Abstract
Classical descriptions of gut development specify subdivision into foregut, midgut, and hindgut together with their derivatives. This is based on the anatomical localisation of the anterior and posterior intestinal portals separating the roof of the yolk sac from the foregut and hindgut diverticulae. When considering the molecular basis of intestinal differentiation, it is necessary to think in terms of the genes involved, and in this respect those containing the homeobox motif are important players in specifying the fate of both the endodermal and mesodermal components of the gut. In this review, evidence is considered for their role, with particular regard to the acquisition of positional information.
Keywords: homeobox genes, gut development
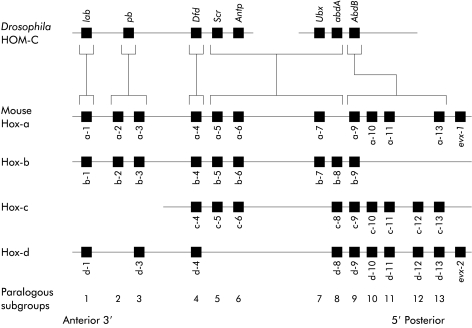
Cells which make up a multicellular organism are subject to constraints that are not imposed upon their protozoan counterparts. In addition to coding for proteins concerned with structural and narrowly functional properties, the DNA of these cells must include genes that impart positional information. This makes possible the complex cell movements that are required for the assumption of form and allows diverse temporally regulated interactions between shifting populations of cells to take place. The latter are necessary for the inductive processes which occur as the organism increases in complexity and result in the hierarchical expression of genes regulating cellular differentiation. The basic mechanisms involved are well illustrated in Drosophila because, compared with higher chordates, this invertebrate retains much of the segmental pattern of more primitive organisms. Thus the definitive anteroposterior pattern of the fly is achieved in stages. Initially, genes of maternal origin transcribe mRNA sequestered in the cytoplasm of the ovum. This is followed by the sequential action of segmentation genes known as gap genes, pair rule genes, and segmental polarity genes. These establish segmental periodicity while the identity of individual segments is specified by homeotic selector genes. The latter are examples of a much larger group of genes containing a conserved “homeobox” sequence coding for a DNA binding homeodomain and thus active as transcription factors. Those that are concerned with axial patterning are clustered in the HOM-C complex and exhibit spatial colinearity—that is, the sites of gene expression along the body axis reflect the relative chromosomal order of the genes. The HOM-C complex has its counterpart in all higher species. In mammals, exemplified by the mouse, it is represented by the Hox1 system consisting of four clusters, Hox-a, Hox-b, Hox-c, and Hox-d, that appear to have arisen by a two step reduplication from an ancestral complex common to mammals and flies.2 There are 13 potential gene sites on each cluster but none contain a gene at every potential locus (fig 1 ▶). Patterns of gene expression in paraxial and lateral plate mesoderm, neural tube, neural crest, hindbrain, and branchial arches demonstrate that spatial colinearity is maintained; in addition, the relative timing of gene expression in a craniocaudal direction also reflects the order of the genes on the chromosome (temporal colinearity).3
Figure 1.
Diagrammatic representation of the HOM-C complex in Drosophila and its phylogenetic homology in the form of four Hox paralogues.
Apart from the Hox clusters, many so-called “dispersed” homeobox genes exist in mice and humans. It is probable that these arise by reduplications that have been transposed away from the Hox cluster and often perform functions other than or additional to coding for positional information. Some form a so-called Para-Hox cluster4 which is thought to be an ancient paralogue of the Hox cluster—both having arisen from a putative ancestral Proto-Hox cluster. Para-Hox genes exhibit colinearity in neural tube and gut endoderm where they also seem to be associated with craniocaudal patterning. In mammals, the cluster consists of Gsh1, Pdx1, and Cdx2 but possibly others await definition.
