Abstract
Background: G protein deficient (Gαi2−/−) mice spontaneously develop an inflammatory bowel disease (IBD) closely resembling ulcerative colitis. Previous studies have demonstrated that gut T cells are hyperreactive to the endogenous microflora in most IBD models.
Aims: The aim of this study was to analyse Peyer's patches (PP), the inductive sites for gut mucosal immune responses.
Subjects and methods: Gαi2−/− mice, an animal model for IBD, were analysed using immunological methods with regard to phenotype and function.
Results: We found significantly decreased numbers of PP in Gαi2−/− mice. Even before the onset of colitis, Gαi2 deficient animals exhibited diminished size of PP, as judged by histology. This involution of PP was associated with strongly increased levels of apoptotic lymphocytes, associated with decreased levels of antiapoptotic intracellular protein Bcl-2. PP T lymphocytes showed highly elevated production of interferon γ in response to the enteric flora compared with PP T cells from wild-type mice, which produced predominantly interleukin 10.
Conclusions: Thus even before the onset of colitis, the PP in Gαi2 deficient mice is a Th1 dominated milieu associated with downregulated levels of Bcl-2, resulting in increased apoptosis of lymphocytes leading to regression of PP. We speculate that this Th1 dominated microenvironment in the inductive site for mucosal immune responses contributes to the development of colitis in Gαi2 deficient mice.
Keywords: inflammatory bowel disease, apoptosis, Peyer's patch, mucosa
Inflammatory bowel disease (IBD) is the common name for ulcerative colitis and Crohn's disease, both chronic bowel diseases of unknown aetiology. A targeted mutation in the gene for the G protein αi2 subunit in mice causes an ulcerative colitis-like disease.1 Intestinal inflammation starts at about 15–25 weeks of age but Gαi2−/− mice have an increased frequency of intestinal CD4+ T cells with a memory phenotype and increased expression of mucosal homing receptors, as well as increased spontaneous production of proinflammatory Th1 cytokines in the intestinal mucosa even before onset of colitis. In addition, they have intestinal flora specific antibodies in large intestinal secretions before the onset of colitis, collectively suggesting that abnormalities in the intestinal immune system are likely to play an important role in the induction of colitis in Gαi2−/− mice.2,3
Peyer's patches (PP) are the primary site for antigen encounter in the gut and specialised sites for initiation of mucosal immune responses. Luminal antigens enter PP via M cells and are transported to underlying B lymphocytes and APCs, which present the antigen to T cells. Effector B and T cells primed in PP then home to mucosal surfaces such as the lamina propria and the epithelium where B cells differentiate into IgA producing plasma cells.4,5
Many studies, both in animal models and patients, now point towards one of the major luminal constituents, the bacterial flora, as a key player in the induction of IBD. Thus all animal models of IBD tested so far remain healthy when kept under germ free conditions6–8 and reconstitution of intestinal bacteria into germ free animals restores intestinal inflammation.9 In patients, antibiotic therapy has been reported to ameliorate disease10 and extend remission in distal Crohn's disease,11 and the disease does not recur after surgery if the faecal stream is diverted.12,13 Duchmann and colleagues14 have shown that normal individuals are tolerant towards their own bacterial flora but respond to the flora of others, and have suggested that IBD may occur when this tolerance to antigens of its own flora is broken.
Several studies have also addressed the role of apoptosis of intestinal lymphocytes in IBD but somewhat contradictory results have been obtained. Thus colonic T cells from patients with ulcerative colitis and Crohn's disease have been shown to be less sensitive to Fas mediated apoptosis15–17 whereas other studies have reported that Fas induced apoptosis participates in damage in ulcerative colitis.18,19 In accordance with the latter, augmented levels of apoptotic cells in the lamina propria was demonstrated in SCID mice with IBD due to transplantation of syngeneic CD4+ T cells.20 Additional studies have demonstrated Fas mediated apoptosis in colonic epithelial cells and mucosal T cells as a possible role in driving the intestinal inflammation in ulcerative colitis patients.18,21 However, to date no study has focused on the function of PP lymphocytes in IBD.
In this study, we demonstrate regression of PP in Gαi2−/− mice associated with downregulated levels of Bcl-2 and increased apoptosis, potentially due to highly increased T cell production of interferon γ (IFN-γ) in response to the intestinal enteric flora.
MATERIALS AND METHODS
Mice
Gαi2 deficient (Gαi2−/−)1 mice were bred and kept at the Department of Clinical Immunology, Göteborg University. Homozygous Gαi2 mutant males were bred with heterozygous females, and the offspring were genotyped by polymerase chain reaction analysis: 100% of Gαi2 deficient mice develop colitis and have to be sacrificed due to severe disease between 15 and 25 weeks of age. Mice were studied before the onset of colitis and compared with age and sex matched 129Sv×C57BL/6 wild-type mice cross bred and subsequently interbred for the same number of generations as the Gαi2 deficient mice.2 The animal facility is kept pathogen free using microisolator cages and sterile workbenches, and mice are routinely monitored by health screening according to FELASA recommendations.
