Abstract
Background and aims: Experimental studies have shown that luminal bacteria may be involved in Crohn's disease. Probiotics are a possible alternative to antibiotics. The aim of this randomised placebo controlled study was to determine if Lactobacillus GG, given by mouth for one year, could prevent Crohn's recurrent lesions after surgery or to reduce their severity.
Methods: Patients operated on for Crohn's disease in whom all of the diseased gut had been removed were randomly allocated to receive 12 billion colony forming units of Lactobacillus or identical placebo for one year. Ileocolonoscopy was performed at the end of the trial or at the onset of symptoms. Endoscopic recurrence was defined as grade 2 or higher of Rutgeerts scoring system.
Results: Eight of 45 patients were excluded from the trial (three for non-compliance and five for protocol violations). Clinical recurrence was ascertained in three (16.6%) patients who received Lactobacillus and in two (10.5%) who received placebo. Nine of 15 patients in clinical remission on Lactobacillus (60%) had endoscopic recurrence compared with six of 17 (35.3%) on placebo (p=0.297). There were no significant differences in the severity of the lesions between the two groups.
Conclusions: Lactobacillus GG seems neither to prevent endoscopic recurrence at one year nor reduce the severity of recurrent lesions.
Keywords: Crohn's disease, probiotics, lactobacillus GG, gut bacteria, endoscopic recurrence
A large body of evidence from both animal models and clinical observations suggests that the most probable inducer of chronic inflammation in Crohn's disease are luminal bacteria.1–4 The inflammatory process is thought to be the result of interaction between the immune response of the host and the enteric flora in susceptible individuals.5 Current methods of treatment vary from blocking the immune response using immunosuppressors to eliminating luminal bacteria by antibiotics.6,7 Among more than 400 species of resident flora, it seems that anaerobic bacteria and Escherichia coli (E coli) play potentially harmful roles.8
Antibiotics are usefully employed in the treatment of active Crohn's disease but side effects and bacterial resistance limit their use long term.
To counterbalance harmful bacteria, manipulation of the bacterial flora with probiotics is an appealing alternative. Probiotics are viable bacteria which, when ingested, offer benefits to human health. Their therapeutic effects may include competitive action with commensal and pathogenic flora and influence on the immune response through various mechanisms.9 Probiotics have been used successfully in the treatment of acute gastrointestinal diseases such as antibiotic associated diarrhoea,10 Clostridium difficile infection,11 traveller's diarrhoea,12 and rotavirus diarrhoea.13 Recently, some investigators have reported success with different strains of probiotics in the treatment of chronic intestinal diseases such as ulcerative colitis,14,15 Crohn's disease,16–18 and pouchitis.19 E coli (Nissle 1917), the yeast Saccharomyces boulardii, Lactobacillus GG, and VSL# 3, a cocktail of eight different strains, have been used successfully in human pathology.
Several significant flaws however limit the importance of many of the probiotic trials, such as inclusion of too few patients,16,17 too low a dose of the control drug,14 too limited a period of observation,14 or the association of the probiotic with other drugs.15–18 Crohn's disease is a heterogeneous condition with different pathological behaviours.20 More than 70% of Crohn's disease patients are operated on during their lifetime, and 70–90% show endoscopic recurrence within one year.21,22 When all of the diseased gut is removed at surgery, the resected patient in remission represents the best candidate for testing a drug for prevention of recurrence. Lactobacillus rhamnosus strain GG (LGG) was discovered in 1985. LGG can survive and colonise the human intestine and adhere to intestinal cells.23
When administered to 14 children with Crohn's disease, LGG was recently shown to increase the mucosal IgA immune response and thereby increase the immunological defences of the gut.24
The aim of this randomised placebo controlled trial was to determine if the probiotic LGG, given by mouth for a period of one year, could prevent the appearance of Crohn's disease recurrent lesions after surgery or reduce their severity.
