Abstract
Background: Increased expression of proinflammatory cytokines, including tumour necrosis factor α, interleukin 6, and interferon γ, as well as activation of proinflammatory signalling molecules such as nuclear factor kappa B, is characteristic of inflammatory bowel disease (IBD).
Aims: To investigate expression and activation of signal transducer and activator of transcription (STAT) 1 in patients with IBD.
Patients: Patients with active IBD (n=42), disease specificity controls (n=8), and normal controls (n=12) were investigated.
Methods: Expression and activation of STAT1 were assessed by western blotting and electrophoretic mobility shift assays in extracts of endoscopic colonic biopsies. Cellular localisation was determined by immunohistochemistry.
Results: Western blots and immunohistochemical staining revealed an increase in STAT1 expression and activation in mucosal samples from ulcerative colitis and to a lesser extend in Crohn's disease patients. High levels of suppressor of cytokine signalling (SOCS)-3 expression, an inhibitor of STAT activation, were observed in Crohn's disease patients and normal controls in western blot experiments whereas no differences were observed for SOCS-1 expression. Phosphorylated (p) STAT1 was mainly detected in monocytic cells and neutrophils in the inflamed mucosa. Induction of remission by systemic glucocorticoids led to a decrease in levels of pSTAT1. In vitro studies indicated a direct effect of steroid treatment on STAT1 activation.
Conclusions: Expression and activation of STAT1 are predominantly heightened in ulcerative colitis and may therefore play an important role in the pathophysiology of colonic inflammation.
Keywords: inflammatory bowel disease, Crohn's disease, ulcerative colitis, signal transducer and activator of transcription
Inflammatory bowel disease (IBD) is characterised by a dysregulated mucosal immune response. The pathophysiology of this regulatory defect is reflected by a distorted balance of regulatory cytokines.1,2 In IBD as well as most animal models resembling IBD, enhanced secretion of proinflammatory cytokines is observed whereas contra-inflammatory cytokines may not be secreted in adequate amounts or their activity inhibited.3–7 The primary regulatory pathways for many of the functional and anatomical alterations found in IBD, which include increased permeability for macromolecules and tissue destruction, are unclear.7,8
The IBD phenotype can be subclassified into Crohn's disease and ulcerative colitis using clinical, endoscopic, histological, and radiological characteristics.9 Given the substantial clinical differences between Crohn's disease and ulcerative colitis, the search for distinctive immunological characteristics in each of these subtypes is of interest. Patterns of cytokine secretion have been investigated for their potential to unveil specific immunological disturbances related to IBD subtypes. In mice, T helper cell type 1 (TH1) driven models of intestinal inflammation were generated which share similarities with human IBD.10,11 However, some investigators have found a preponderance of TH1 cytokine secretion in patients with Crohn's disease and of TH2 cytokine secretion in ulcerative colitis.12–14 Other data indicate increased production of the TH2 cytokine interleukin (IL)-413 as a critical step in the development of early lesions of Crohn's disease. The TH1 cytokine interferon γ (IFN-γ) and cytokines with an IL-12-like activity may be major components of the pathophysiology of pouchitis and ulcerative colitis, respectively.14,15 Therefore, interpretation of ex vivo cytokine assessments and of data from animal models of colitis with regard to human IBD appears to be difficult.
Most cytokines specifically activate transcription factors to regulate expression of specific genes. Whereas tumour necrosis factor α (TNF-α) and IL-1β preferentially induce activation of the nuclear factor kappa B (NFκB) system,16,17 many other growth factors and cytokines activate proteins of the signal transducer and activator of transcription (STAT) family.18,19 STAT proteins are dormant cytoplasmic transcription factors which become activated after phosphorylated by Janus kinases (JAK) or other kinases in response to binding of cytokine or growth factor receptors.20,21 The activated protein migrates into the nucleus and binds to specific promoter elements to regulate gene expression. STAT regulated genes include the Fcγ receptor,22 inducible nitric oxide synthetase,23 major histocompatibility class II,24 and intercellular adhesion molecule 1 (ICAM-1).25 The cophosphorylation of other STAT family molecules appears to be an important mechanism to confer the specificity of transcriptional regulation. In 1997, a new group of proteins was described which specifically inhibit activation of members of the STAT family.26 These proteins are called suppressors of cytokine signalling (SOCS). SOCS-1 and -3 have been shown to inhibit phosphorylation of STAT1 and STAT3.27,28
Signal transduction abnormalities have been demonstrated previously in Crohn's disease and animal models of IBD—for example, for the NFκB system.29,30 We have assessed activation of STAT1 in IBD because many immune regulatory genes contain STAT binding sites in their promoter regions. In addition, activation of STATs is important for signalling of many cytokine and growth factor receptors. The status of STAT activation and its expression were assessed in snap frozen tissue from diseased human intestine with different methodologies. The data presented here demonstrate the increased nuclear accumulation of STAT1 in IBD. A preponderance of STAT1 activation is seen in ulcerative colitis in comparison with Crohn's disease, indicating an important role for this protein and eventually TH1 driven immunity in the pathophysiology of colonic inflammation.
MATERIAL AND METHODS
Patients
A total of 14 patients with clinically active Crohn's disease and 28 patients with active ulcerative colitis participated in the study. Patients were recruited from the outpatient clinics of the Charité University Hospital, Berlin, the Tabea Hospital for Inflammatory Bowel Disease, Hamburg, and the Hospital of the Christian-Albrechts-University, Kiel, Germany. All patients were seen because of increased clinical activity. At the time mucosal biopsies were obtained, 8/14 patients with Crohn's disease and 24/28 patients with ulcerative colitis received treatment with oral salicylates (mesalazine—Salofalk, Claversal, or Pentasa; salazosulphapyridine—Azulfidine or Colopleon; olsalazine—Dipentum) in doses up to 4.5 g/day. None of the patients received steroids and/or cytotoxic drugs, immunosuppressives, or antibiotics. All IBD patients underwent sigmoidoscopy or colonoscopy for routine clinical evaluation. Diagnosis had been established by clinical, endoscopic, histological, and/or radiological criteria. The presence of infection or parasites was excluded by stool cultures, microscopic stool examination, and serology (Yersinia, Campylobacter). Nine patients with ulcerative colitis were followed until glucocorticoid induced remission. Normal controls (n=12) were age and sex matched healthy volunteers. Disease specificity controls included patients with diverticulitis, salmonellosis, and infectious enterocolitis. The study was approved by the institutional review board. Patients gave written informed consent 24 hours prior to the procedures.
