Abstract
Familial adenomatous polyposis (FAP) is characterised by the development of numerous colorectal adenomatous polyps. Other extracolonic benign or malignant lesions have been reported previously in association with FAP but precancerous lesions in the pancreas have never been described. We report the first case of intraductal papillary and mucinous pancreatic tumour (IPMT) in a patient with FAP. A 48 year old man with a well documented past history of FAP was admitted for epigastric pain, weight loss, and new onset diabetes mellitus. Spiral computed tomography scan revealed a large tumour in the pancreatic head with upstream main pancreatic duct dilatation. Endoscopic ultrasonography confirmed these data. Mucous secretion was seen at duodenoscopy and a lesion in the main pancreatic duct was confirmed by retrograde pancreatography. The patient underwent a pancreaticoduodenectomy for suspected IPMT. Histological examination of the resected specimen confirmed an IPMT with in situ carcinoma. Twelve months after resection, the patient remained free of tumour relapse. Genetic analysis showed loss of the wild allele of the adenomatous polyposis coli gene in IPMT, causing inactivation of both alleles and demonstrating that IPMT was not incidental in this patient. IPMT should be included in the extracolonic localisation of FAP.
Keywords: adenomatous polyposis coli, familial adenomatous polyposis, intraductal papillary and mucinous tumour
Familial adenomatous polyposis (FAP) is an autosomal dominant disease caused by germline mutation of the adenomatous polyposis coli (APC) gene located on chromosome 5q21. This gene encodes a large protein with multiple functional domains that mediates binding to many intracellular proteins, including β-catenine, γ-catenine, glycogen synthase kinase-3β, axin, and tubulin.1 Alteration in the APC protein results in disruption of complex formation and increases cytoplasmic levels of β-catenine which upregulate T cell factor responsive genes critical for proliferation and transformation of epithelial cells.1 APC mutations occur early during colonic carcinogenesis. There is a correlation between genotype and phenotype, characterised by a more severe form when the mutation is at the 3` extremity of the gene.2 FAP is characterised by the development of hundreds to thousands of colorectal adenomas, which develop when the second copy of the APC gene is lost or mutated, causing APC gene inactivation. Patients with FAP face inevitable colorectal cancer, usually before 40 years of age, if prophylactic colectomy is not performed. Early screening of FAP and prophylactic colectomy explain the decrease in mortality due to colorectal cancer in these patients. More and more other extracolonic lesions have thus been reported, including benign (osteomas, odontomas, epidermoid cysts, desmoid tumours, congenital hypertrophy of the retinal pigment epithelium, fundic gland polyposis) and malignant (adenocarcinoma of the duodenum, thyroid, pancreas, biliary tract, stomach, and tumours of the liver or central nervous system) lesions.3–6
Precancerous lesions in the pancreas have never been described in association with FAP. Intraductal papillary and mucinous tumours (IPMT) of the pancreas are rare. They are considered as pancreatic adenomas leading to adenocarcinomas through the dysplasia-carcinoma sequence.7 We report here the first case of pancreatic IPMT with both APC alleles inactivated in a patient with FAP.
CASE REPORT
A 48 year old man was admitted in March 2000 for investigation of recent epigastric pain with dorsal irradiation associated with weight loss of 17 kg and new onset diabetes mellitus. He had previously undergone a colectomy with ileorectal anastomosis in 1974 for FAP (six cases described in his family) and an abdominoperineal resection with preoperative radiotherapy in 1985 for rectal adenocarcinoma (stage B1 in the Astler-Coller classification).