MATERIALS AND METHODS
Patients
Patients were eligible for the study if they were aged at least 18 years and were scheduled for curative resection for Crohn's disease. Inclusion criteria were: a diagnosis of Crohn's disease, defined by the criteria adopted by Lennard-Jones25 and confirmed by surgical specimens; complete resection of all diseased intestine, as shown by inspection at surgery; ability to start oral nutrition and therefore the trial itself within 10 days of operation; and informed written consent.
Exclusion criteria were: pregnancy and lactation; postoperative septic complications; presence of other concomitant important disease; active perianal disease; presence of Crohn's disease in other intestinal tracts; need for antibiotics for more than 10 days after surgery; intake of steroids for more than 30 days after operation; total parenteral nutrition or elemental diet; and use of other drugs possibly active in Crohn's disease. Antidiarrhoeals such as loperamide or other opiates, and colestiramine, were allowed provided their use had been calculated in the Crohn's disease activity index (CDAI).26
Study drugs
LGG (Dicoflor 60; Dicofarm, Rome, Italy) consisted of 2.46 g bags each containing LGG 6 billion colony forming units (cfu) and was administered at a dose of 6 billion cfu twice daily. LGG belongs to Lactobacillus casei subspecies rhamnosus, isolated by Goldin and Gorbach.
The placebo consisted of bags of identical appearance to the probiotic. Each bag contained maltodextrines 2.060 mg, sorbitol 400 mg, and silicic dioxide 5 mg. The taste and smell of the active substance and placebo were the same.
Study design
The study was performed as a single centre, 52 week, prospective, randomised, double blind, placebo controlled trial.
Using computerised randomisation in blocks of two, patients were allocated to receive bags of either Dicoflor 60 or placebo. The study drugs were administered orally, one bag twice daily, morning and afternoon, dissolved in half a glass of water, for 52 weeks. Treatment was started as soon as patients could take solid food by mouth after operation but not later than 10 days after surgery. Follow up visits were carried out after 13, 26, 39, and 52 weeks of treatment. Compliance with the study drugs was checked by the investigator by counting the number of the bags returned at each visit. Ileocolonoscopy was performed at the end of the trial or at any period in case of recurrent symptoms. Treatment failure during the study period was defined as the appearance of Crohn's disease symptoms and/or signs which needed additional medical treatment or operation. Failure was also defined as an increase in CDAI to more than 150 points, confirmed at a second visit a week later. The CDAI was calculated at all postoperative visits. Patients were provided with a diary card which was completed by the patients themselves during the week before the visit. In the event of treatment failure, endoscopy with biopsies was performed to confirm recurrence. The study was conducted in compliance with Good Clinical Practice and international research ethics standards.
Outcome measurements
The primary parameter for determination of drug efficacy was reduction of endoscopic recurrence rate at 12 months or reduction in the severity of recurrent lesions. To evaluate the degree of recurrent inflammation, the endoscopic scoring system (0–4) of Rutgeerts et al was used.21 Endoscopic recurrence was defined as the presence of grade 2 or higher, and severe recurrence as grade 3 or 4.21 Colonoscopy with inspection of the ileocolonic anastomosis and the neoterminal ileum was carried out after 52 weeks of treatment or when the patient was withdrawn from the study because of clinical symptoms.
The secondary outcome measure was reduction of clinical recurrence rate. Clinical recurrence was defined as an increase in CDAI to more than 150 points, confirmed by endoscopic signs of inflammation.
Laboratory assessment
Complete blood count, and serum iron and ferritin were analysed at each clinic visit for assessment of inflammatory activity. Serum creatinine, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, and urine analysis were also evaluated at each visit to assess possible toxicity.
Statistical analysis
To analyse differences between treatment groups, we used a two sided test for the difference in proportions: Z tests were reported when the expected frequency for each cell in the crosstabulation was five or greater. When the expected frequency was less than five in at least one cell, Fisher's exact test was reported. Values for all of these tests were considered significant at p<0.05.