Extracts
For electrophoretic mobility shift assays (EMSAs), snap frozen biopsies were pulverised in liquid nitrogen and nuclear and cytosolic extracts were prepared as previously described.29 Protein concentration was assessed by a modified Bradford protein assay (Biorad, Hercules, California, USA) and all samples were adjusted to an equal protein concentration.
Isolation of peripheral blood monocytes
Peripheral blood monocytes were isolated as previously described.6 Briefly, 15 ml of Lymphoprep solution (Nycomed, Denmark) were placed into 50 ml Leucosep tubes (Greiner, Germany) and centrifuged for two minutes at 100 g until the entire solution was below the frit. Whole blood (30 ml) containing 1 mM EDTA as anticoagulant was directly poured onto the frit followed by centrifugation at 800 g for 15 minutes at room temperature. The resulting interphase was collected and washed twice with sterile phosphate buffered saline (PBS). The cells were counted, resuspended in RPMI with 10% fetal calf serum, plated on six well plates, and incubated for two hours at 37°C. Non-adherent cells were removed by washing with 2 ml of ice cold PBS and the remaining monocytes were overlayed with 2 ml of fresh medium (37°C). The viability of the cells was determined by trypan blue staining and purity was examined by flow cytometry according to standard procedures and reached 80–90%.
Western blotting and quantification
Total protein (10 μg per lane) was separated on a 10% denaturing polyacrylamide gel and transferred to a polyvinylidene difluoride membrane (0.8 mA/cm2, 75 minutes, transfer buffer: 25 mM Tris, 190 mM glycine, 20% MeOH, 0.5% sodium dodecyl sulphate) by semidry electroblotting (Biorad). The membrane was blocked for 60 minutes at room temperature with 5% non-fat dry milk in TTBS (10 mM Tris (7.5), 100 mM NaCl, 0.1% Tween-20). The primary antibody was diluted 1:250 to 1:1000 in blocking buffer and placed on the membrane for 60 minutes at room temperature. After washing with TTBS, the membrane was incubated with a peroxidase conjugated secondary antibody, which was diluted 1:1000 in blocking buffer for 20 minutes at room temperature. Detection was performed by incubation with ECL Plus (Amersham, Germany) and exposed to radio film. Bands were quantified by densitometry (ImageQuaNT; Molecular Dynamics, Sunnyvale, California, USA). Western blots were standardised by addition of geometrically diluted appropriate cell lysates. On the basis of these standards, the bands of the scanned films were normalised to an arbitrary intensity unit.
Electrophoretic mobility shift assay (EMSA)
Extract (2 μl=5 μg of protein) was incubated with 0.5 ng of 33P-labelled GAS containing oligonucleotide (from the promoter of the human FcγRI gene: 5`-GTATTTCCCAGAAAAGGAAC-3`) in a total reaction volume of 10 μl supplemented with 2 μl of 5× Gelshift binding buffer (Promega, Madison, Wisconsin, USA) for 20 minutes at room temperature. After addition of 1 μl of 10× loading buffer the entire reaction mixture was run for 45 minutes on a 4% polyacrylamide gel with 0.5× TBE (1.8 mM Tris (8.3), 50 μM EDTA, 1.8 mM borate) at 300 V. The gel was dried, exposed over night at room temperature to an imaging plate, and finally analysed with a Fuji FLA3700 phosphoimager. Supershift or competition experiments were performed by adding 1 μl of antibody or a 50-fold molar excess of unlabelled oligonucleotide (β-casein-gamma interferon activation site (GAS): AGATTTCTAGGAATTCAAATC; NFκB: AGTTGAGGGGACTTTCCCAGGC; activator protein 1 (AP-1): CGCTTGATGAGTCAGCCGGAA) to the binding reaction.
Immunofluorescence and grading of histology
Biopsies were embedded in cryomatrix and snap frozen in liquid nitrogen. Cryostat sections (7 μm) were thaw mounted on Superfrost slides (Menzel/Merck Ltd, Poole, UK), postfixed for five minutes in acetone, air dried, and stored at −20°C before staining. Two slides of each biopsy were stained with haematoxylin-eosin for routine histopathology. On the other slides, tissue sections were made permeable with 0.1% Triton X-100 in 0.1 M PBS, washed three times in PBS, and blocked non-specifically with 0.75% bovine serum albumin in PBS. Subsequently, sections were incubated for 60 minutes with the appropriate antibody diluted 1:100 to 1:200 in 0.75% bovine serum albumin. After washing in PBS, tissue bound antibody was detected using biotinylated goat-antimouse IgG antibodies (Vector Laboratories, Burlingame, California, USA), followed by avidin-FITC (Vector Laboratories), both diluted at 1:100 in 5% human serum. Stainings with an irrelevant primary antibody, only with secondary antibody, and avidin-FITC served as controls. Nuclear counterstaining with bisbenzimide was performed. Fluorescence was visualised using an Axiophot microscope (Zeiss, Jena, Germany) with the appropriate filter systems, and photos were taken on Provia 1600 colour films (Fuji, Dusseldorf, Germany).
Inflammatory activity was graded as follows (haematoxylin-eosin stained slides): 0, no or non-significant inflammatory activity; 1, significant inflammation present; and 2, severe changes. Parameters were intensity of the inflammatory infiltrate (neutrophils, mononuclear cells) and severity of destruction of the normal micro architecture. In order to be assigned to the “severe” category, a dense inflammatory infiltrate had to be present in conjunction with significant alterations of the micro architecture.
Other biochemicals
Fetal calf sera were purchased from Gibco (Grand Island, New York, USA) or Sigma (St Louis, Missouri, USA). All antibodies were purchased either from Santa Cruz Biotechnology (Santa Cruz, California, USA), BD Pharmingen (San Diego, California, USA), Dianova (Hamburg, Germany), or New England Biolabs (Beverly, Massachusetts, USA). All other chemicals were obtained from Sigma if not otherwise specified.
Expression of data
The symbol n refers to the number of experiments. All experiments were carried out three or more times. Normal distribution of the data was evaluated by calculating Lilliefors probabilities based on the Komolgorov-Smirnov test.31,32 Statistical significance of the differences for non-normally distributed data was tested using the Mann-Whitney U test or the Wilcoxon matched pairs test.33 Results are expressed as mean (SD) if data followed a normal distribution or as median (interquartile range) if data were non-normally distributed.