Histological and morphometric assessment
Swiss rolls of the small and large intestine were made from four wild-type, five Gαi2−/− mice with no clinical symptoms of colitis—that is, no increase in mononuclear cells or alteration in crypt architecture or other signs of colitis—and five Gαi2−/− mice with established colitis demonstrating infiltration of mononuclear cells and polymorphonuclear lymphocytes into the lamina propia, crypt abscesses, disturbed glandular architecture, and epithelial dysplasia. Tissue was fixed in 4% paraformaldehyde, embedded in paraffin, sectioned, and stained with haematoxylin-eosin, and one 5 μm section was made from each of the colon specimens for classification into a colitis or non-colitis group based on the criteria described above. From each of the swiss rolls of the small intestine, five sections (5 μm thick) of the entire length of the intestine were made 100 μm apart. The number of lymphoid follicles was counted in each section and the results are given as the mean number of follicles per cross sectional tissue area of the entire small intestine. Microscopic images of the lymphoid follicles were captured onto a MacIntosh G3 computer and the cross sectional area of the follicles was assessed using the public domain program NIH Image 1.61 (written by Wayne Rasband at NIH and available from the Internet by anonymous FTP at zippy.nih.gov). Results are given as mean cross sectional follicle area per small intestine (mm2).
Immunohistochemistry
Frozen sections of PP dissected from Gαi2 deficient mice before the onset of colitis and wild-type mice were prepared on microslides using a cryostat (model 1720; Leitz, Wetzlar, Germany) and frozen at −70°C. Slides were fixed in 50% acetone for 30 seconds followed by 100% acetone for five minutes at 4°C and five minutes in room temperature. After washing in phosphate buffered saline (PBS), slides were treated with 20% horse serum in PBS for 15 minutes in a humid chamber. For detection and analysis of germinal centre formation in PP, fixed sections were double labelled for the presence of IgA positive B cells versus germinal centre B cells using the following reagents: goat antimouse IgA conjugated to Texas Red (Southern Biotechnology, Birmingham, Alabama, USA) and biotinylated peanut (Arachis hypogaea) haemagglutinin (PNA) (Sigma, St Louis, Missouri, USA), followed by streptavidin conjugated to fluorescein isothiocyanate (FITC; Southern Biotechnology). The sections were evaluated and photographed using an Axioskop microscope (Zeiss, Cambridge, UK).
Preparation of cells
PP resected from the small intestine were cut into pieces and incubated in Hank's solution containing 1 mg/ml collagenase/dispase (Roche, Palo Alto, California, USA), 250 U/ml DNase (Roche), and 50 μg/ml gentamycin (Sigma) for 20 minutes at 37°C with occasional stirring. PP and mesenteric lymph nodes were washed in 2% fetal calf serum (FCS)/PBS and thereafter passed through nylon net, and further washed twice in 2% FCS/PBS. PP T lymphocytes were purified by passing the cell suspension through mouse T cell enrichment columns (R&D Systems, Minneapolis, Minnesota, USA) as described previously22 or by using Dynabeads pan B (CD220+) (Dynal AS, Oslo, Norway) for negative selection of the T cells, according to the manufacturer's protocol. The resulting T cell population was at least 94% pure, as estimated by FACS analysis. Peritoneal macrophages (PM) were isolated from wild-type mice injected with 1 ml thioglycollate (BD, San Jose, California, USA) 3–4 days before sacrifice. PM were collected by flushing the peritoneal cavity with PBS. After washing the PM twice with PBS, cells were irradiated at 2200 rad to inhibit cell proliferation and delete contaminating lymphocytes.
FACS analysis
Freshly isolated cells 0.5–5×105/100 μl in 1% FCS/PBS were incubated with FITC or phycoerythrin (PE) conjugated monoclonal antibodies (PharMingen, San Diego, California, USA) for 30 minutes at 4°C, and thereafter washed thoroughly in 1% FCS/PBS. Lymphocytes analysed for intracellular expression of Bcl-2 were incubated with specific hamster anti-Bcl-2 FITC conjugated antibodies (PharMingen) and anti-CD19 or anti-CD3 PE conjugated antibodies (PharMingen). For Bcl-2 detection, cells were incubated in a buffer containing 0.1% saponin (Sigma). Lymphocytes were analysed for apoptosis using the In Situ Cell Death Detection Kit/TUNEL technique (Boehringer Mannheim, Indianapolis, Indiana, USA). For these investigations, cells were fixed in 4% paraformaldehyde for 30 minutes at room temperature, washed, and permeabilised (0.1% Triton-X 100) for two minutes on ice. After washing, cells were colabelled with anti-CD19 or anti-CD3-PE conjugated antibodies, incubated with the TUNEL reaction mixture for one hour at 37°C, and analysed for the level of FITC-dUTP expression in CD19 and CD3 gated cells, respectively. Lymphocytes were also analysed for apoptosis/necrosis using TACS Annexin V-FITC Apoptosis Detection Kit (R&D Systems). Cells were incubated in a buffer containing 1% Annexin V-FITC/10% propidium iodide for 15 minutes in the dark and thereafter diluted in binding buffer. Analysis was performed on 10 000 live lymphocytes per sample as defined by forward and side scatter on a FACScan (BD). Data were analysed using WinMDI 26 software.