HOMEOBOX GENE EXPRESSION IN THE GUT
Genes belonging to the Hox cluster are expressed in a colinear manner along the length of the lateral plate mesodermal component of the gut during development.5 Deschamp et al describe three stages of Hox gene expression in the mouse.3 The process is initiated in the posterior part of the primitive streak in late streak stage embryos. Expression then spreads anteriorly in sequence for individual Hox genes to involve the cells in the anterior part of the streak and Hensen's node. This extension is not due to clonal expansion but takes the form of a spreading wave. As the expression domains of various Hox genes “arrive” at the anterior region of the streak at different times, this results in linear expression of positional information along the anteroposterior axis as well as in the components of the lateral plate mesoderm which make up the mesodermal tissues of the gut and associated viscera. Thereafter, further expression of Hox genes is clonally transmitted by the progeny of cells originating in the streak. Finally, mechanisms that maintain and in a sense “lock in” homeobox gene expression become operative to ensure continued spatial fidelity of expression. In Drosophila, the polycomb, trithorax, and brahma group of genes serve this purpose and their homologues have been identified in vertebrates.
Dispersed homeobox genes belonging to the Nkx group are also differentially expressed in specific mesodermal regions of the gut. As yet the picture is not clear and may vary between species but it has been suggested that they influence epithelial growth and differentiation through target genes coding for secreted growth factors such as those of the BMP family.6
“Genes belonging to the Hox cluster are expressed in a colinear manner along the length of the lateral plate mesodermal component of the gut during development”
Although Hox and Nkx genes are strongly expressed in intestinal mesoderm during development, they do not feature prominently in the endodermal layer caudal to the stomach mucosa. There are a few exceptions, principally involving the endoderm of the cloacal region where Hoxd13 (as well as Hoxa13 in the chick) is expressed in the endoderm as well as in the underlying mesoderm, possibly in response to Sonic hedgehog. There is also report of Hoxa 8 expression in the small and large intestinal endoderm while numerous Hox genes are expressed in the endodermal layers of the stomach and oesophagus. Accounts of Hox gene expression sites in the gut are summarised by Sekimoto and colleagues7 and by Beck and colleagues.5 By contrast, two members of the Para-Hox group are strongly transcribed and translated in the postgastric intestinal epithelium during the greater part of gestation and in the adult mouse. Pdx1 expression8 begins at 8.5 days of gestation in the dorsal cells of the gut. At nine days it is restricted to the presumptive duodenal region and to the cells of the dorsal (and later also the ventral) pancreatic bud. By 13.5 days virtually all of the endodermal components of the pancreatic duct and the pancreatic primordial produce Pdx1 but as development proceeds gene expression is largely restricted to the beta cells of the endocrine pancreas and to the epithelium of the duodenal villi. Cdx2 which is closely linked to Pdx1 on mouse chromosome 59 is also strongly expressed in the postgastric gut endodermal nuclei.10 Initially it is expressed in the trophoblastic lineage and if it is inactivated implantation does not occur. Subsequently, expression in unsegmented paraxial mesoderm and the posterior neural tube enables the gene to play a part in axial patterning, possibly as a cis acting transaction factor responsible in part for the regulation of Hox gene expression. Cdx2 is a homologue of the Drosophila gene Caudal (Cad) and in the fly it is concerned with patterning of the posterior segments; an extended function in vertebrates may be connected to the fact that the genes of maternal origin and the segmentation genes (see above) are not greatly involved in axial specification and a greater role for Cdx2 in regulating Hox gene expression is thus conceivable. From day 12 of mouse gestation, Cdx2 expression is confined to the intestinal epithelium (fig 2 ▶) from a region just rostral to the hepatic diverticulum to the distal colon. In the adult, high levels of expression persist in the proximal colon gradually falling off in the ileum and jejunum cranially and in the distal colon caudally. The function of Cdx2 during development and in the adult is discussed below.
Figure 2.
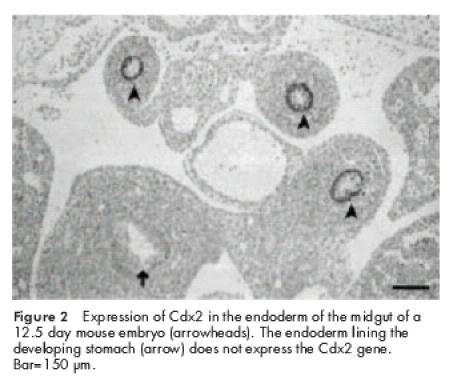
Expression of Cdx2 in the endoderm of the midgut of a 12.5 day mouse embryo (arrowheads). The endoderm lining the developing stomach (arrow) does not express the Cdx2 gene. Bar=150 μm.