RESULTS
Nuclear expression levels of STAT1 are increased in IBD and correlate with the endoscopic and histological activity of the disease
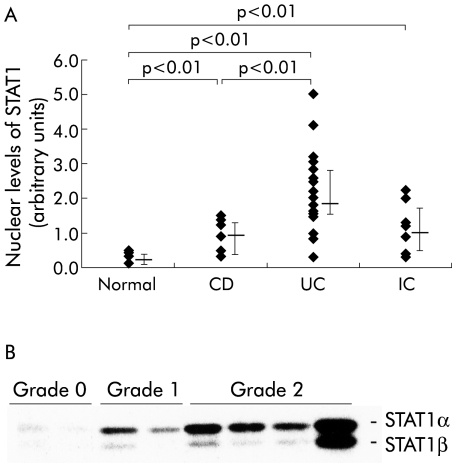
During activation, STAT proteins translocate into the nucleus. The amount of STAT1 in nuclear extracts of snap frozen colonic mucosal biopsy tissues was determined by western blot followed by densitometric measurement (fig 1A ▶). Increased nuclear amounts of STAT1 (median 1.73 (interquartile range 1.4–2.8) arbitrary units, n=22) were detected in biopsies from patients with ulcerative colitis in comparison with both Crohn's disease patients (0.92 (0.45–1.21), n=10; p<0.01) and normal controls (0.20 (0.16–0.28), n=12; p<0.01). Nuclear levels of STAT1 in biopsies from inflamed mucosa were not different between similarly inflamed anatomical regions (for example, left sided colon versus right sided colon in ulcerative colitis; not shown). Patients with infectious colitis were examined to determine the specificity of our findings. In patients with non-IBD colonic inflammation, nuclear levels of STAT1 were increased (1.06 (0.6–1.6), n=8) in comparison with normal controls (p<0.01) although still considerably lower than in ulcerative colitis.
Figure 1.
(A) Levels of signal transducer and activator of transcription 1 (STAT1) in the nuclear extracts of colonic biopsies. Extracts were examined by western blot with specific monoclonal antibodies. CD, Crohn's disease; UC, ulcerative colitis; IC, inflammatory controls. (B) Nuclear levels of STAT1 were determined by western blot in colonic mucosal biopsies from UC patients in relation to the histological degree of inflammation (grade 0, mild; grade 1, moderate; grade 2, severe). Representative of three experiments with identical findings.
High total protein expression levels of STAT1 were found in unfractionated intestinal mucosal samples of patients with ulcerative colitis (2.19 (1.39–2.75), n=20) in comparison with patients suffering from Crohn's disease (0.95 (0.23–1.44), n=10; p<0.05) and normal controls (0.17 (0.05–0.23), n=12; p<0.001) (not shown). Total expression of STAT1 in Crohn's disease was statistically different from normal controls (p<0.005). Samples were obtained from the same anatomical sites as the biopsies used for nuclear extracts.
To examine whether nuclear levels of STAT1 correlate with disease activity, biopsies were obtained from patients with different degrees of endoscopic activity. Endoscopically non-involved mucosa from patients with ulcerative colitis showed increased levels of STAT1 in nuclear extracts (median 0.76 (interquartile range 0.53–1.13), n=9) in comparison with biopsies from normal controls (0.2 (0.16–0.28), n=12; p<0.05). Levels in endoscopically inflamed mucosa were considerably higher in comparison with samples from macroscopically non-inflamed tissue of the same patient (1.4 (0.73–2.3), n=9; p=0.011). Comparison of different degrees of histological activity is demonstrated in the western blot in fig 1B ▶. This indicates that the degree of histological inflammation, as rated by an independent examiner, was related to nuclear accumulation of STAT1. The endoscopically “non-involved” mucosa, as described above, was associated with a histological degree of inflammation of 0 or 1 (on a scale of 0–2).
Phosphorylation and DNA binding activity of STAT1 is predominantly increased in ulcerative colitis
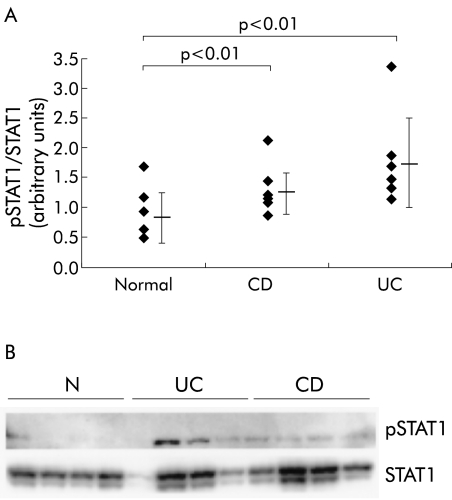
Activation of STAT1 as a regulator of transcription requires both phosphorylation and nuclear translocation of the factor, and leads to binding of specific DNA sites. To examine whether increased nuclear accumulation of STAT1 in IBD is associated with an increased level of activated (phosphorylated) STAT1, tyrosine phosphorylation of STAT1 was determined by western blot with a PY701-STAT1 specific antibody. All membranes were stripped and reprobed with a STAT1 specific antibody. STAT1 phosphorylation was then expressed as the ratio between the amount of phosphorylated (p) STAT1 and the total amount of STAT1 in the biopsies (fig 2A ▶). Patients with ulcerative colitis exhibited higher levels of tyrosine phosphorylated STAT1 in the intestinal mucosa (mean 1.73 (SD 0.75), n=7; p=0.0048) in comparison with normal controls (0.84 (0.45), n=7) or Crohn's disease (1.29 (0.4), n=7; p=0.0017). An example western blot of these sets of experiments is shown in fig 2B ▶.
Figure 2.
(A) Phosphorylation of signal transducer and activator of transcription 1 (pSTAT1) in the intestinal mucosa from patients with ulcerative colitis (UC) or Crohn's disease (CD) and normal controls (N). STAT1 phosphorylation was expressed as the ratio of pSTAT1 to total STAT1. Every membrane was probed with an anti-PY701-STAT1 antibody and afterwards reprobed with a STAT1 specific antibody (an example is shown in (B)).