Preparation of enteric flora antigens
The caecal and colonic contents from wild-type and Gαi2 deficient mice before the onset of colitis were used as sources of intestinal flora. The intestines were opened lengthwise and placed in PBS/0.05% Tween 20. After careful vortexing, insoluble material was removed. The remaining bacterial suspension was centrifuged twice for 10 minutes at 600 g, and the resulting supernatant was centrifuged at 16 000 g. The pellet was resuspended in PBS, sonicated to disrupt the cells, and the lysate was sterilised by 0.2 μm syringe filter (Sartorius, Göttingen, Germany).
T lymphocyte stimulation
Irradiated PM were seeded at 1×105 cells per well in Iscove's total medium in flat bottomed microtitre plates and pulsed for 24 hours with 200 μg/ml enteric flora or keyhole limpet haemocyanin (Calbiochem, San Diego, California, USA). Following removal of unprocessed antigen by careful washing of the plates with PBS, 2×105 T lymphocytes were added per well in a total volume of 200 μl.23 Cells were cultured for 48 hours, after which supernatants were collected and stored at −70°C.
Measurement of cytokine production
Interleukin (IL)-10 and IFN-γ levels were determined by PharMingen OptEIA Set cytokine kits (PharMingen), according to the manufacturer's recommendations.
Statistical analysis
The Mann-Whitney U test for unmatched data was used for analysis of significance. p values <0.05 were regarded as significant.
RESULTS
Gαi2 deficient mice have reduced numbers of PP which decrease in size after the onset of colitis
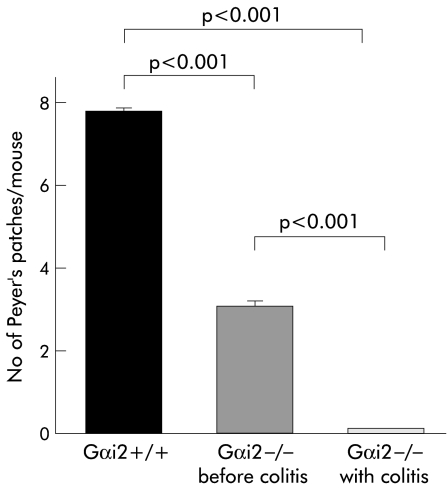
On examination of the number of macroscopically visible PP, Gαi2 deficient mice were found to have significantly fewer PP prior to the onset of colitis compared with wild-type mice. Interestingly, Gαi2 deficient mice with established colitis were found to lack macroscopically visible PP (fig 1 ▶). The number of microscopically visible PP was not different between Gαi2 deficient mice before and after colitis. However, the number of lymphoid follicles was significantly decreased in Gαi2 deficient mice both before as well as after the onset of colitis compared with wild-type mice (fig 2A ▶). In addition, the mean area of the PP was significantly decreased in Gαi2 deficient mice with colitis compared with Gαi2 deficient mice and wild-type mice whereas mean follicle area in Gαi2 deficient mice before colitis was not significantly decreased compared with wild-type mice (fig 2B ▶).
Figure 1.
Gαi2 deficient mice had a significantly decreased number of macroscopically visible Peyer's patches in the small intestine both before and after the onset of colitis compared with wild-type mice. At least 15 mice were examined in each group. Mice were sacrificed at 4–6 weeks of age (wild-type and Gαi2 deficient mice before the onset of colitis) or at 15–25 weeks of age (Gαi2 deficient mice with established colitis).
Figure 2.
