppression per se. In this respect, a number of groups have identified an apparent association of severity of recurrent infection in the liver graft with severity and frequency of antecedent acute rejection and/or its treatment.7,12–16 We did not find an association of fibrosis rate with rejection or its treatment. However, only one third of patients required treatment for rejection, and fewer than 10% suffered more than one treated rejection episode. None received OKT3.
We identified an association between liver warm ischaemic time and subsequent fibrosis progression. Baron et al, examining the severity of recurrent hepatitis (primarily histological grade) during the first post-transplant year, also observed an association with liver warm ischaemic time.26 Indeed, analysis of a large US database including patients transplanted for all acute and chronic liver diseases identified an association between warm ischaemic time and patient and graft survival following LT.27 Thus prolongation of warm ischaemic time may have detrimental short and long term effects on the graft in HCV infected and other transplant recipients.
In conclusion, we have selected post-transplant biopsies that specifically reflect the effects of recurrent HCV infection. Our analysis of donor and recipient parameters suggests that donor characteristics, principally age, may be important determinants of graft outcome. These observations, if confirmed by others, shed important light on the natural history of HCV infection in immunocompetent patients and in liver transplant recipients, and have very important implications for donor organ allocation. When the use of old donor livers for HCV positive recipients cannot be avoided, aggressive hepatitis can be anticipated, and such recipients should be considered for antiviral therapy following transplantation.
Abbreviations
HCV, hepatitis C virus
LT, liver transplantation
HBV, hepatitis B virus
HBsAg, hepatitis B surface antigen
REFERENCES
- 1.Gane EJ, Portmann BC, Naoumov NV, et al. Long-term outcome of hepatitis C infection after liver transplantation. N Engl J Med 1996;334:815–20. [DOI] [PubMed] [Google Scholar]
- 2.Feray C, Caccamo L, Alexander GJ, et al. European collaborative study on factors influencing outcome after liver transplantation for hepatitis C. European Concerted Action on Viral Hepatitis (EUROHEP) Group. Gastroenterology 1999;117:619–25. [DOI] [PubMed] [Google Scholar]
- 3.Schluger LK, Sheiner PA, Thung SN, et al. Severe recurrent cholestatic hepatitis C following orthotopic liver transplantation. Hepatology 1996;23:971–6. [DOI] [PubMed] [Google Scholar]
- 4.Taga SA, Washington MK, Terrault N, et al. Cholestatic hepatitis C in liver allografts. Liver Transpl Surg 1998;4:304–10. [DOI] [PubMed] [Google Scholar]
- 5.Rosen HR, Gretch DR, Oehlke M, et al. Timing and severity of initial hepatitis C recurrence as predictors of long-term liver allograft injury. Transplantation 1998;65:1178–82. [DOI] [PubMed] [Google Scholar]
- 6.Papatheodoridis GV, Patch D, Dusheiko GM, et al. The outcome of hepatitis C virus infection after liver transplantation—is it influenced by the type of immunosuppression? J Hepatol 1999;30:731–8. [DOI] [PubMed] [Google Scholar]
- 7.Berenguer M, Ferrell L, Watson J, et al. HCV-related fibrosis progression following liver transplantation: increase in recent years. J Hepatol 2000;32:673–84. [DOI] [PubMed] [Google Scholar]
- 8.Boker KH, Dalley G, Bahr MJ, et al. Long-term outcome of hepatitis C virus infection after liver transplantation. Hepatology 1997;25:203–10. [DOI] [PubMed] [Google Scholar]
- 9.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet 1997;349:825–32. [DOI] [PubMed] [Google Scholar]