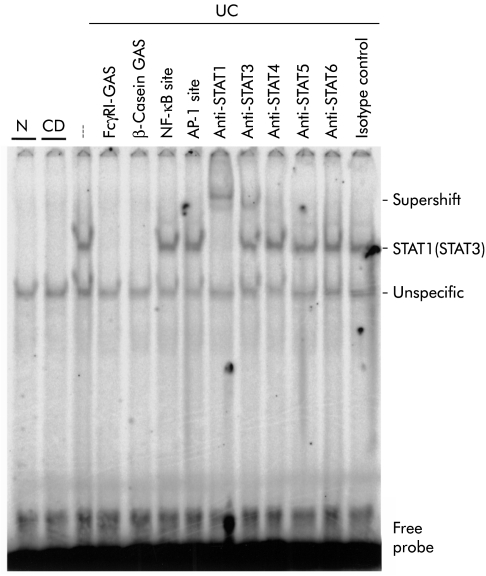
To assess the DNA binding activity of STAT1 in IBD, EMSAs were performed. Nuclear extracts from four different normal control biopsies or biopsies from ulcerative colitis (n=5) and Crohn's disease (n=4) patients were incubated with a 33P-labelled oligonucleotide containing the GAS site from the FcγRI promoter, and DNA/protein complexes were separated on an acrylamide gel (see methods). Figure 3 ▶ shows a representative result obtained with one extract from each investigated group. A strong DNA binding complex was detected in ulcerative colitis homogenates but not (as shown), or to a significantly lesser degree, in Crohn's disease and normal control samples. STAT binding to the 33P-labelled oligonucleotide was characterised by competition experiments with an excess of different unlabelled specific (FcγRI-GAS, β-casein-GAS) and unspecific (NFκB binding site, AP-1 binding site) oligonucleotides or coincubation with different specific anti-STAT antibodies, as outlined in the figure legend. The specificity of each antibody used has been reported previously. These experiments demonstrate that the DNA binding complex detected in IBD mainly contains STAT1 but also lower amounts of STAT3.
Figure 3.
Representative electrophoretic mobility shift assay for assessment of signal transducer and activator of transcription (STAT)/DNA binding complexes in colonic mucosal biopsies from a control (N) subject, and two patients with ulcerative colitis (UC) or Crohn's disease (CD). The specificity of the complex was examined by cold competition with a 25-fold molar excess of unlabelled FcγRI-GAS (lane 4), β-casein-GAS (lane 5), nuclear factor kappa B (NF-κB) binding (lane 6), or activator protein 1 (AP-1) binding (lane 7) oligonucleotides. The existence of STAT proteins in the DNA binding complex was assessed by parallel use of different anti-STAT antibodies (lanes 8–12). The last lane represents a control with an isotype specific control antibody.
Increased STAT1 expression in ulcerative colitis is mainly restricted to infiltrating neutrophils and monocytes in the lamina propria
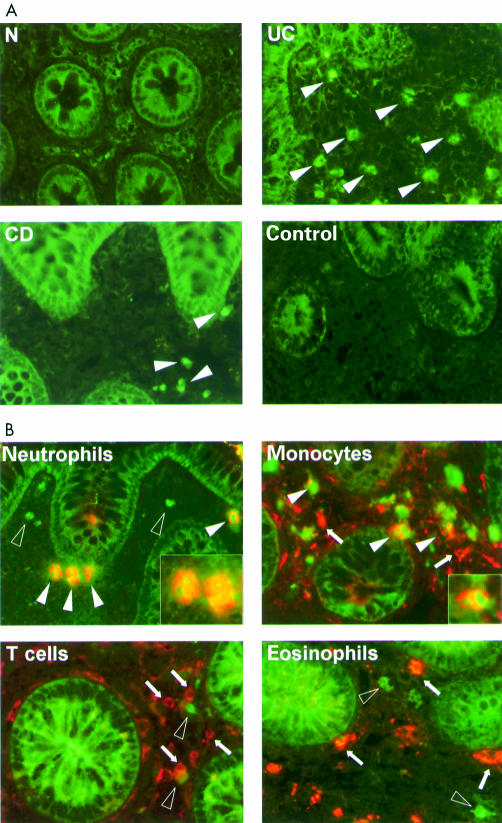
To localise the anatomical site and type of cells in the lamina propria which contribute to the high expression and activation of STAT1, immunofluorescence studies were performed. Frozen sections from normal controls (n=5) and from patients with ulcerative colitis (n=6) and Crohn's disease (n=5), which were obtained in parallel from the same sites as the biopsies used for the cell biology studies, were stained with an anti-PY701-STAT1 antibody (fig 4A ▶). Large numbers of cells were stained by the anti-pSTAT1 antibody in patients with ulcerative colitis but considerably less positive cells were detected in patients with Crohn's disease and only rarely in normal controls. Positive cells were exclusively found in the lamina propria. To investigate whether activation of STAT1 in ulcerative colitis is restricted to particular cell types, we used an anti-pSTAT1 antibody in combination with different cell type specific antibodies against neutrophils (human neutrophilic peptide), eosinophils (eosinophilic peroxidase), monocytes/macrophages (CD68), and T cells (CD3) (fig 4B ▶). Detection of pSTAT1 was clearly associated with neutrophils and monocytic cells but not with eosinophils or T cells. The same results were obtained when the fluorescence dyes of the secondary antibodies were inverted, which demonstrated the specificity of our results.
Figure 4.
(A) Immunostaining for phosphorylated signal transducer and activator of transcription 1 (pSTAT1). Distinct staining with high numbers of positive cells were detected in ulcerative colitis (UC) in comparison with Crohn's disease (CD) or normal controls (N). The control shows a staining experiment with an irrelevant isotype antibody in a patient with ulcerative colitis. Arrowheads indicate the position of stained cells. (B) Double staining of UC biopsies with an anti-pSTAT1 antibody (green) and cell type specific antibodies (red) (open arrowheads, cells exclusively stained with the anti-pSTAT1 antibody; arrowheads: double stained cells; arrows, cells exclusively stained with cell type specific antibody).
Expression of the suppressor of cytokine signalling (SOCS)-3 in IBD
Altered levels of STAT1 activation could be based on different levels of SOCS-1 and SOCS-3 expression in ulcerative colitis and Crohn's disease. Therefore, levels of both proteins were examined by western blot in total extracts of biopsies from active IBD patients and compared with controls (example blot shown in fig 5 ▶). Colonic biopsies from patients with active ulcerative colitis showed lower protein levels of SOCS-3 protein expression (median 0.5 (interquartile range 0.3–0.9) arbitrary units, n=9) than active Crohn's disease patients (2.2 (1.2–3.1), n=9; p=0.004) or normal controls (2.0 (1.54–2.75), n=8; p=0.005). The same extracts were also investigated for SOCS-1 expression but all signals remained near or even less than the detection levels of the antibodies used (not shown). SOCS-1 and SOCS-3 could not be detected by immunohistology.
Figure 5.
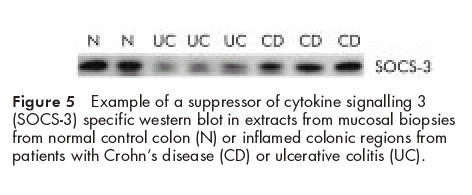
Example of a suppressor of cytokine signalling 3 (SOCS-3) specific western blot in extracts from mucosal biopsies from normal control colon (N) or inflamed colonic regions from patients with Crohn's disease (CD) or ulcerative colitis (UC).