Two other Caudal homologues exist in vertebrates. In the mouse these are Cdx1 and Cdx4. They are not linked to the Para-Hox cluster but both are expressed in gut endoderm. Cdx1 is demonstrable in the primitive streak and at other sites during early mouse development11 and has been shown to play a role in axial specification.12 However, it is not seen in gut endoderm until day 15.13,14 It is upregulated when developmental maturation of the gut occurs.13 In the adult it is principally expressed in the intestinal crypts and it is possible that it is principally concerned with maturation of the stem cells in the intestine although no gut phenotype has been described in null mutant mice.
Cdx4 is transiently expressed between days 7 and 10 of mouse development in the posterior part of the primitive streak moving anteriorly as development proceeds to find later expression in the posterior neurectoderm, presegmental paraxial mesoderm, and lateral plate mesoderm.15 This distribution, reminiscent of Hox expression, is suggestive of a role in axial specification similar to that described for Cdx1 and Cdx2 but the null mutant phenotype has not been described. Although no information concerning expression later in development is available, transcription of the gene in the endoderm of the hindgut suggests the possibility that it might (like Cdx2) be involved in gut lining specification.
EVIDENCE THAT HOMEOBOX GENES PLAY A ROLE IN GUT DEVELOPMENT
Individual gene expression at a specific site does not necessarily imply an important role in development. This can only be shown by demonstration of an abnormal phenotype resulting from over expression or null mutation of a gene. Even then, the considerable degree of redundancy arising from regular coexpression of two or more Hox gene paralogues in the same region of the gut serve to cloud the picture. Genetic redundancy can also extend in a linear manner making it necessary for several contiguous Hox genes to be inactivated in order to generate a recognisable gut phenotype. Furthermore, it must be borne in mind that anteroposterior gut specification is not as clear over relatively short distances as it is for the axial skeleton or the central nervous system. Histologically similar intestinal morphology extends over relatively large regions whereas individual rhombomeres or somite derivatives are more readily distinguishable from their neighbours. Considerable alteration of positional specification of intestinal segments might not exhibit an obvious phenotypic change.
“Genetic redundancy can also extend in a linear manner making it necessary for several contiguous Hox genes to be inactivated in order to generate a recognisable gut phenotype”
As might be expected, the results obtained following mutation or over expression of Hox genes affect mesodermal components of the gut although it is often not possible to anticipate the phenotype from a knowledge of wild-type gene expression. For example, Wohlgemuth and colleagues16 reported megacolon in transgenic mice expressing a Hoxa4 transgene designed to produce normal Hoxa4 expression from a construct distinguishable from the wild-type. The result was a greatly extended region of Hoxa4 transcription leading to abnormal mesodermal development resulting in megacolon. Pollock and colleagues17 used the regulatory sequence of Hoxa4 to drive a Hoxc8 transgene thereby expressing the gene more anteriorly than in the wild-type. This resulted in the development of multiple hamartomatous lesions in the gastric mucosa of the transgenic mice which the authors attribute to ectopic expression of Hoxc8. However, there are no reports of Hoxa4 (which was used to drive the transgene) expression in wild-type gastric epithelium although the gene is expressed in the mesodermal layers. Zakany and Duboule18 produced mice in which Hoxd4 through to Hoxd13 were inactivated. The animals lacked an ileocaecal sphincter and showed abnormalities in the region of both the pyloric and anal sphincters. As mutation of single Hox genes within this series had no effect on the midgut, the authors concluded that several neighbouring Hox-d genes are required to make normal ileocaecal sphincters. However, Kondo and colleagues19 have convincingly demonstrated that Hoxd12 and Hoxd13 mutants each lack normal anal sphincters leading to rectal prolapse while Boulet and colleagues20 have shown that inactivation of Hoxc4 results in blockage of the oesophageal lumen and disruption of its musculature.
The general conclusion that Hox-d genes are involved in the development of intestinal sphincters while other paralogues have different functions in gut development mainly confined to the mesodermal layers seems justified.
The functional implications of expression of the Nkx group of genes in the intestinal mesoderm is of interest. It is illustrated by the observation that inactivation of Nkx2.3 results in delayed villous development followed by increased (possibly compensatory) growth of crypt cells and hyperproliferation of gut epithelium in adult mutants, principally affecting the jejunum.6 These observations support the conclusion, discussed later, that mesodermal factors influence epithelial differentiation.