Clinical examination was normal as were serum pancreatic enzymes and biochemical hepatic tests. At upper gastrointestinal endoscopy, mucous protrusion through the major papilla was observed, as well as a 20 mm ampullary tumour, with no other duodenal polyp. Histological examination of ampullar biopsies demonstrated a papillary adenoma with low grade dysplasia. Abdominal ultrasonography showed dilatation of the main pancreatic duct above a tumour located in the pancreatic head. Spiral computed tomography scan found pancreatic calcifications in the body and tail and confirmed a heterogeneous mass in the head of the pancreas measuring 60×35 mm, with proximal main pancreatic duct dilatation, a 20 mm ampullary lesion, and a mass (50×35 mm) in the left adrenal gland (fig 1 ▶). At endoscopic ultrasonography, the pancreatic tumour was seen to develop in the cephalic part of the main pancreatic duct, which was markedly enlarged, both distal and proximal. The common bile duct was partially stenosed by the lesion. The surrounding vessels seemed to be respected and there were no lymph nodes. Finally, lateral duodenoscopy with endoscopic retrograde cholangiopancreatography (ERCP) was performed and showed: (a) mucous spreading through the major papilla, (b) a polypoid lesion of the major papilla, (c) a large intraductal friable mass of the pancreatic head extending through the papilla after sphincterotomy, and (d) stenosis of the common bile duct (fig 2A, B ▶). An adenomatous proliferation with high grade dysplasia was demonstrated by transpapillary biopsies. Pancreatic magnetic resonance imaging confirmed these data (fig 3 ▶). Serum carcinoembryonic antigen and carbohydrate antigen 19.9 values were normal.
Figure 1.
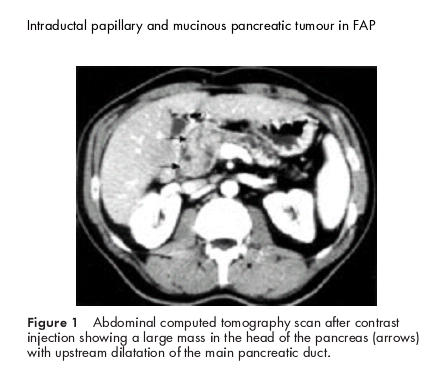
Abdominal computed tomography scan after contrast injection showing a large mass in the head of the pancreas (arrows) with upstream dilatation of the main pancreatic duct.
Figure 2.
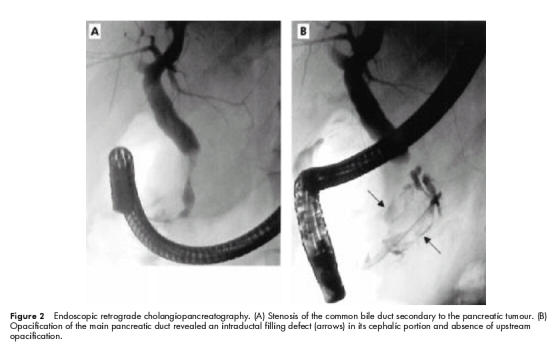
Endoscopic retrograde cholangiopancreatography. (A) Stenosis of the common bile duct secondary to the pancreatic tumour. (B) Opacification of the main pancreatic duct revealed an intraductal filling defect (arrows) in its cephalic portion and absence of upstream opacification.
Figure 3.
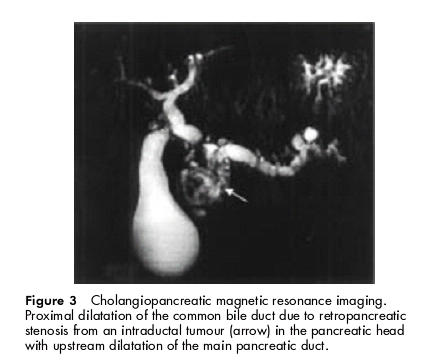
Cholangiopancreatic magnetic resonance imaging. Proximal dilatation of the common bile duct due to retropancreatic stenosis from an intraductal tumour (arrow) in the pancreatic head with upstream dilatation of the main pancreatic duct.
The diagnosis of IPMT localised in the pancreatic head with possible malignant transformation (suggested by the large intraductal vegetation) was suspected. Pancreatic metastasis from colorectal cancer was reasonably excluded as: (1) rectal adenocarcinoma was a stage B1 Astler-Coller, (2) there was a long delay between the two events (15 years), (3) intraductal location of the pancreatic tumour, (4) absence of other metastatic lesions, and (5) presence of mucous, pathognomonic of IPMT.