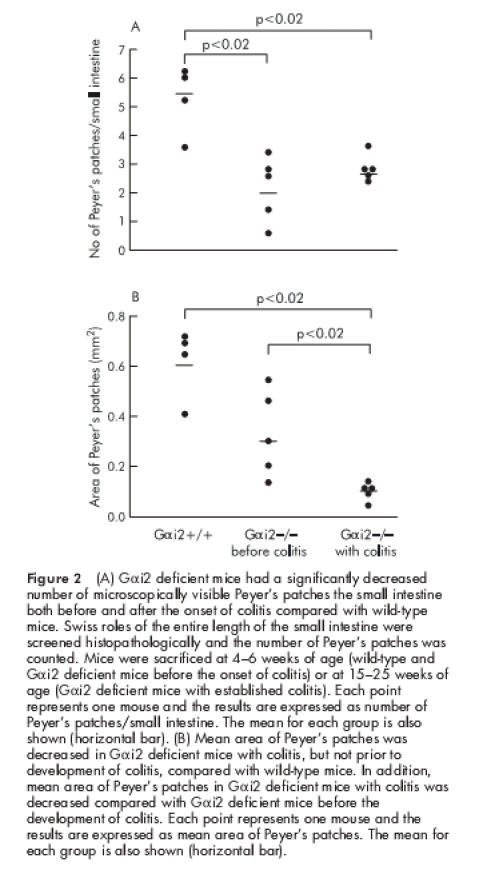
(A) Gαi2 deficient mice had a significantly decreased number of microscopically visible Peyer's patches the small intestine both before and after the onset of colitis compared with wild-type mice. Swiss roles of the entire length of the small intestine were screened histopathologically and the number of Peyer's patches was counted. Mice were sacrificed at 4–6 weeks of age (wild-type and Gαi2 deficient mice before the onset of colitis) or at 15–25 weeks of age (Gαi2 deficient mice with established colitis). Each point represents one mouse and the results are expressed as number of Peyer's patches/small intestine. The mean for each group is also shown (horizontal bar). (B) Mean area of Peyer's patches was decreased in Gαi2 deficient mice with colitis, but not prior to development of colitis, compared with wild-type mice. In addition, mean area of Peyer's patches in Gαi2 deficient mice with colitis was decreased compared with Gαi2 deficient mice before the development of colitis. Each point represents one mouse and the results are expressed as mean area of Peyer's patches. The mean for each group is also shown (horizontal bar).
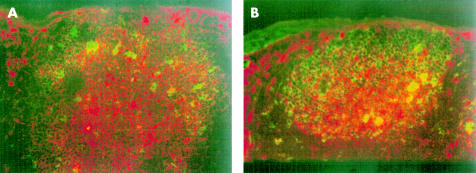
Germinal centre formations in the PP of Gαi2 deficient mice were analysed immunohistochemically. Frozen sections were double labelled with anti-IgA and PNA. PNA+ germinal centre, to which IgA+ cells colocalised, were found both in Gαi2 deficient mice and wild-type mice (fig 3 ▶), suggesting that germinal centre reactions are not impaired in the PP of Gαi2 deficient mice.
Figure 3.
Light level micrographs demonstrating the presence of germinal centres in Peyer's patches. B cells brightly staining for both surface IgA (phycoerythrin/red) and peanut agglutinin (fluorescein isothiocyanate/green) were found in both wild-type mice (A) and Gαi2 deficient mice (B) before the onset of colitis. This indicates the presence of IgA+ B cells (yellow) colocalising to germinal centres in Gαi2 deficient mice before the onset of colitis.
Reduced expression of Bcl-2 and increased apoptosis in T and B lymphocytes in Gαi2 deficient mice
Although the total number of lymphocytes in PP from Gαi2 deficient mice were greatly reduced, neither the relative frequency of CD19+ B or CD3+ T lymphocytes, nor the frequency of CD4+ or CD8+ T cells was significantly altered in PP in Gαi2 deficient mice, supporting the fact that no selective alteration occurred in Gαi2 deficient mice prior to the onset of colitis. In addition, the frequency of PP lymphocytes expressing the mucosal homing receptor integrin β7 and CD62L (L-selectin) was increased compared with wild-type mice, allowing lymphocytes to actively home to the PP (not shown). Thus the diminished size of PP was not due to alterations in these B or T cell subsets in the PP of Gαi2 deficient mice prior to colitis.
Rather, we observed dramatically decreased levels of the intracellular antiapototic protein Bcl-2 in the lymphocyte populations (fig 4 ▶). Downregulated levels of Bcl-2 were most marked in the B cell population but reduced levels were also recorded in the T cell subset. In accordance with this, Gαi2−/− mice exhibited increased frequencies of apoptotic Annexin V+ (not shown) as well as TUNEL+ T and B lymphocytes compared with wild-type mice (fig 4 ▶). Expression of Fas/CD95 was also increased, indicating a high level of activated lymphocytes (fig 4 ▶). Thus the decreased level of Bcl-2 in PP lymphocytes, associated with increased frequencies of apoptotic lymphocytes, is a likely explanation for regression of PP in Gαi2 deficient mice prior to colitis.
Figure 4.