STAT1 phosphorylation is decreased after glucocorticoid treatment
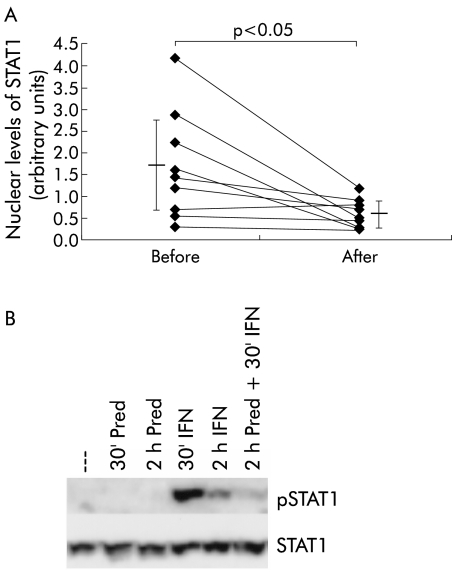
Biopsies from nine IBD patients were derived before and 2–4 weeks after beginning steroid therapy, and pSTAT1 levels were investigated by western blot. All patients received prednisolone (equivalent) in a dose of 60 mg for two weeks, which was then reduced to 40 mg (one week) and 30 mg (one week). Patients reached clinical remission after conclusion of the treatment. IBD patients before therapy (ratio between pSTAT1 and STAT1: mean 1.68 (SD 1.12)) showed significant downregulation of STAT1 phosphorylation after treatment (0.59 (0.3); p<0.05) (fig 6A ▶). To examine whether downregulation of STAT1 phosphorylation is a direct effect induced by glucocorticoids or is secondary to the reduced level of inflammation, further in vitro assays were performed by treating isolated peripheral blood monocytes for different times with IFN-γ (1000 U/ml) and/or 10 μM of prednisolone (fig 6B ▶). These experiments indicated that glucocorticoids directly inhibited IFN-γ mediated STAT1 phosphorylation, as shown in the last lane (see fig 6B ▶). Simultaneous stimulation of the cells with IFN-γ and prednisolone also resulted in marked loss of STAT1 phosphorylation (not shown). Similar results were obtained in additional experiments with monocytic THP-1 cells (not shown).
Figure 6.
(A) Downregulation of signal transducer and activator of transcription 1 (STAT1) phosphorylation after therapy with glucocorticoids. Colonic mucosal STAT1 levels were examined before and 2–4 weeks after treatment of ulcerative colitis (UC) patients with systemic glucocorticoids (n=9) was initiated. Results were obtained by western blot and analysed densitometrically. (B) In vitro stimulation of isolated peripheral blood monocytes with interferon γ (IFN 1000 U/ml) and prednisolone (pred 10 μM) for the indicated times (30 minutes and two hours). pSTAT1 (top panel) was detected by western blot and total amounts of STAT1 were examined by reprobing of the same membrane (bottom panel).
DISCUSSION
The normal non-inflamed mucosal immune system is characterised by a delicate balance between pro- and contra-inflammatory cytokines. The preponderance of proinflammatory cytokines, which is observed in IBD, has led to the development of therapeutic approaches that aim to readjust the balance (for example, the introduction of monoclonal antibodies directed against TNF-α34,35 or systemic application of the contra-inflammatory cytokine IL-106,36,37). Analysis of cytokine dysregulation by assessment of cytokine secretion or expression in the mucosa may not completely represent the pathophysiological process. The biological activity of cytokines is dependent on interaction between mediators with their receptors and on the microenvironment of secretion.
Analysis of cytokine induced transcription factor activation may reveal additional insights into key steps of regulation of the complex cytokine network. Activation of the NFκB system as a major cytokine signalling pathway has recently been described in different chronic inflammatory disorders, including Crohn's disease.29,30,38 However, activation of this system does not completely explain the nature of the inflammatory process. Many immunoregulatory genes contain multiple binding sites for transcription factors, including members of the STAT family. The mechanisms resulting in specificity of transcriptional regulation by STATs (in particular STAT1), which are involved in signalling of many cytokine receptors, are still unclear. Specificity may be reached through the sequential and cooperative binding of multiple transcription factors.39
Specific activation of STAT1 was demonstrated previously in other chronic inflammatory diseases, including asthma and rheumatoid arthritis.40,41 In this study, we demonstrated that ulcerative colitis and Crohn's disease are characterised by increased expression and activation of STAT1 in the intestinal lamina propria. Activation of this transcription factor was found consistently using different assays targeting nuclear accumulation, phosphorylation, or functional activity of STAT1, respectively. The predominance of STAT1 activation in ulcerative colitis in comparison with Crohn's disease was not due to differences in inflammatory activity, as demonstrated by similar histological activity and by high levels of NFκB p65 in Crohn's disease in the same set of nuclear extracts of biopsy tissue (data not shown). Most interestingly, STAT1 can also inhibit activation of NFκB under certain conditions.42
Immunohistology localised high levels of phosphorylated STAT1 to neutrophils and monocytic cells in the lamina propria. Increased infiltration with neutrophils and macrophages is a hallmark of the intestinal immunopathology of IBD. We therefore conclude that the observed elevation of STAT1 activation and expression in the intestinal mucosa is partially due to increased infiltration with STAT1 expressing cells. However, the heightened ratio between phosphorylated and total STAT1, as depicted in fig 2A ▶, indicates an over proportional increase in STAT1 activation in ulcerative colitis. Therefore, both an increase in total levels but also a true activation are seen. We suggest that increased STAT1 activation in ulcerative colitis and (to a lesser extent) in Crohn's disease may reflect an immunoregulatory difference of cytokine action in vivo. This would indicate that the animal model derived simplified hypothesis of a TH1 driven response in Crohn's disease and a TH2 driven immunity in ulcerative colitis does not reflect human pathophysiology.
Various cytokines can induce activation of STAT1. In addition to the interferons, these include epidermal growth factor, platelet derived growth factor, IL-6, or colony stimulating factor 1.21,43,44 The predominance of STAT1 activation seen in ulcerative colitis could be consistent with reports that have suggested that IFN-γ is an important mediator in both human IBD and in animal models of colitis.5,11,45–47 However, it should be noted that increased expression of IFN-γ has not been described consistently in human IBD. Although high levels of IFN-γ have been reported as a specific characteristic of Crohn's disease3 and in pouchitis,15 the IFN-γ gene does not appear to be a candidate for a primary genetic cause of IBD.48 Therefore, it is unlikely that differences in the cytokine patterns are the only explanation for differences in STAT1 activation.