As no vascular or lymph node involvement was found at the preoperative investigations, the patient underwent a duodenopancreatectomy with pancreaticogastric anastomosis. No postoperative complications occurred. Complete histological examination of the resected specimen demonstrated: (a) IPMT with severe atypia corresponding to high grade dysplasia/carcinoma in situ in the cephalic part of the main pancreatic duct (figs 4, 5A, B ▶ ▶), (b) absence of tumour and calcified chronic pancreatitis at the section cut, (c) an ampullary tumour with low grade dysplasia, which was independent of the IPMT (these entities were separated by 10 mm of normal main pancreatic duct), and (d) a microscopic duodenal adenoma with low grade dysplasia. The adrenal biopsy showed a benign adenoma. Twelve months after surgery, the patient was asymptomatic and there was no evidence of tumour relapse.
Figure 4.
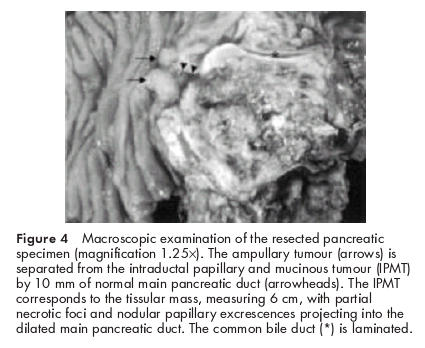
Macroscopic examination of the resected pancreatic specimen (magnification 1.25×). The ampullary tumour (arrows) is separated from the intraductal papillary and mucinous tumour (IPMT) by 10 mm of normal main pancreatic duct (arrowheads). The IPMT corresponds to the tissular mass, measuring 6 cm, with partial necrotic foci and nodular papillary excrescences projecting into the dilated main pancreatic duct. The common bile duct (*) is laminated.
Figure 5.
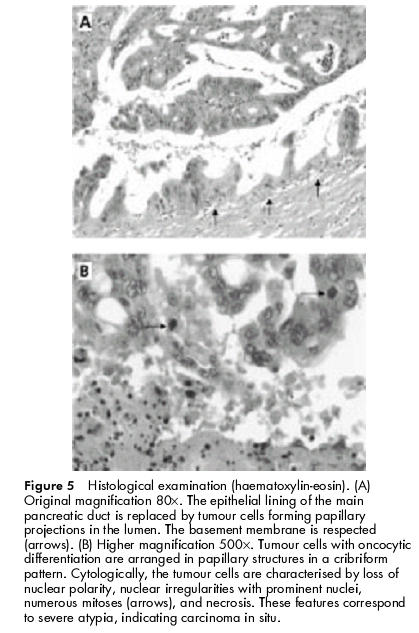
Histological examination (haematoxylin-eosin). (A) Original magnification 80×. The epithelial lining of the main pancreatic duct is replaced by tumour cells forming papillary projections in the lumen. The basement membrane is respected (arrows). (B) Higher magnification 500×. Tumour cells with oncocytic differentiation are arranged in papillary structures in a cribriform pattern. Cytologically, the tumour cells are characterised by loss of nuclear polarity, nuclear irregularities with prominent nuclei, numerous mitoses (arrows), and necrosis. These features correspond to severe atypia, indicating carcinoma in situ.
Genetic analysis showed that the germline mutation was a 3921del5 mutation in exon 15 of the APC gene. Loss of heterozygosity was evidenced in the pancreatic tumour, causing inactivation of both alleles in the APC gene.
DISCUSSION
Extracolonic malignant tumours have previously been reported in patients with FAP. Malignant transformation of duodenal adenomas is the best known. The frequency of duodenal adenomas is approximately 90% with a malignant potential between 1% and 5%.8 The lifetime risk of other extracolonic tumours has been estimated as follows: pancreatic adenocarcinoma (2%), thyroid cancer (2%), gastric adenocarcinoma (0.5%), and hepatoblastoma in children less than five years of age (1.6%).9 In a cohort study of 1391 patients with FAP, the relative risks of pancreatic and thyroid cancer were, respectively, 4.5 (95% confidence interval (CI) 1.2–11.4) and 7.6 (95% CI 2.5–17.7).10 Two cases of gastric adenocarcinoma occurring in patients with fundic gland polyposis have also been reported.11,12 Finally, one case each of pancreatic cystic and pseudopapillary tumour,13 pancreatic glucagonoma,14 and duodenal endocrine tumour15 have been described.