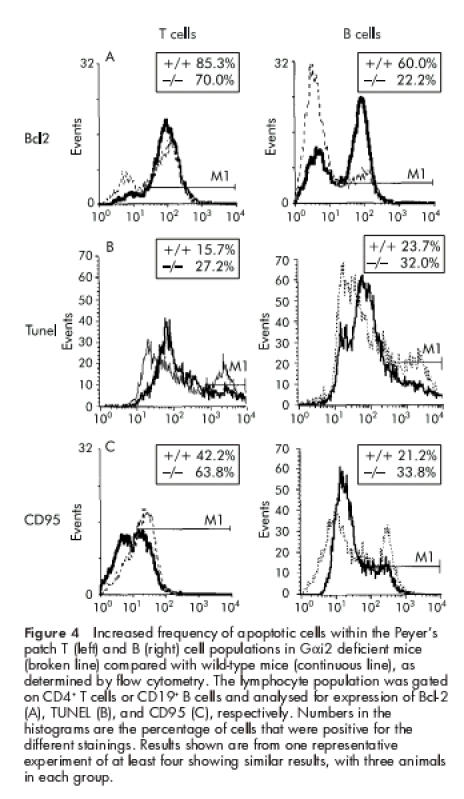
Increased frequency of apoptotic cells within the Peyer's patch T (left) and B (right) cell populations in Gαi2 deficient mice (broken line) compared with wild-type mice (continuous line), as determined by flow cytometry. The lymphocyte population was gated on CD4+ T cells or CD19+ B cells and analysed for expression of Bcl-2 (A), TUNEL (B), and CD95 (C), respectively. Numbers in the histograms are the percentage of cells that were positive for the different stainings. Results shown are from one representative experiment of at least four showing similar results, with three animals in each group.
Upregulated intestinal flora specific TH1 cytokine production in PP of Gαi2 deficient mice
As several studies has pointed towards the bacterial flora as being involved in disease pathogenesis,6,7 we measured intestinal flora specific cytokine production from T cells from the PP in Gαi2 deficient mice prior to the onset of colitis.
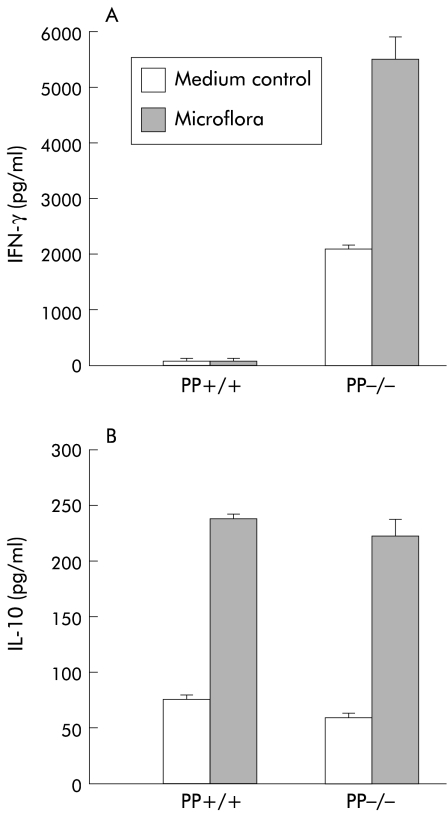
PP T lymphocytes from Gαi2 deficient mice as well as wild-type mice produced large amounts of the Th2 cytokine IL-10 in response to restimulation with enteric flora in vitro (fig 5 ▶), the flora specific production of IL-10 being of similar magnitude in Gαi2 deficient and wild-type mice. Interestingly, PP T lymphocytes also showed spontaneous production of IL-10, irrespective of the Gαi2 genotype. However, in sharp contrast with wild-type mice, PP T lymphocytes from Gαi2 deficient mice exhibited very high production of IFN-γ.
Figure 5.
Strong spontaneous and intestinal flora specific Th1 response in Peyer's patch (PP) T lymphocytes of Gαi2 deficient mice. Intestinal flora specific interferon γ (IFN-γ) (A) and interleukin 10 (IL-10) (B) production from Peyer's patch T cells were measured in Gαi2 deficient mice before the onset of colitis and compared with wild-type animals. Sonicated and sterile filtered bacteria from the caecum and colon were processed and presented by irradiated peritoneal macrophages to the T cell population. Supernatants were analysed for cytokine content after 48 hours of culture. One representative experiment of at least four is shown with three to six animals in each group.
PP T lymphocytes from Gαi2 deficient mice also showed significant spontaneous production of IFN-γ, without stimulation with flora, indicating that these cells are already activated in vivo (fig 5 ▶). Stimulation of PP T cells with KLH as a control antigen did not result in cytokine production exceeding that seen in unstimulated cultures (not shown). Thus in contrast with Th2 cytokine dominated T helper activity normally seen within the PP, Gαi2 deficient mice harbour increased numbers of IFN-γ producing T helper cells within their PP.
DISCUSSION
To our knowledge this is the first report demonstrating that wild-type mice normally express high levels of Bcl-2 in PP, and that decreased levels of Bcl-2 in PP lymphocytes is associated with an increased frequency of T and B cells undergoing apoptosis, most likely participating in regression of PP, in an animal model of colitis.
Gene knockout studies have indicated important roles for lymphotoxin (LT) αβ and LTβR, as well as IL-7Rα in PP organogenesis: the current hypothesis is that an as yet unidentified ligand stimulates IL-7Rα+ cells to produce LTαβ, which in turn activates LTβR+ cells to form PP.24 In addition, the B lymphocyte chemoattractant (BLC/BCA1) and its receptor CXCR5 are required for normal development of PP and most lymph nodes,25 and it was suggested that BLC functions by recruiting CXCR5+ CD3−IL-7R+ cells and inducing them to upregulate LTαβ. Moreover, mice deficient in the mucosal homing receptor integrin β7 have dramatically reduced size and cellularity in PP.26 PP lymphocytes from Gαi2 deficient mice did however have increased expression of this homing receptor, implying that PP of Gαi2−/− mice probably have a normal, or even increased, recruitment of lymphocytes.