RESULTS
Patients
Forty five consecutive patients (29 men and 16 women) who fulfilled the enrolment criteria entered into the study between May 1998 and March 2000. Twenty three were randomised to receive LGG and 22 to receive placebo. Demographic and disease characteristics did not differ significantly between the two groups but a higher percentage of patients treated with LGG were smokers (p=0.410) (table 1 ▶). Although not significant, there was also a difference with respect to disease location: 16 patients (69.6%) who received LGG had ileitis compared with 19 (86.4%) who received placebo. The most common indication for surgery in both groups was obstructive symptoms.
Table 1.
Baseline characteristics of patients in the Lactobacillus GG (LGG) and placebo groups
| LGG (n=23) | Placebo (n=22) | p Value | |
| Male | 14 (60.8%) | 15 (68.2%) | 0.841 |
| Age (y) (mean (range)) | 37.3 (22–71) | 36.2 (22–64) | |
| Smokers | 10 (43.5%) | 6 (27.3%) | 0.410 |
| Disease duration (y) (mean (range)) | 6.5 (0.6–21) | 7.4 (1–19) | |
| Disease location | |||
| Ileum | 16 (69.6%) | 19 (86.4%) | 0.319 |
| Ileum-colon | 5 (21.7%) | 2 ( 9.1%) | 0.448 |
| Colon | 2 ( 8.7%) | 1 ( 4.5%) | 0.968 |
| Previous resection | 5 (21.7%) | 6 (27.3%) | 0.932 |
| Primary indication for surgery | |||
| Obstruction | 17 (74.0%) | 15 (68.2%) | 0.924 |
| Fistula | 6 (26.0%) | 4 (18.2%) | 0.780 |
| Failure of medical therapy | 0 | 3 (13.6%) | 0.217 |
There were no statistically significant differences.
Patient withdrawal
Overall, 13 (28.8%) of the randomised patients (8 (34.7%) in the LGG group and 5 (22.7%) in the placebo group) withdrew before completing the trial (table 2 ▶). Non-compliance with the study procedures caused early termination in three patients.
Table 2.
Clinical and endoscopic remission in the Lactobacillus GG (LGG) and placebo groups
| LGG (n=23) | Placebo (n=22) | p Value | |
| Non-compliance (%) | 2 (8.7) | 1 (4.5) | 0.968 |
| Protocol violation (%) | 3 (13.0) | 2 (9.0) | 1.000 |
| Clinical remission* (%) | 15 (83.3) | 17 (89.4) | 0.948 |
| Endoscopic remission† (%) | 6 (40.0) | 11 (64.7) | 0.243 |
| Score 0 | 1 | 9 | |
| 1 | 5 | 2 | |
| Endoscopic recurrence† (%) | 9 (60.0) | 6 (35.3) | 0.297 |
| Score 2 | 3 | 3 | |
| 3 | 2 | 0 | |
| 4 | 4 | 3 |
*Crohn's disease activity index ≤150 after 52 weeks of therapy.
†Rutgeerts score: 0–1=remission; 2–4=recurrence.
There were no statistically significant differences.
Five patients discontinued the study for protocol violations: three (two in the LGG group and one in the placebo group) withdrew from the study because of complications after surgery which needed antibiotic therapy. Other reasons for protocol violation were the presence of residual disease in the ileum diagnosed after operation in one patient receiving LGG and the use of antibiotics for a perianal abscess in a patient who received placebo.
Clinical recurrence was suspected in three patients (16.6%) on LGG, one at 24 weeks, one at 29 weeks, and one at 46 weeks (mean CDAI 222), and in two patients (10.5%) on placebo, one at 13 weeks and one at 15 weeks (mean CDAI 216) (fig 1 ▶). Ileocolonoscopy confirmed severe recurrence (scores 3–4) in all patients with symptoms. No patient was withdrawn for adverse events.
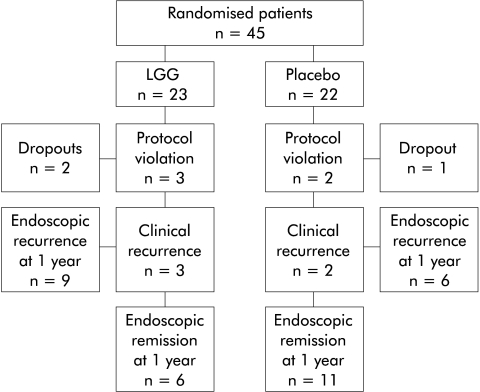
Figure 1.