“Mesodermal factors influence epithelial differentiation”
As previously stated, Para-Hox genes are exclusively expressed in the gut endoderm during the later stages of development and null mutation of these result in phenotypes more clearly related to sites of embryonic expression than is the case with Hox genes. This may be because the absence of a complex series of paralogues minimises the possibility of redundancy and also perhaps because the small number of genes involved implies that each may influence large regions of the gut. Null mutation of Pdx1 results in pancreatic and duodenal abnormalities.21,22 Pancreatic ducts begin to develop but fail to form a pancreas. The primordia lack insulin and amylase positive cells but glucagon expressing cells may be formed. The foregut region of the duodenal mucosa fails to form villi, remains lined by cuboidal epithelium, and lacks Brunner's glands. The extent of the abnormalities thus closely corresponds to sites of gene expression during development.
Chawengsaksophak et al made Cdx2 null mutant mice.23 Homozygous Cdx2−/− embryos die at implantation and this correlates with early expression of the gene in the trophoblast. Heterozygotes display an anterior homeotic shift involving the axial skeleton and this is in keeping with early expression of the gene in the presegmental paraxial mesoderm. Most importantly, these animals have multiple polyp-like lesions with the highest frequency in the caecum, decreasing in incidence both proximally and distally but involving the whole of the intestinal region which expresses Cdx2 during development. Lesions do not occur elsewhere in the intestinal tract. Histologically, the polyps contain normal gastric mucosa (fig 3 ▶) with villi and Paneth cells interposed proximally and distally between the stomach mucosa and the surrounding colonic epithelium. The gastric mucosa is arranged in an orderly array passing from stratified squamous epithelium of forestomach-type through mucous glands of the cardia to gastric glands of the corpus and finally to mucous antral-type cells as one passes both proximally and distally.24 This phenotype represents an anterior homeotic shift in which intestinal epithelium has the character of a more rostral phenotype due to localised areas of Cdx2 haplo-insufficiency. The “default” state is forestomach epithelium which in the normal animal does not express Cdx2. Intercalary growth subsequently results in the orderly appearance of the appropriate tissue types to “fill in” the histological discontinuity between gastric and colonic epithelia. This is the only example of homeosis in the mammalian gut so far described and the first report of intercalary regeneration in mammals.
Figure 3.
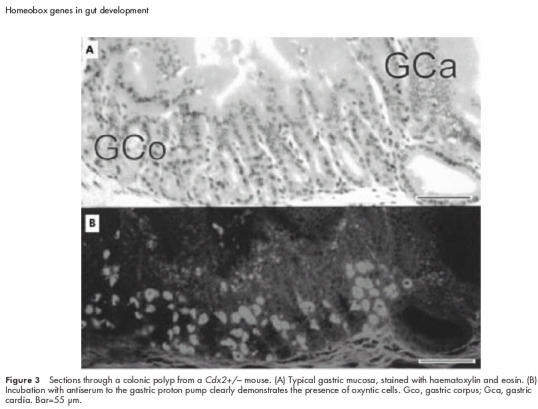
Sections through a colonic polyp from a Cdx2+/− mouse. (A) Typical gastric mucosa, stained with haematoxylin and eosin. (B) Incubation with antiserum to the gastric proton pump clearly demonstrates the presence of oxyntic cells. Gco, gastric corpus; Gca, gastric cardia. Bar=55 μm.
The Cdx1 gene has also been mutated.12 Heterozygotes in this case are normal but homozygotes have an anterior homeotic shift involving the axial skeleton but no reported gut abnormalities. It seems therefore that overlap of function between Cdx1 and 2 occurs in the paraxial mesoderm but does not extend to the gut endoderm. No information concerning Cdx4 inactivation is available.
Accompanying the evolutionary formation of new structures, the function of existing genes is frequently extended to control processes unrelated to their original roles. A good example is provided by Cdx2. In the adult intestine there is evidence that Cdx2 binds to cis elements of genes coding for various digestive enzymes such as lactase-phlorizin hydrolase,25 sucrase-isomaltase,26 carbonic anhydrase,27 and calbindin-D9K.28 These only begin to be expressed after completion of morphological gut differentiation. The specialisation of Hox-d paralogues in the formation of gut sphincters discussed above is another example of such gene recruitment.