Our results showed that regression of PP in Gαi2 deficient mice may be caused by excessive apoptosis. Bcl-2 is an antiapoptotic protein that when downregulated can induce programmed cell death.27 In accordance with this, expression of Bcl-2 was downregulated in both T and B cells within PP, and increased levels of apoptotic lymphocytes were found in the PP of Gαi2 deficient mice. No other study has investigated Bcl-2 levels in murine PP, but sheep PP B lymphocytes are rescued from apoptosis by induction of Bcl-2 expression.28
We believe that the PP lymphocytes of Gαi2−/− mice are stimulated by the luminal flora, and undergo activation induced cell death to a greater extent than wild-type mice. This is supported by Ayala and colleagues29 who reported that increased apoptosis in PP is due to increased exposure to luminal antigens following septic gut barrier breakdown. Moreover, a recent study showed that lipopolysaccharide causes atrophy of PP due to increased apoptosis.30
A striking finding in this study was the increased spontaneous as well as enteric flora specific IFN-γ production within PP of Gαi2 deficient mice. The normal immune response in the mucosa is Th2, and oral immunisation selectively induces Th2 cells in PP.31,32 In fact, one of the unique features of murine, but not human, PP is their ability to induce T helper cells to produce Th2 and Th3 (that is, transforming growth factor β) cytokines, thought to be important for the production of IgA and generation of oral tolerance.33 Freshly isolated PP, but not spleen, dendritic cells produce IL-10 and induce differentiation of Th2 cells.34
Interestingly, a number of studies have reported that IFN-γ mediates an increased susceptibility to apoptosis, whereas Th2 cytokines such as IL-4 and IL-10 protect from apoptosis35 by incomplete processing of caspase-8 at the death inducing signalling complex36 in Th2 cells. Thus peroral infection with Toxoplasma gondii was found to induce profound apoptosis in PP T cells which could be prevented by treatment with anti-IFN-γ antibodies.37 In addition, the outcome of thyroid autoimmunity is dependent on regulation of apoptotic proteins via T helper cytokines, in that in Hashimoto's thyroiditis thyrocytes are destroyed by apoptosis due to IFN-γ, whereas in Graves' disease IL-4 and IL-10 prevent apoptosis of thyrocytes.38
We therefore hypothesise that an aberrant Th1 response to the normal flora in the PP of Gαi2 deficient mice results in increased susceptibility to apoptosis, and consequently decreased cellularity in the patch.
Why do PP T lymphocytes from Gαi2 deficient mice respond to the enteric flora with such marked IFN-γ production in addition to normal IL-10 production? Pertussis toxin inhibits Gi proteins39 and thus mimics the situation in Gαi2 deficient mice. Pertussis toxin has been shown to induce a Th1 dominated T cell response40,41 and increase the severity of disease in animal models of Th1 mediated autoimmunity.42,43 In addition, He and colleagues44 recently provided direct evidence that Gαi2−/− mice exhibit enhanced production of IL-12, but not IL-10, from stimulated splenocytes, most probably enhancing IFN-γ production.
In contrast with regression of PP with the development of colitis in Gαi2 deficient mice, the available studies on the relationship between IBD and inductive mucosal immunological tissues rather point at a detrimental role of these tissues for disease initiation. Thus, trinitrobenezene sulphonic acid induced colitis in BALB/c mice results in hypertrophy of colonic patches, morphologically similar to PP.45 In addition, appendectomy—that is, deliberate removal of an inductive mucosal immunological tissue—of TcR-α mutant mice suppressed the development of IBD in these mice.46 However, whereas colitis in Gαi2−/− mice is a Th1 associated disease,3 the hapten induced colonic inflammation observed in BALB/c mice and TcR-α mutant mice are Th2 associated colitis models. In other words, inductive mucosal immune tissues such as PP, cryptopatches, and appendix seem supportive of a Th2 dominated response in the intestinal mucosa.
In an ongoing study, we are investigating whether there is an inherent ability of Gαi2−/− to switch the Th1 dominated inflammatory mucosal immune response to a more protective Th2 response, after immunisation with microbial products known to induce a Th2 response. We have found that a significant shift in cytokine production to a more Th2 dominated response caused significantly less colitis. Th2 induced mice also showed increased numbers of PP, underscoring the relevance of the findings presented in this study (Ohman Bache et al, manuscript in preparation).