This is the first observation of an association between FAP and IPMT. IPMT is a rare type of pancreatic tumour, first described by Ohhashi and colleagues.16 This disease is defined by adenomatous proliferation of the epithelium lining of the pancreatic ducts, with excessive mucous production. All gradations from benign appearing epithelium to in situ or invasive carcinoma may be encountered. Because of its well known malignant potential, surgical resection is warranted whenever possible.
Carcinogenesis of IPMT and colorectal adenomatous polyps are strikingly similar: (1) epithelial hyperplasia precedes the development of adenomas with increasing grades of dysplasia and then invasive cancer and metastases, and (2) common genetic alterations including p53 and K-ras mutations (with gradual increase from normal pancreatic duct to adenoma and adenocarcinoma in IPMT).17,18
APC somatic mutations have previously been reported in extracolonic tumours associated with FAP. Toyooka and colleagues19 found a somatic mutation of the second copy of the APC gene in 69% of duodenal, periampullary, or gastric tumours in 21 patients with FAP. In addition, somatic mutations in the APC gene have been described in 30–47% of sporadic pancreatic cancers.20–23 To our knowledge, such mutations have never been reported in IPMT. In the case described here, we showed loss of heterozygosity of the APC gene in pancreatic IPMT. This result implicates the APC gene in the development of this tumour.
Screening for duodenal cancer (upper gastrointestinal endoscopy, including side viewing examinations, every 1–3 years, starting from age 20–25 years) is warranted in patients with FAP because of a well documented increased risk.9 In contrast, screening for pancreatic tumours seems unwarranted as pancreatic tumours are rare (2%) and often difficult to diagnose with non-invasive techniques. However, IPMT diagnosis should be considered in cases of acute pancreatitis, diabetes, or steatorrhoea in patients with FAP.
In conclusion, IMPT should be added to the list of extracolonic premalignant tumours that can occur in patients with FAP. The presence of a mutation in the second allele of the APC gene in IPMT in our case argues in favour of a common carcinogenic process in both IPMT and colic adenomas.
Abbreviations
APC, adenomatous polyposis coli
ERCP, endoscopic retrograde cholangiopancreatography
FAP, familial adenomatous polyposis
IPMT, intraductal papillary and mucinous tumour
REFERENCES
- 1.Chung DC. The genetic basis of colorectal cancer: insights into critical pathways of tumorigenesis. Gastroenterology 2000;119:854–65. [DOI] [PubMed] [Google Scholar]
- 2.Giardiello FM, Petersen GM, Piantadosi S, et al. APC gene mutations and extraintestinal phenotype of familial adenomatous polyposis. Gut 1997;40:521–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mao C, Huang Y, Howard JM. Carcinoma of the ampulla of Vater and mesenteric fibromatosis (desmoid tumor) associated with Gardner's syndrome: problems in management. Pancreas 1995;10:239–45. [DOI] [PubMed] [Google Scholar]
- 4.DiMagno EP. Pancreatic cancer: clinical presentation, pitfalls and early clues. Ann Oncol 1999;10(suppl 4):140–2. [PubMed] [Google Scholar]
- 5.Spigelman AD, Farmer KC, James M, et al. Tumours of the liver, bile ducts, pancreas and duodenum in a single patient with familial adenomatous polyposis. Br J Surg 1991;78:979–80. [DOI] [PubMed] [Google Scholar]
- 6.Kartheuser A, Walon C, West S, et al. Familial adenomatous polyposis associated with multiple adrenal adenomas in a patient with a rare 3` APC mutation. J Med Genet 1999;36:65–7. [PMC free article] [PubMed] [Google Scholar]