The surprising finding in this study that STAT1 appears to be mostly activated in ulcerative colitis and to a lesser degree in Crohn's disease has led to the question, which molecular mechanisms are responsible for this phenomenon in addition to cytokines? Downregulation of STAT activity is not caused primarily by degradation of signal transducers but is mostly due to active shutdown mechanisms, such as those mediated by phosphatases (that is, SHP-1).49 Another recently described family of cytokine induced inhibitors of the JAK-STAT signal cascade are the SOCS.26,50,51 Western blot experiments revealed that SOCS-3 levels appeared to be higher in the majority of Crohn's disease and control samples in comparison with the ulcerative colitis samples (fig 5 ▶). However, SOCS-1 and SOCS-3 were not detectable by immunohistology. This is most likely due to technical reasons (that is, the monoclonal antibodies available do not work for this application or the concentration is below detection levels for this technique). Therefore, it is unclear whether SOCS-3 expression is increased in the same cells which show low STAT1 activity. SOCS-1 does not appear to be upregulated in the intestinal mucosa of patients with IBD (not shown). This is consistent with recently published findings from Suzuki et al demonstrating high expression of SOCS-3 but not SOCS-1 in IBD samples and also in murine colitis models.52
Increased activation of STAT1 was recently shown to correlate with expression of ICAM-1.40 In IBD, upregulated expression of ICAM-1 among other adhesion molecules is one of the hallmarks of inflammatory activity.53 Increased expression of ICAM-1 is a pivotal factor for the homing of large numbers of activated phagocytes into intestinal lesions. Levels of STAT1 activation paralleled the endoscopic and histological presentation of inflammatory activity in patients with ulcerative colitis. Patients in remission showed nuclear levels of STAT1 which approached normal non-inflamed controls. STAT1 regulated genes such as major histocompatibility class II, Fcγ receptor, inducible nitric oxide synthase, or ICAM-1 also correspond in their expression to the overall inflammatory activity.53–57 Activation of STAT1 (in addition to the TNF/NFκB system58,59) may therefore contribute to many of the pathophysiological features observed in IBD.
This study also showed that glucocorticoids actively inhibit phosphorylation of STAT1. We found that prednisolone inhibited in vitro phosphorylation of STAT1 in isolated peripheral monocytes as well as in the THP-1 cell line. In addition, treatment of IBD patients with systemic glucocorticoids resulted in a decrease in pSTAT1 levels in the intestinal mucosa. While these experiments cannot prove a primary role for STAT1 activation in the inflammatory pathophysiology, they suggest that inhibition of the STAT system may be an important part of the anti-inflammatory efficacy of glucocorticoids. Patients with glucocorticoid induced remission had lower nuclear levels of STAT1 (fig 6A ▶). In addition, the action of glucocorticoids may explain the discrepancy between increased levels of STAT1 activation found in IBD in this study and the work by Suzuki et al who did not report increased phosphorylation of STAT1 in IBD.52 It should be pointed out that IBD patients in the study of Suzuki et al were pretreated with anti-inflammatory drugs and that only small numbers (two patients with ulcerative colitis, one patient with Crohn's disease) were investigated.
Microinvasion of Escherichia coli has been described as an important event in both IBD60,61 as well as colonic adenocarcinoma.62 The hypothesis has been raised that bacterial invasion into the mucosa may be an important early event in the onset of the inflammatory reaction. The heightened levels of nuclear accumulation of STAT1, which are seen in infectious colitis and diverticulitis, could point to a role for intestinal microorganisms in the mechanism of activation of this transcription factor. The role of microorganisms in the activation of STAT1 in ulcerative colitis is therefore under intense investigation. In this regard it will be important to evaluate genetic susceptibility as a potential permissive factor.63–65
In summary, we have shown that STAT1 expression and activation are significantly upregulated in the colonic mucosa of patients with active ulcerative colitis. Detection of phosphorylated STAT1 is mostly restricted to neutrophils and monocytes in the lamina propria and appears to be downregulated by glucocorticoids. Analysis of the intracellular signalling responses may reveal important novel aspects in complex inflammatory diseases such as human IBD. Activation of STAT1 and (as already demonstrated) other STAT family members could contribute to the pathophysiology of the disease. Further studies will demonstrate whether STATs are promising target molecules for future therapeutic interventions in colonic inflammation.
Acknowledgments
This work was supported by grants from the Deutsche Forschungsgemeinschaft (DFG), the BMBF competence network “Inflammatory Bowel Disease”, as well as a training and mobility of researchers grant of the European Commission (ERBFMRXCT980240). The authors are grateful to J Sievers (Department of Anatomy, Christian-Albrechts-University, Kiel, Germany) for discussions and access to the immunofluorescence equipment. The contribution of the endoscopy staff at the Charité University Hospital, Tabea Centre for Inflammatory Bowel Diseases, and the Hospital of the Christian-Albrechts-University in obtaining colonic tissue as well as the excellent technical help of AM Wenner, S Eidner, and I Woywod are gratefully acknowledged.