Flow chart describing the outcome of patients during the trial.
Endoscopic recurrence
In all patients the anastomosis was reached by endoscopy. After 52 weeks of treatment, 15 patients (83.3%) treated with LGG and 17 (89.4%) treated with placebo remained in clinical remission (CDAI ≤150). Among patients remaining in clinical remission, nine of 15 allocated to the LGG group (60.0%) showed recurrent endoscopic lesions compared with six of 17 patients in the placebo group (35.3%) (p=0.297).
Six of 15 patients who received LGG (40.0%) had severe endoscopic recurrence compared with three of 17 patients who received placebo (17.6%) (p=0.313).
Adverse events
Two patients who received LGG and six who received placebo suffered adverse events. A suture stitch suppuration and a mild increase in alanine aminotransferase were reported both in the LGG group and in the placebo group. In the placebo group, acne (one case), nausea (one case), mild haematuria (one case), and a depressive state (one case) were also recorded. In all cases the events were not considered trial related and in no patient did they cause interruption of the study.
Among patients in clinical remission, diarrhoea, bloating, and meteorism did not differ between the two groups.
DISCUSSION
The pathogenic role of bacteria in Crohn's disease is supported by both experimental and clinical data.1,2,4,5 The most striking clinical observations are: (1) the efficacy of antibiotics in modulating the intestinal flora in the treatment of active Crohn's disease,3,6,7,27 and (2) the fact that recurrence of lesions in patients operated on for complicated Crohn's disease followed the reintroduction of luminal content into the gut.28,29 The reduction in the harmful effect of the bacterial flora by antibiotics has been shown to be useful not only in the active phases of disease3,6,7 but also in reducing the rate of ileal postoperative recurrence and clinical symptoms in operated patients.30
Experimental models have suggested that certain bacteria—for example, bacteroides species—are particularly pathogenic while the lactobacilli species seem to have a protective effect.31 When all of the diseased gut is removed by surgery, the operated patient provides an optimal testing ground for assessing the effect of luminal bacteria in causing new lesions. In fact in a recent trial, metronidazole, an antibiotic active against bacteriodes and clostridia, given immediately after surgery, reduced the appearance of recurrent lesions and their severity at three months.30
Probiotics have been shown to be effective in ulcerative colitis15 and in the prevention of pouchitis recurrence.19 Few data however have been reported with regard to Crohn's disease.16–18
The probiotic used in our study was LGG. LGG, which is of human origin, has been shown to survive in and colonise the human intestine.23 It can adhere to the colonic mucosa32,33 and has been shown to be effective in treating several forms of acute diarrhoea, including rotavirus diarrhoea, travellers' diarrhoea, and relapsing Clostridium difficile infection.11–13 In Crohn's disease, a study has suggested that LGG may have the potential to promote the gut's immunological defence.24 Moreover, LGG has been shown to decrease the response towards the body's own and foreign Bacteroides fragilis and E coli in healthy volunteers.34
To our knowledge this is the first randomised controlled trial that has used a probiotic alone in the prevention of Crohn's disease recurrence after surgery. It is also the first trial which has given a clear negative result among other more positively slanted studies.
The basic idea of the study was that counterbalancing the harmful gut flora (the possible cause of recurrent lesions in Crohn's disease) with a beneficial bacterium would prevent the appearance of lesions or reduce their severity.
Because of the small number of patients, this study should be considered a pilot trial. However, the results are strengthened because of the strict criteria adopted for patient enrolment, surgery, and endoscopic control which was performed in only one centre.