In this study, we showed that as colitis develops in Gαi2 deficient mice, the size and number of PP are reduced. We believe that this is due to an aberrant enteric flora driven Th1 response, leading to activation induced cell death of PP lymphocytes associated with decreased levels of Bcl-2. It is possible, although not proved, that the lack of Th2 dominated mucosa tissues (that is, PP) in Gαi2 deficient mice is of critical importance for their susceptibility to chronic intestinal inflammation.
Acknowledgments
This study was supported by the Swedish Medical Research Council (grant No K99-71X-12174-03A), EU grant No QLRT-199-0050, the Society for Strategic Research, Swedish Society of Medicine, Magnus Bergwall Foundation, OE and Edla Johanssons Foundation, Professor Nanna Svartz Foundation, Sigurd and Elsa Golje Foundation, Clas Groschinsky Foundation, Örebro Medical Center Research Foundation, Kungl. and Hvitfeldtska Foundation, Ragnhild and Einar Lundströms Foundation, Ollie and Elof Ericsson Foundation, and Adlerbertska Foundation. We thank Professor N Lycke, Professor H Sjövall, Professor S Petterson, and Dr O H Hultgren for comments on the manuscript.
Abbreviations
PP, Peyer's patches
IBD, inflammatory bowel disease
LT, lymphotoxin
IFN-γ, interferon γ
PBS, phosphate buffered saline
FCS, fetal calf serum
PM, peritoneal macrophages
FITC, fluorescein isothiocyanate
PE, phycoerythrin
IL, interleukin
PNA, peanut agglutinin
REFERENCES
- 1.Rudolph U, Finegold MJ, Rich SS, et al. Ulcerative colitis and adenocarcinoma of the colon in G alpha i2-deficient mice. Nat Genet 1995;10:143–50. [DOI] [PubMed] [Google Scholar]
- 2.Öhman L, Franzen L, Rudolph U, et al. Immune activation in the intestinal mucosa before onset of colitis in Gai2-deficient mice. Scand J Immunol 2000;52:80–90. [DOI] [PubMed] [Google Scholar]
- 3.Hornquist CE, Lu X, Rogers-Fani PM, et al. G(alpha)i2-deficient mice with colitis exhibit a local increase in memory CD4+ T cells and proinflammatory Th1-type cytokines. J Immunol 1997;158:1068–77. [PubMed] [Google Scholar]
- 4.Corthesy-Theulaz IE, Hopkins S, Bachmann D, et al. Mice are protected from Helicobacter pylori infection by nasal immunization with attenuated Salmonella typhimurium phoPc expressing urease A and B subunits. Infect Immun 1998;66:581–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacDonald T. Effector and regulatory lymphoid cells and cytokines in mucosal sites. Curr Top Microbial Immunol 1998;236:113–35. [DOI] [PubMed] [Google Scholar]
- 6.Sadlack B, Merz H, Schorle H, et al. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell 1993;75:253–61. [DOI] [PubMed] [Google Scholar]
- 7.Taurog JD, Richardson JA, Croft JT, et al. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med 1994;180:2359–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sartor B. Microbial factors in the pathogenesis of Crohn's disease, ulcerative colitis, and experimental intestinal inflammation, 5th edn. Philadelphia: WB Saunders Company, 2000.
- 9.Rath HC, Herfarth HH, Ikeda JS, et al. Normal luminal bacteria, especially Bacteroides species, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human beta2 microglobulin transgenic rats. J Clin Invest 1996;98:945–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leiper K, Morris AI,Rhodes JM. Open label trial of oral clarithromycin in active Crohn's disease. Aliment Pharmacol Ther 2000;14:801–6. [DOI] [PubMed] [Google Scholar]
- 11.Rutgeerts P, Hiele M, Geboes K, et al. Controlled trial of metronidazole treatment for prevention of Crohn's recurrence after ileal resection. Gastroenterology 1995;108:1617–21. [DOI] [PubMed] [Google Scholar]
- 12.Rutgeerts P, Goboes K, Peeters M, et al. Effect of faecal stream diversion on recurrence of Crohn's disease in the neoterminal ileum. Lancet 1991;338:771–4. [DOI] [PubMed] [Google Scholar]
- 13.D'Haens GR, Geboes K, Peeters M, et al. Early lesions of recurrent Crohn's disease caused by infusion of intestinal contents in excluded ileum. Gastroenterology 1998;114:262–7. [DOI] [PubMed] [Google Scholar]
- 14.Duchmann R, Kaiser I, Hermann E, et al. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD) . Clin Exp Immunol 1995;102:448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Itoh J, de La Motte C, Strong SA, et al. Decreased Bax expression by mucosal T cells favours resistance to apoptosis in Crohn's disease. Gut 2001;49:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boirivant M, Marini M, Di Felice G, et al. Lamina propria T cells in Crohn's disease and other gastrointestinal inflammation show defective CD2 pathway-induced apoptosis. Gastroenterology 1999;116:557–65. [DOI] [PubMed] [Google Scholar]
- 17.Neurath MF, Finotto S, Fuss I, et al. Regulation of T-cell apoptosis in inflammatory bowel disease: to die or not to die, that is the mucosal question. Trends Immunol 2001;22:21–6. [DOI] [PubMed] [Google Scholar]
- 18.Strater J, Wellisch I, Riedl S, et al. CD95 (APO-1/Fas)-mediated apoptosis in colon epithelial cells: a possible role in ulcerative colitis. Gastroenterology 1997;113:160–7. [DOI] [PubMed] [Google Scholar]
- 19.Ueyama H, Kiyohara T, Sawada N, et al. High Fas ligand expression on lymphocytes in lesions of ulcerative colitis. Gut 1998;43:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bregenholt S, Reimann J,Claesson MH. Proliferation and apoptosis of lamina propria CD4+ T cells from scid mice with inflammatory bowel disease. Eur J Immunol 1998;28:3655–63. [DOI] [PubMed] [Google Scholar]
- 21.Asakura H, Suzuki A, Ohtsuka K, et al. Gut-associated lymphoid tissues in ulcerative colitis. JPEN J Parenter Enteral Nutr 1999;23:S25–8. [DOI] [PubMed] [Google Scholar]
- 22.Wigzell H. In vitro methods in cell-mediated and tumor immunity. In: Bloom BR, Davids JR, eds. In vitro methods in cell-mediated and tumor immunity. New York: Academic Press, 1976: 245.