- 10.Prieto M, Berenguer M, Rayon JM, et al. High incidence of allograft cirrhosis in hepatitis C virus genotype 1b infection following transplantation: relationship with rejection episodes. Hepatology 1999;29:250–6. [DOI] [PubMed] [Google Scholar]
- 11.Berenguer M, Prieto M, Rayon JM, et al. Natural history of clinically compensated hepatitis C virus-related graft cirrhosis after liver transplantation. Hepatology 2000;32:852–8. [DOI] [PubMed] [Google Scholar]
- 12.Berenguer M, Prieto M, Cordoba J, et al. Early development of chronic active hepatitis in recurrent hepatitis C virus infection after liver transplantation: association with treatment of rejection. J Hepatol 1998;28:756–63. [DOI] [PubMed] [Google Scholar]
- 13.Testa G, Crippin JS, Netto GJ, et al. Liver transplantation for hepatitis C: recurrence and disease progression in 300 patients. Liver Transpl 2000;6:553–61. [DOI] [PubMed] [Google Scholar]
- 14.Sheiner PA, Schwartz ME, Mor E, et al. Severe or multiple rejection episodes are associated with early recurrence of hepatitis C after orthotopic liver transplantation. Hepatology 1995;21:30–4. [PubMed] [Google Scholar]
- 15.Rosen HR, Shackleton CR, Higa L, et al. Use of OKT3 is associated with early and severe recurrence of hepatitis C after liver transplantation. Am J Gastroenterol 1997;92:1453–7. [PubMed] [Google Scholar]
- 16.Shuhart MC, Bronner MP, Gretch DR, et al. Histological and clinical outcome after liver transplantation for hepatitis C. Hepatology 1997;26:1646–52. [DOI] [PubMed] [Google Scholar]
- 17.Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatology 1995;22:696–9. [DOI] [PubMed] [Google Scholar]
- 18.Herrero JI, de la Pena A, Quiroga J, et al. Risk factors for recurrence of hepatitis C after liver transplantation. Liver Transpl Surg 1998;4:265–70. [DOI] [PubMed] [Google Scholar]
- 19.Charlton M, Seaberg E, Wiesner R, et al. Predictors of patient and graft survival following liver transplantation for hepatitis C. Hepatology 1998;28:823–30. [DOI] [PubMed] [Google Scholar]
- 20.Pelletier SJ, Raymond DP, Crabtree TD, et al. Hepatitis C-induced hepatic allograft injury is associated with a pretransplantation elevated viral replication rate. Hepatology 2000;32:418–26. [DOI] [PubMed] [Google Scholar]
- 21.Benhamou Y, Bochet M, Di Martino V, et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology 1999;30:1054–8. [DOI] [PubMed] [Google Scholar]
- 22.Hanafusa T, Ichikawa Y, Kishikawa H, et al. Retrospective study on the impact of hepatitis C virus infection on kidney transplant patients over 20 years. Transplantation 1998;66:471–6. [DOI] [PubMed] [Google Scholar]
- 23.Legendre C, Garrigue V, Le Bihan C, et al. Harmful long-term impact of hepatitis C virus infection in kidney transplant recipients. Transplantation 1998;65:667–70. [DOI] [PubMed] [Google Scholar]
- 24.Ong JP, Barnes DS, Younossi ZM, et al. Outcome of de novo hepatitis C virus infection in heart transplant recipients. Hepatology 1999;30:1293–8. [DOI] [PubMed] [Google Scholar]
- 25.Lim HL, Lau GK, Davis GL, et al. Cholestatic hepatitis leading to hepatic failure in a patient with organ-transmitted hepatitis C virus infection. Gastroenterology 1994;106:248–51. [DOI] [PubMed] [Google Scholar]
- 26.Baron PW, Sindram D, Higdon D, et al. Prolonged rewarming time during allograft implantation predisposes to recurrent hepatitis C infection after liver transplantation. Liver Transpl 2000;6:407–12. [DOI] [PubMed] [Google Scholar]
- 27.Seaberg EC, Belle SH, Beringer KC, et al. Liver transplantation in the United States from 1987–1998: updated results from the Pitt-UNOS Liver Transplant Registry. Clin Transpl 1998;12:17–37. [PubMed] [Google Scholar]