Moreover, the trial had a certain number of non-evaluable patients because of protocol violations. Unfortunately, protocol violations are a relatively frequent event when patients are enrolled immediately after operation. In four of our five cases, complications after surgery requiring antibiotic therapy were the cause. Another criticism could be that no evaluation of the faecal microflora was performed but we do not know whether the bacterial flora in stools reflects the flora found in the mucosa.35
The percentage recurrence in our study, both symptomatic and endoscopic, was lower than that reported in other studies.21,22,36 Consequently, a high placebo response may have obscured the efficacy of the probiotic. Our trial however selected patients with a relatively milder disease because of the exclusion criteria: patients who needed antibiotics or who were unable to stop steroids after surgery were excluded from the trial. Other exclusion criteria eliminated patients unable to take oral food by 10 days, or with concomitant perianal disease. Moreover, more than 70% of the study population had ileal disease and were operated on for obstruction, two characteristics with a lower recurrence tendency.37,38 All of these exclusions produced a group of patients who should have had a reduced one year rate of recurrence. Sixty five per cent of patients who received placebo were in remission at 12 months compared with 40% who received LGG. More severe endoscopic recurrences also occurred in the LGG group. We believe that these non-statistically significant differences are due to chance and/or to the higher number of smokers in the LGG group.
We can also speculate however that any form of bacterium can become an antigenic stimulus and consequently be the cause of the increased recurrences and severe recurrent lesions found in the group treated with the probiotic.
The discouragingly negative result of this first well controlled study on a probiotic is in sharp contrast with previous positive studies. How can we reconcile these diverging data?
Firstly, the positive results with probiotics in other studies are open to question: in some there were be too few patients,16,17 in another too short a period of observation,14 and in others still a concomitant use of other drugs active in Crohn's disease.16,17 In the VSL# 3 trial on prevention of recurrence of Crohn's disease after surgery,18 the antibiotic administered for three months could have reduced the recurrence rate at one year, as in fact occurred in the metronidazole trial.30
The different type of lesion and possibly also the difference in pathogenesis could be another explanation for the discrepant responses to probiotic therapy in ulcerative colitis and Crohn's disease.
Furthermore, differences between our negative result and other positive studies could be explained by the different types of probiotics used. Direct comparison between different probiotic strains has not been performed and consequently it is not advisable to extrapolate the results from one strain to other stains. We have learned from laboratory studies that the efficacy of one probiotic may not be the same in all patients, or even in the same patient at different stages of disease.39 In a recent study, development of colitis in interleukin 10 deficient mice was attenuated by Lactobacillus plantarum but not by LGG.40
Given that the resident human gut flora is composed of approximately 400–500 bacterial strains, one strain alone might not exert a competitive action in the human intestine. In addition, VSL# 3, which was efficaciously employed in the study on pouchitis, was given at a dosage of 1800 billion of eight bacterial strains while we administered 12 billion cfu of only one strain (LGG).
Could a higher bacterial concentration and a mixture of various strains enhance the competitive interaction with commensal and pathogenic flora? The answer to this question can only come from investigations into the mechanism by which probiotics may prevent inflammation, followed by more randomised trials.
Acknowledgments
The authors thank Claudio Fiocchi for helpful discussion, and Dicofarm spa-Rome-Italy for providing Lactobacillus GG and placebo, and for supporting the trial.