REGULATION OF HOMEOBOX GENES CONCERNED WITH GUT DEVELOPMENT
The mechanisms by which the colinearity exhibited by Hox genes expressed in the gut is established is not yet understood but the fact that control elements for individual genes are frequently embedded in adjacent members of the cluster is undoubtedly relevant. Numerous cofactors, enhancers, and modifiers are involved29 and a degree of tissue specificity for some of these elements leads to their typical expression pattern in the lateral plate mesoderm.30 In a general review of the genesis of Hox gene expression in the mouse, Deschamps and colleagues3 define a number of cis acting regulatory sequences among which retinoic acid receptor regulatory elements appear to play an important role but with decreasing sensitivity in the ability of retinoic acid to induce the more caudally expressed Hox genes.1 Many Hox genes contain control elements which bind the mouse homologues of Cad and the cdx genes are thus strong candidates for the regulation of Hox expression. Initial expression of the three mouse cdx genes is in the posterior part of the primitive streak and their transcription might generate a set of expression domains overlapping posteriorly and creating a stepwise gradient of cdx gene products with a posterior maximum. This may be reflected in serial expression of Hox genes in the paraxial mesoderm.5 The possible involvement of embryonic fibroblast growth factor arises from the observations of Pownall and colleagues31 and Isaacs and colleagues32 who showed that Hox gene expression in anteroposterior specification of Xenopus is dependent on a fibroblast growth factor gradient which may activate the Caudal homologue Xcad3.
“When considering gut formation it is necessary to pay attention to the interaction between the endoderm and mesoderm”
When considering gut formation it is necessary to pay attention to the interaction between the endoderm and mesoderm. Each of these layers probably contains positional information prior to the establishment of their topographical juxtaposition which allows “cross talk” between them to be established. That such interactions occur was shown by Le Dourain who established that grafting pharyngeal endodermal anlagen into avian somatopleuric mesenchyme induced the development of oesophagus, stomach, and small intestine at implantation sites. These and other studies by Haffen and colleagues33 supply evidence in favour of a primary commitment of endoderm in gut differentiation. But there is also evidence that the visceral (splanchnopleuric) layer of the lateral plate mesoderm influences the patterning of the endoderm. Duluc and colleagues 34 found that mouse colonic endoderm combined with small intestinal mesoderm showed small intestine-like differentiation although colonic mesoderm was not able to alter differentiation when combined with small intestinal endoderm. For technical reasons the experiments were performed on 14 day embryonic mouse gut—that is, at a stage when specific transcription factors such as Cdx2 have already had the opportunity of acting to influence endodermal phenotype.
It has been shown that endodermally secreted Sonic hedgehog is also active in (avian) gut development. Using virally mediated transgenesis, Roberts and colleagues35,36 showed that Sonic hedgehog induced expression of Bmp4 and Hoxd13 in the hindgut mesoderm. Furthermore, when Hoxd13 is mis-expressed in primitive midgut mesoderm there is transformation of the endoderm to the morphology of hindgut, again showing the importance of “cross talk” between the layers.
The Cdx2 “knock out” studies detailed above show that this gene is central to differentiation of midgut endoderm. They lead to the conclusion that decreased levels of Cdx2 expression result in rostralisation of gut differentiation with gastric mucosa constituting the “default” condition in which there is no expression of Cdx2. However, as mentioned above, mesodermal factors may in their turn also influence endodermal differentiation. Cdx1 and 2 levels are higher in endodermal grafts cocultured with A1:F1 mesenchymal cells which support colonic-type morphogenesis than with F1:G9 mesenchymal cells which support small intestine-like differentiation.37,38
SUMMARY
Null mutation and gain of function experiments show that genes bearing the homeobox motif are important in gut development. There is good evidence to suggest that determination of endodermal fate provides the initial stimulus for positional values in gut morphogenesis. Pdx1 and Cdx2, members of the so-called Para-Hox cluster, are strong candidates for mediation of this process, at least in specific intestinal regions.
“Genes bearing the homeobox motif are important in gut development”
In the gut, mesenchyme genes of the Nkx and Hox groups appear to be important in regional specification but it is not clear whether their action is permissive or primarily instructive. Indeed, their importance may vary in different gut regions, as exemplified by the role of Hox-d genes in sphincter formation. It is also possible that homeobox gene expression in the mesodemal component of the intestine serves to stabilise information from other sources.
It is obvious that a large variety of genes other than those considered in this review are involved in gut development; the challenge for the future lies in determining their interrelationships and control mechanisms.