Abbreviations
IBD, inflammatory bowel disease
STAT, signal transducer and activator of transcription
SOCS, suppressor of cytokine signalling
IFN-γ, interferon γ
TH1, TH2, T helper cell types 1 and 2
IL, interleukin
TNF-α, tumour necrosis factor α
NFκB, nuclear factor kappa B
JAK, Janus kinases
EMSA, electrophoretic mobility shift assay
PBS, phosphate buffered saline
AP-1, activator protein 1
ICAM-1, intercellular adhesion molecule 1
REFERENCES
- 1.Hodgson HJ. Pathogenesis of Crohn's disease. Baillieres Clin Gastroenterol 1998;12:1–17. [DOI] [PubMed] [Google Scholar]
- 2.Abreu-Martin MT, Targan SR. Regulation of immune responses of the intestinal mucosa. Crit Rev Immunol 1996;16:277–309. [DOI] [PubMed] [Google Scholar]
- 3.MacDonald TT, Hutchings P, Choy MY, et al. Tumour necrosis factor-alpha and interferon-gamma production measured at the single cell level in normal and inflamed human intestine. Clin Exp Immunol 1990;81:301–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuhn R, Lohler J, Rennick D, et al. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 1993;75:263–74 [DOI] [PubMed] [Google Scholar]
- 5.Powrie F, Leach MW, Mauze S, et al. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity 1994;1:553–62. [DOI] [PubMed] [Google Scholar]
- 6.Schreiber S, Heinig T, Thiele HG, et al. Immunoregulatory role of interleukin 10 in patients with inflammatory bowel disease. Gastroenterology 1995;108:1434–44 [DOI] [PubMed] [Google Scholar]
- 7.Wyatt J, Vogelsang H, Hubl W, et al. Intestinal permeability and the prediction of relapse in Crohn's disease. Lancet 1993;341:1437–9. [DOI] [PubMed] [Google Scholar]
- 8.Pender SL, Breese EJ, Gunther U, et al. Suppression of T cell-mediated injury in human gut by interleukin 10: role of matrix metalloproteinases. Gastroenterology 1998;115:573–83. [DOI] [PubMed] [Google Scholar]
- 9.Podolsky DK. Inflammatory bowel disease (1). N Engl J Med 1991;325:928–37. [DOI] [PubMed] [Google Scholar]
- 10.Bregenholt S, Claesson MH. Increased intracellular Th1 cytokines in scid mice with inflammatory bowel disease. Eur J Immunol 1998;28:379–89. [DOI] [PubMed] [Google Scholar]
- 11.Strober W, Ludviksson BR, Fuss IJ. The pathogenesis of mucosal inflammation in murine models of inflammatory bowel disease and Crohn disease. Ann Intern Med 1998;128:848–56. [DOI] [PubMed] [Google Scholar]
- 12.Niessner M, Volk BA. Altered Th1/Th2 cytokine profiles in the intestinal mucosa of patients with inflammatory bowel disease as assessed by quantitative reversed transcribed polymerase chain reaction (RT-PCR). Clin Exp Immunol 1995;101:428–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desreumaux P, Brandt E, Gambiez L, et al. Distinct cytokine patterns in early and chronic ileal lesions of Crohn's disease. Gastroenterology 1997;113:118–26. [DOI] [PubMed] [Google Scholar]
- 14.Christ AD, Stevens AC, Koeppen H, et al. An interleukin 12-related cytokine is up-regulated in ulcerative colitis but not in Crohn's disease. Gastroenterology 1998;115:307–13 [DOI] [PubMed] [Google Scholar]
- 15.Stallmach A, Schafer F, Hoffmann S, et al. Increased state of activation of CD4 positive T cells and elevated interferon gamma production in pouchitis. Gut 1998;43:499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol 1994;12:141–79. [DOI] [PubMed] [Google Scholar]
- 17.Baldwin AS Jr. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol 1996;14:649–83. [DOI] [PubMed] [Google Scholar]
- 18.Shuai K, Schindler C, Prezioso VR, et al. Activation of transcription by IFN-gamma: tyrosine phosphorylation of a 91-kD DNA binding protein. Science 1992;258:1808–12. [DOI] [PubMed] [Google Scholar]
- 19.Darnell JE Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 1994;264:1415–21. [DOI] [PubMed] [Google Scholar]
- 20.Sadowski HB, Shuai K, Darnell JE Jr, et al. A common nuclear signal transduction pathway activated by growth factor and cytokine receptors. Science 1993;261:1739–44. [DOI] [PubMed] [Google Scholar]
- 21.Zhong Z, Wen Z, Darnell JE Jr. Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science 1994;264:95–8. [DOI] [PubMed] [Google Scholar]
- 22.Paquette RL, Minosa MR, Verma MC, et al. An interferon-gamma activation sequence mediates the transcriptional regulation of the IgG Fc receptor type IC gene by interferon-gamma. Mol Immunol 1995;32:841–51. [DOI] [PubMed] [Google Scholar]
- 23.Singh K, Balligand JL, Fischer TA, et al. Regulation of cytokine-inducible nitric oxide synthase in cardiac myocytes and microvascular endothelial cells. Role of extracellular signal-regulated kinases 1 and 2 (ERK1/ERK2) and STAT1 alpha. J Biol Chem 1996;271:1111–17. [DOI] [PubMed] [Google Scholar]
- 24.Lee YJ, Benveniste EN. Stat1 alpha expression is involved in IFN-gamma induction of the class II transactivator and class II MHC genes. J Immunol 1996;157:1559–68. [PubMed] [Google Scholar]
- 25.Jahnke A, Johnson JP. Intercellular adhesion molecule 1 (ICAM-1) is synergistically activated by TNF-alpha and IFN-gamma responsive sites. Immunobiology 1995;193:305–14. [DOI] [PubMed] [Google Scholar]
- 26.Starr R, Willson TA, Viney EM, et al. A family of cytokine-inducible inhibitors of signalling. Nature 1997;387:917–21. [DOI] [PubMed] [Google Scholar]
- 27.Ito S, Ansari P, Sakatsume M, et al. Interleukin-10 inhibits expression of both interferon alpha- and interferon gamma-induced genes by suppressing tyrosine phosphorylation of STAT1. Blood 1999;93:1456–63. [PubMed] [Google Scholar]
- 28.Sasaki A, Yasukawa H, Suzuki A, et al. Cytokine-inducible SH2 protein-3 (CIS3/SOCS3) inhibits Janus tyrosine kinase by binding through the N-terminal kinase inhibitory region as well as SH2 domain. Genes Cells 1999;4:339–51. [DOI] [PubMed] [Google Scholar]
- 29.Schreiber S, Nikolaus S, Hampe J. Activation of nuclear factor kappa B inflammatory bowel disease. Gut 1998;42:477–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rogler G, Brand K, Vogl D, et al. Nuclear factor kappaB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology 1998;115:357–69. [DOI] [PubMed] [Google Scholar]
- 31.Lilliefors HW. On the Komolgorov-Smirnov test for normality with mean and variance unknown. J Am Stat Assoc 1967;64:399–402. [Google Scholar]
- 32.Sachs L. Angewandte Statistik. Heidelberg: Springer, 1992.