Abbreviations
CDAI, Crohn's disease activity index
LGG, Lactobacillus GG
cfu, colony forming units
REFERENCES
- 1.Sartor RB. Role of intestinal microflora in initiation and perpetuation of inflammatory bowel disease. Can J Gastroenterol 1990;4:271–7. [Google Scholar]
- 2.Sartor RB. Enteric microflora in IBD: pathogens or commensals? Inflamm Bowel Dis 1997;3:23–35. [PubMed] [Google Scholar]
- 3.Colombel JF, Lemann M, Cassagnou M, et al. A controlled trial comparing ciprofloxacin with mesalazine for the treatment of active Crohn's disease. Am J Gastroenterol 1999;94:674–8. [DOI] [PubMed] [Google Scholar]
- 4.Prantera C, Scribano ML, Berto E, et al. Antibiotic use in Crohn's disease. Why and how? Bio Drugs 1997;8:293–306. [DOI] [PubMed] [Google Scholar]
- 5.Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology 1998;115:182–205. [DOI] [PubMed] [Google Scholar]
- 6.Prantera C, Zannoni F, Scribano ML, et al. An antibiotic regimen for the treatment of active Crohn's disease: a randomized, controlled clinical trial of metronidazole plus ciprofloxacin. Am J Gastroenterol 1996;91:328–33. [PubMed] [Google Scholar]
- 7.Prantera C, Berto E, Scribano ML, et al. Use of antibiotics in the treatment of active Crohn's disease: experience with metronidazole and ciprofloxacin. Ital J Gastroenterol Hepatol 1998;30:602–6. [PubMed] [Google Scholar]
- 8.D' Haens G, Rutgeerts P. Postoperative recurrence of Crohn's disease: pathophysiology and prevention. Inflamm Bowel Dis 1999;5:295–303. [DOI] [PubMed] [Google Scholar]
- 9.Shanahan F. Probiotics and inflammatory bowel disease: is there a scientific rationale? Inflamm Bowel Dis 2000;6:107–15. [DOI] [PubMed] [Google Scholar]
- 10.Surawicz CM, Elmer GW, Speelman P, et al. Prevention of antibiotic-associated diarrhea by Saccharomyces boulardii: a prospective study. Gastroenterology 1989;96:981–8. [DOI] [PubMed] [Google Scholar]
- 11.Gorbach SL, Chang TW, Goldin B. Successful treatment of relapsing Clostridium difficile colitis with Lactobacillus GG. Lancet 1987;2:1519. [DOI] [PubMed] [Google Scholar]
- 12.Hilton E, Kolakowski P, Smith M, et al. Efficacy of Lactobacillus GG as a diarrheal preventative in travelers diarrhea. J Travel Med 1996;4:41–3. [DOI] [PubMed] [Google Scholar]
- 13.Saavedra JM, Bauman NA, Oung I, et al. Feeding of Bifidobacterium bifidum and Streptococcus thermophilus to infants in hospital for prevention of diarrhoea and shedding of rotavirus. Lancet 1994;344:1046–9. [DOI] [PubMed] [Google Scholar]
- 14.Kruis W, Schutz E, Fric P, et al. Double-blind comparison of an oral Escherichia coli preparation and mesalazine in maintaining remission of ulcerative colitis. Aliment Pharmacol Ther 1997;11:853–8. [DOI] [PubMed] [Google Scholar]
- 15.Rembacken BJ, Snelling AM, Hawkey PM, et al. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomised trial. Lancet 1999;354:635–9. [DOI] [PubMed] [Google Scholar]
- 16.Malchow HA. Crohn's disease and Escherichia coli. A new approach in therapy to maintain remission of colonic Crohn's disease? J Clin Gastroenterol 1997;25:653–8. [DOI] [PubMed] [Google Scholar]
- 17.Guslandi M, Mezzi G, Sorghi M, et al. Saccharomyces boulardii in maintenance treatment of Crohn's disease. Dig Dis Sci 2000;45:1462–4. [DOI] [PubMed] [Google Scholar]
- 18.Campieri M, Rizzello F, Venturi A, et al. Combination of antibiotic and probiotic treatment is efficacious in prophylaxis of post-operative recurrence of Crohn's disease: a randomized controlled study vs mesalamine. Gastroenterology 2000;118:A781. [Google Scholar]
- 19.Gionchetti P, Rizzello F, Venturi A, et al. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology 2000;119:305–9. [DOI] [PubMed] [Google Scholar]
- 20.Sachar DB, Andrews HA, Farmer RG, et al. Proposed classification of patient subgroups in Crohn's disease. Gastroenterol Int 1992;5:141–54. [Google Scholar]