Acknowledgments
I am grateful to the Medical Research Council and the Leverhulme Trust for grants in aid of research
REFERENCES
- 1.Krumlauf R. Hox genes in vertebrate development. Cell 1994;78:191–201. [DOI] [PubMed] [Google Scholar]
- 2.Kappen C, Schughart K, Ruddle FH. Two steps in the evolution of Antennapedia-class vertebrate homeobox genes. Proc Natl Acad Sci USA 1989;86:5459–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deschamps J, Van den Akker E, et al. Initiation, establishment and maintenance of Hox gene expression patterns in the mouse. Int J Dev Biol 1999;43:635–90. [PubMed] [Google Scholar]
- 4.Brooke NM, Garcia-Fernandez J, Holland PWH. The paraHox gene cluster is an evolutionary sister of the Hox gene cluster. Nature 1998;392:920–2. [DOI] [PubMed] [Google Scholar]
- 5.Beck F, Tata F, Chawengsaksophak K. Homeobox genes and gut development. Bioessays 2000;22:431–41. [DOI] [PubMed] [Google Scholar]
- 6.Pabst O, Zweigerdt R, Arnold H-H. Targeted disruption of the homeobox transcription factor Nkx2–3 in mice results in postnatal lethality and abnormal development of the small intestine and spleen. Development 1999;126:2215–25. [DOI] [PubMed] [Google Scholar]
- 7.Sekimoto T, Yoshinobu K, Yoshida M, et al. Region specific expression of murine Hox genes implies the Hox code-mediated patterning of the digestive tract. Genes Cells 1998;3:51–64. [DOI] [PubMed] [Google Scholar]
- 8.Guz Y, Montminy MR, Stein R, et al. Expression of murine STF-1, a putative insulin gene transcription factor, in beta cells of pancreas, duodenal epithelium, and pancreatic exocrine and endocrine progenitors during ontogeny. Development 1995;121:11–18. [DOI] [PubMed] [Google Scholar]
- 9.Chawengsaksophak K, Beck F. Chromosomal localization of Cdx2, a murine homologue of the Drosophila gene caudal, to mouse chromosome 5. Genomics 1996;34:270–1. [DOI] [PubMed] [Google Scholar]
- 10.Beck F, Erler T, James R. Expression of Cdx-2 in the mouse embryos and placenta: possible role in patterning of the extrabmbryonic membranes. Dev Dyn 1995;204:217–29. [DOI] [PubMed] [Google Scholar]
- 11.Meyer BI, Gruss P. Mouse Cdx-1 expression during gastrulation. Development 1993;117:191–203. [DOI] [PubMed] [Google Scholar]
- 12.Subramanian V, Meyer B, Gruss P. Disruption of the murine homeobox gene Cdx1 affects axial skeletal isentities by altering the mesodermal expression domians of Hox genes. Cell 1995;83:641–53. [DOI] [PubMed] [Google Scholar]
- 13.Hu YL, Kazenwadel J, James R. Isolation and characterization of the murine homeobox gene Cdx-1 regulation of expression in intestinal epithelial cells. J Biol Chem 1993;268:27214–25. [PubMed] [Google Scholar]
- 14.Duprey P, Chowdhury K, Dressler GR, et al. A mouse gene homologous to the Drosophila gene caudal is expressed in epithelial cells from the embryonic intestine. Genes Dev 1988;2:1647–54. [DOI] [PubMed] [Google Scholar]
- 15.Gamer L, Wright CVE. Murine Cdx-4 bears striking similarities to the Drosophila caudal gene in its homeodomain sequence and early expression pattern. Mech Dev 1993;43:71–81. [DOI] [PubMed] [Google Scholar]
- 16.Wohlgemuth DJ, Behringer RR, Mostoller MP, et al. Transgenic mice overexpressing the mouse homoeobox-containing gene Hox-1.4 exhibit abnormal gut development. Nature 1989;337:464–7. [DOI] [PubMed] [Google Scholar]
- 17.Pollock RA, Jay G, Bieberich CJ. Altering the boundaries of Hox3.1 expression: evidence for antipodal gene regulation. Cell 1992;71:911–23. [DOI] [PubMed] [Google Scholar]
- 18.Zakany J, Duboule D. Hox genes and the making of sphincters. Nature 1999;401:761–2 [DOI] [PubMed] [Google Scholar]
- 19.Kondo T, Dollé P, Zákány J, et al. Function of posterior HoxD genes in the morphogenesis of the anal spincter. Development 1996;122:2651–9. [DOI] [PubMed] [Google Scholar]