- 7.Loftus EV Jr, Olivares-Pakzad BA, Batts KP, et al. Intraductal papillary-mucinous tumors of the pancreas: clinicopathologic features, outcome, and nomenclature. Gastroenterology 1996;110:1909–18. [DOI] [PubMed] [Google Scholar]
- 8.Jagelman DG, DeCosse JJ, Bussey HJ. Upper gastrointestinal cancer in familial adenomatous polyposis. Lancet 1988;1:1149–51. [DOI] [PubMed] [Google Scholar]
- 9.Burt RW. Colon cancer screening. Gastroenterology 2000;119:837–53. [DOI] [PubMed] [Google Scholar]
- 10.Giardiello FM, Offerhaus GJ, Lee DH, et al. Increased risk of thyroid and pancreatic carcinoma in familial adenomatous polyposis. Gut 1993;34:1394–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hofgartner WT, Thorp M, Ramus MW, et al. Gastric adenocarcinoma associated with fundic gland polyps in a patient with attenuated familial adenomatous polyposis. Am J Gastroenterol 1999;94:2275–81. [DOI] [PubMed] [Google Scholar]
- 12.Zwick A, Munir M, Ryan CK, et al. Gastric adenocarcinoma and dysplasia in fundic gland polyps of a patient with attenuated adenomatous polyposis coli. Gastroenterology 1997;113:659–63. [DOI] [PubMed] [Google Scholar]
- 13.Le Borgne J, Bouvier S, Fiche M, et al. Cystic and papillary tumor of the pancreas: diagnostic and developmental uncertainties. Chirurgie 1997;122:31–4. [PubMed] [Google Scholar]
- 14.Stewart CJ, Imrie CW, Foulis AK. Pancreatic islet cell tumour in a patient with familial adenomatous polyposis. J Clin Pathol 1994;47:860–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.July LV, Northcott KA, Yoshida EM, et al. Coexisting carcinoid tumors in familial adenomatous polyposis-associated upper intestinal adenomas. Am J Gastroenterol 1999;94:1091–4. [DOI] [PubMed] [Google Scholar]
- 16.Ohhashi K, Murakami F, Maruyama M. Four cases of mucous secreting pancreatic cancer. Prog Dig Endosc 1982;20:348–51. [Google Scholar]
- 17.Islam HK, Fujioka Y, Tomidokoro T, et al. Immunohistochemical analysis of expression of molecular biologic factors in intraductal papillary-mucinous tumors of pancreas. Diagnostic and biologic significance. Hepatogastroenterology 1999;46:2599–605. [PubMed] [Google Scholar]
- 18.Z'graggen K, Rivera JA, Compton CC, et al. Prevalence of activating K-ras mutations in the evolutionary stages of neoplasia in intraductal papillary mucinous tumors of the pancreas. Ann Surg 1997;226:491–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toyooka M, Konishi M, Kikuchi-Yanoshita R, et al. Somatic mutations of the adenomatous polyposis coli gene in gastroduodenal tumors from patients with familial adenomatous polyposis. Cancer Res 1995;55:3165–70. [PubMed] [Google Scholar]
- 20.Horii A, Nakatsuru S, Miyoshi Y, et al. Frequent somatic mutations of the APC gene in human pancreatic cancer. Cancer Res 1992;52:6696–8. [PubMed] [Google Scholar]
- 21.Neuman WL, Wasylyshyn ML, Jacoby R, et al. Evidence for a common molecular pathogenesis in colorectal, gastric, and pancreatic cancer. Genes Chromosomes Cancer 1991;3:468–73. [DOI] [PubMed] [Google Scholar]
- 22.Achille A, Baron A, Zamboni G, et al. Chromosome 5 allelic losses are early events in tumours of the papilla of Vater and occur at sites similar to those of gastric cancer. Br J Cancer 1998;78:1653–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imai Y, Oda H, Tsurutani N, et al. Frequent somatic mutations of the APC and p53 genes in sporadic ampullary carcinomas. Jpn J Cancer Res 1997;88:846–54. [DOI] [PMC free article] [PubMed] [Google Scholar]