- 21.Rutgeerts P, Geboes K, Vantrappen G, et al. Predictability of the postoperative course of Crohn's disease. Gastroenterology 1990;99:956–63. [DOI] [PubMed] [Google Scholar]
- 22.Olaison G, Smedh K, Sjodahl R. Natural course of Crohn's disease after ileocolic resection: endoscopically visualised ileal ulcers preceeding symptoms. Gut 1992;33:331–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldin BR, Gorbach SL, Saxelin M, et al. Survival of Lactobacillus species (strain GG) in human gastrointestinal tract. Dig Dis Sci 1992; 37:121–8. [DOI] [PubMed] [Google Scholar]
- 24.Malin M, Suomalainen H, Saxelin M, et al. Promotion of IgA immune response in patients with Crohn's disease by oral bacteriotherapy with Lactobacillus GG. Ann Nutr Metab 1996;40:137–45. [DOI] [PubMed] [Google Scholar]
- 25.Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol 1989;24:2–6. [DOI] [PubMed] [Google Scholar]
- 26.Best WR, Becktel JM, Singleton JW, et al. Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology 1976;70:439–44. [PubMed] [Google Scholar]
- 27.Prantera C, Scribano ML. Crohn's disease: the case for bacteria. Ital J Gastroenterol Hepatol 1999;31:244–6. [PubMed] [Google Scholar]
- 28.Rutgeerts P, Geboes K, Peeters M, et al. Effect of faecal stream diversion on recurrence of Crohn's disease in the neoterminal ileum. Lancet 1991;338:771–4. [DOI] [PubMed] [Google Scholar]
- 29.D' Haens GR, Geboes K, Peeters M, et al. Early lesions of recurrent Crohn's disease caused by infusion of intestinal content in excluded ileum. Gastroenterology 1998;114:262–7. [DOI] [PubMed] [Google Scholar]
- 30.Rutgeerts P, Hiele M, Geboes K, et al. Controlled trial of metronidazole treatment for prevention of Crohn's recurrence after ileal resection. Gastroenterology 1995;108:1617–21. [DOI] [PubMed] [Google Scholar]
- 31.Madsen KL, Doyle JS, Jewell LD, et al. Lactobacillus species prevents colitis in interleukin 10 gene-deficient mice. Gastroenterology 1999;116:1107–14. [DOI] [PubMed] [Google Scholar]
- 32.Alander M, Korpela R, Saxelin M, et al. Recovery of Lactobacillus rhamnosus GG from human colonic biopsies. Preliminary results. Nutr Today 1996;31:47–8S. [DOI] [PubMed] [Google Scholar]
- 33.Saxelin M. Colonization of the human gastrointestinal tract by probiotic bacteria. Nutr Today 1996;31:5–8S. [Google Scholar]
- 34.Schultz M, Linde HJ, Staudner H, et al. Oral administration of Lactobacillus GG (L.GG) induces an antiinflammatory, TH-2 mediated systemic immune response towards intestinal organisms. Gastroenterology 1998;114:A781. [Google Scholar]
- 35.Horing E, Gopfert D, Schroter G, et al. Frequency and spectrum of microorganisms isolated from biopsy specimens in chronic colitis. Endoscopy 1991;23:325–7. [DOI] [PubMed] [Google Scholar]
- 36.Hellers G, Cortot A, Jewell D, et al. Oral budesonide for prevention of postsurgical recurrence in Crohn's disease. Gastroenterology 1999;116:294–300. [DOI] [PubMed] [Google Scholar]
- 37.Mekhjian HS, Switz DM, Watts HD, et al. National Cooperative Crohn's Disease Study: factors determining recurrence of Crohn's disease after surgery. Gastroenterology 1979;77:907–13. [PubMed] [Google Scholar]
- 38.Lautenbach E, Berlin JA, Lichtenstein GR. Risk factors for early postoperative recurrence of Crohn's disease. Gastroenterology 1998;115:259–67. [DOI] [PubMed] [Google Scholar]
- 39.Shanahan F. Inflammatory bowel disease: immunodiagnostics, immunotherapeutics, and ecotherapeutics. Gastroenterology 2001; 120:622–35. [DOI] [PubMed] [Google Scholar]
- 40.Veltkamp C, Tonkonogy SL, Schultz M, et al. Lactobacillus plantarum is superior to Lactobacillus GG in preventing colitis in IL-10 deficient mice. Gastroenterology 1999;11:A838. [Google Scholar]