- 20.Boulet AM, Capecchi MR. Targeted disruption of Hoxc-4 causes esophageal defects and vertebral transformations. Dev Biol 1996;177:232–49. [DOI] [PubMed] [Google Scholar]
- 21.Jonsson J, Carlsson L, Edlund T, et al. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature 1994;371:606–9. [DOI] [PubMed] [Google Scholar]
- 22.Offield MF, Jetton TL, Labosky PA, et al. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development 1996;122:983–95. [DOI] [PubMed] [Google Scholar]
- 23.Chawengsaksophak K, James R, Hammond VE, et al. Homeosis and intestinal tumours in Cdx2 mutant mice. Nature 1997;386:83–7. [DOI] [PubMed] [Google Scholar]
- 24.Beck F, Chawengsaksophak K, Waring P, et al. Reprogramming of intestinal differentiation and intercalary regeneration in Cdx2 mutant mice. Proc Natl Acad Sci USA 1999;96:7318–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Troelsen JT, Mitchelmore C, Spodsberg N, et al. Regulation of lactase-phlorizin hydrolase gene expression by the caudal-related homoeodomain protein Cdx-2. Biochem J 1997;322:833–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suh E, Traber PG. An intestine-specific homeobox gene regulates proliferation and differentiation. Mol Cell Biol 1996;16:619–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drummond F, Sowden J, Morrison K, et al. The caudal-type homeobox protein Cdx-2 binds to the colon promoter of the carbonic anhydrase 1 gene. Eur J Biochem 1996;236:670–81. [DOI] [PubMed] [Google Scholar]
- 28.Lambert M, Colnot SSE, L'Horset F, et al. cis-Acting elements and transcription factors involved in the intestinal specific expression of the rat calbindin-D9K gene: binding of the intestine-specific transcription factor Cdx-2 to the TATA box. Eur J Biochem 1996;236:778–88. [DOI] [PubMed] [Google Scholar]
- 29.Gellon G, Mc Ginnis W. Shaping animal body plans in development and evolution by modulation of Hox expression patterns. Bioessays 1998;20:116–25. [DOI] [PubMed] [Google Scholar]
- 30.Shashikant CS, Ruddle FH. Combinations of closely situated cis-acting elements determine tissue-specific patterns and anterior extent of early Hoxc8 expression. Proc Natl Acad Sci USA 1996;93:12364–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pownall ME, Tucker AS, Slack JM, et al. eFGF, Xcad3 and Hox genes form a molecular pathway that establishes the anteroposterior axis in Xenopus. Development 1996;122:3881–92. [DOI] [PubMed] [Google Scholar]
- 32.Isaacs HV, Pownall ME, Slack JM. Regulation of Hox gene expression and posterior development by the Xenopus caudal homologue Xcad3. EMBO J 1998;7:3413–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haffen K, Kedinger M, Simon-Assmann P. In: Lebenthal E, ed. Human gastrointestinal development. New York: Raven Press, 1989:19–38.
- 34.Duluc I, Freund JN, Leberquier C, et al. Fetal endoderm primarily holds the temporal and positional information required for mammalian intestinal development. J Cell Biol 1994;126:211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts DJ, Johnson RL, Burke AC, et al. Sonic hedgehog is an endodermal signal inducing Bmp-4 and Hox genes during induction and regionalization of the chick hindgut. Development 1995;121:3163–74. [DOI] [PubMed] [Google Scholar]
- 36.Roberts DJ, Smit, DM, Goff DJ, et al. Epithelial-mesenchymal signalling during the regionalization of the chick gut. Development 1998;125:2791–801. [DOI] [PubMed] [Google Scholar]
- 37.Duluc I, Lorentz O, Fritsch C, et al. Changing intestinal connective tissue interactions alters homeobox gene expression in epithelial cells. J Cell Sci 1997;110:1317–24. [DOI] [PubMed] [Google Scholar]
- 38.Fritsch C, Simon-Assmann P, Kedinger M, et al. Cytokines modulate fibroblast phenotype and epithelial-stroma interactions in rat intestine. Gastroenterology 1997;112:826–38. [DOI] [PubMed] [Google Scholar]