- 33.Mann HB, Whitney DR. On a test of whether one of two random variables is stochastically larger than the other. Ann Math Statistics 1947;18:50–60. [Google Scholar]
- 34.Targan SR, Hanauer SB, van Deventer, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's Disease cA2 Study Group. N Engl J Med 1997;337:1029–35. [DOI] [PubMed] [Google Scholar]
- 35.Stack WA, Mann SD, Roy AJ, et al. Randomised controlled trial of CDP571 antibody to tumour necrosis factor-alpha in Crohn's disease. Lancet 1997;349:521–4. [DOI] [PubMed] [Google Scholar]
- 36.Fedorak RN, Gangl A, Elson CO, et al. Recombinant human interleukin 10 in the treatment of patients with mild to moderately active Crohn's disease. The Interleukin 10 Inflammatory Bowel Disease Cooperative Study Group. Gastroenterology 2000;119:1473–82. [DOI] [PubMed] [Google Scholar]
- 37.Schreiber S, Fedorak RN, Nielsen OH, et al. Safety and efficacy of recombinant human interleukin 10 in chronic active Crohn's disease. Crohn's Disease IL-10 Cooperative Study Group. Gastroenterology 2000;119:1461–72. [DOI] [PubMed] [Google Scholar]
- 38.Nikolaus, Raedler A, Stikas N, et al. Mechanisms in failure of infliximab for Crohn's disease. Lancet2000;356:1475–79. [DOI] [PubMed] [Google Scholar]
- 39.Xu X, Sun YL, Hoey T. Cooperative DNA binding and sequence-selective recognition conferred by the STAT amino-terminal domain. Science 1996;273:794–7. [DOI] [PubMed] [Google Scholar]
- 40.Sampath D, Castro M, Look DC, et al. Constitutive activation of an epithelial signal transducer and activator of transcription (STAT) pathway in asthma. J Clin Invest 1999;103:1353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yokota A, Narazaki M, Shima Y, et al. Preferential and persistent activation of the STAT1 pathway in rheumatoid synovial fluid cells. J Rheumatol 2001;28:1952–9. [PubMed] [Google Scholar]
- 42.Wang Y, Wu TR, Cai S, et al. Stat1 as a component of tumor necrosis factor alpha receptor 1-TRADD signaling complex to inhibit NF-kappaB activation. Mol Cell Biol 2000;20:4505–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gerhartz C, Heesel B, Sasse J, et al. Differential activation of acute phase response factor/STAT3 and STAT1 via the cytoplasmic domain of the interleukin 6 signal transducer gp130. I. Definition of a novel phosphotyrosine motif mediating STAT1 activation. J Biol Chem 1996;271:12991–8. [DOI] [PubMed] [Google Scholar]
- 44.Vignais ML, Sadowski HB, Watling D, et al. Platelet-derived growth factor induces phosphorylation of multiple JAK family kinases and STAT proteins. Mol Cell Biol 1996;16:1759–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Powrie F, Correa-Oliveira R, Mauze S, et al. Regulatory interactions between CD45RBhigh and CD45RBlow CD4+ T cells are important for the balance between protective and pathogenic cell-mediated immunity. J Exp Med 1994;179:589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rudolph U, Finegold MJ, Rich SS, et al. Gi2 alpha protein deficiency: a model of inflammatory bowel disease. J Clin Immunol 1995;15:101–5S. [DOI] [PubMed] [Google Scholar]
- 47.Berg DJ, Davidson N, Kuhn R, et al. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest 1996;98:1010–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hampe J, Hermann B, Bridger S, et al. The interferon-gamma gene as a positional and functional candidate gene for inflammatory bowel disease. Int J Colorectal Dis 1998;13:260–3. [DOI] [PubMed] [Google Scholar]
- 49.Klingmuller U, Lorenz U, Cantley LC, et al. Specific recruitment of SH-PTP1 to the erythropoietin receptor causes inactivation of JAK2 and termination of proliferative signals. Cell 1995;80:729–38. [DOI] [PubMed] [Google Scholar]
- 50.Naka T, Narazaki M, Hirata M, et al. Structure and function of a new STAT-induced STAT inhibitor. Nature 1997;387:924–9. [DOI] [PubMed] [Google Scholar]
- 51.Endo TA, Masuhara M, Yokouchi M, et al. A new protein containing an SH2 domain that inhibits JAK kinases. Nature 1997;387:921–4. [DOI] [PubMed] [Google Scholar]
- 52.Suzuki A, Hanada T, Mitsuyama K, et al. CIS3/SOCS3/SSI3 plays a negative regulatory role in STAT3 activation and intestinal inflammation. J Exp Med 2001;193:471–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Malizia G, Dino O, Pisa R, et al. Expression of leukocyte adhesion molecules in the liver of patients with chronic hepatitis B virus infection. Gastroenterology 1991;100:749–55. [DOI] [PubMed] [Google Scholar]
- 54.Smolen JS, Gangl A, Polterauer P, et al. HLA antigens in inflammatory bowel disease. Gastroenterology 1982;82:34–8. [PubMed] [Google Scholar]
- 55.Hommes DW, Meenan J, de Haas M, et al. Soluble Fc gamma receptor III (CD 16) and eicosanoid concentrations in gut lavage fluid from patients with inflammatory bowel disease: reflection of mucosal inflammation. Gut 1996;38:564–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singer II, Kawka DW, Scott S, et al. Expression of inducible nitric oxide synthase and nitrotyrosine in colonic epithelium in inflammatory bowel disease. Gastroenterology 1996;111:871–85. [DOI] [PubMed] [Google Scholar]
- 57.Nakamura S, Ohtani H, Watanabe Y, et al. In situ expression of the cell adhesion molecules in inflammatory bowel disease. Evidence of immunologic activation of vascular endothelial cells. Lab Invest 1993;69:77–85. [PubMed] [Google Scholar]
- 58.Schreiber S, Nikolau S, Haupe J, et al. Tumor necrosis factor - α and interleukin - 1,3 in relapse of Crohn's disease. Lancet 1999;353:459–61. [DOI] [PubMed] [Google Scholar]
- 59.Kullman F, Renmann H, Alt M, et al. Clinical and histopathological features of dextran sulfate sodium induced acute and chronic colitis associated with dysplasia in rats. Int J Colorectal Dis 2001;16:238–46. [DOI] [PubMed] [Google Scholar]
- 60.Darfeuille-Michaud A, Neut C, Barnich N, et al. Presence of adherent Escherichia coli strains in ileal mucosa of patients with Crohn's disease. Gastroenterology 1998;115:1405–13. [DOI] [PubMed] [Google Scholar]
- 61.Swidsinski A, Ladehoff A, Pernthaller A, et al. Mucosal flora in inflammatory bowel disease. Gastroenterology 2002; 122:44–54. [DOI] [PubMed] [Google Scholar]
- 62.Swidsinski A, Khilkin M, Kerjaschki D, et al. Association between intraepithelial Escherichia coli and colorectal cancer. Gastroenterology 1998;115:281–6. [DOI] [PubMed] [Google Scholar]
- 63.Hugot JP, Chamaillard M, Zouali H, et al. Association of NOD2 leucin-rich repeat variants with susceptibility to Crohn's disease. Nature 2001;411:599–602. [DOI] [PubMed] [Google Scholar]
- 64.Ogura Y, Bonen DK, Inohara N. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature 2001;411:603–5. [DOI] [PubMed] [Google Scholar]
- 65.Hampe J, Cuthbert A, Grouchu PJP, et al. An insertion mutation in the NOD2 gene predisposes to Crohn's disease in German and British populations. Lancet 2001;357:1925–8. [DOI] [PubMed] [Google Scholar]