- 23.Hornquist E, Lycke N. Cholera toxin adjuvant greatly promotes antigen priming of T cells. Eur J Immunol 1993;23:2136–43. [DOI] [PubMed] [Google Scholar]
- 24.Nishikawa S, Honda K, Hashi H, et al. Peyer's patch organogenesis as a programmed inflammation: a hypothetical model. Cytokine Growth Factor Rev 1998;9:213–20. [DOI] [PubMed] [Google Scholar]
- 25.Ansel KM, Ngo VN, Hyman PL, et al. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature 2000;406:309–14. [DOI] [PubMed] [Google Scholar]
- 26.Wagner N, Lohler J, Kunkel EJ, et al. Critical role for beta7 integrins in formation of the gut-associated lymphoid tissue. Nature 1996;382:366–70. [DOI] [PubMed] [Google Scholar]
- 27.Ruvolo PP, Deng X,May WS. Phosphorylation of Bcl2 and regulation of apoptosis. Leukemia 2001;15:515–22. [DOI] [PubMed] [Google Scholar]
- 28.Motyka B, Reynolds JD. Rescue of ileal Peyer's patch B cells from apoptosis is associated with the induction of Bcl-2 expression. Immunology 1995;84:383–7. [PMC free article] [PubMed] [Google Scholar]
- 29.Ayala A, Xin Xu Y, Ayala CA, et al. Increased mucosal B-lymphocyte apoptosis during polymicrobial sepsis is a Fas ligand but not an endotoxin-mediated process. Blood 1998;91:1362–72. [PubMed] [Google Scholar]
- 30.Manhart N, Vierlinger K, Habel O, et al. Lipopolysaccharide causes atrophy of Peyer's patches and an increased expression of CD28 and B7 costimulatory ligands. Shock 2000;14:478–83. [DOI] [PubMed] [Google Scholar]
- 31.Xu-Amano J, Aicher WK, Taguchi T, et al. Selective induction of Th2 cells in murine Peyer's patches by oral immunization. Int Immunol 1992;4:433–45. [DOI] [PubMed] [Google Scholar]
- 32.Wilson AD, Bailey M, Williams NA, et al. The in vitro production of cytokines by mucosal lymphocytes immunized by oral administration of keyhole limpet hemocyanin using cholera toxin as an adjuvant. Eur J Immunol 1991;21:2333–9. [DOI] [PubMed] [Google Scholar]
- 33.Daynes RA, Araneo BA, Dowell TA, et al. Regulation of murine lymphokine production in vivo. III. The lymphoid tissue microenvironment exerts regulatory influences over T helper cell function. J Exp Med 1990;171:979–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iwasaki A, Kelsall BL. Freshly isolated Peyer's patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. J Exp Med 1999;190:229–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lemaire C, Andrau K, Fraisse CS, et al. IL-4 inhibits apoptosis and prevents mitochondrial damage without inducing the switch to necrosis observed with caspase inhibitors. Cell Death Differ 1999;6:813–20. [DOI] [PubMed] [Google Scholar]
- 36.Varadhachary AS, Peter ME, Perdow SN, et al. Selective up-regulation of phosphatidylinositol 3`-kinase activity in Th2 cells inhibits caspase-8 cleavage at the death-inducing complex: a mechanism for Th2 resistance from Fas-mediated apoptosis. J Immunol 1999;163:4772–9. [PubMed] [Google Scholar]
- 37.Liesenfeld O, Kosek JC, Suzuki Y. Gamma interferon induces Fas-dependent apoptosis of Peyer's patch T cells in mice following peroral infection with Toxoplasma gondii. Infect Immun 1997;65:4682–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stassi G, Di Libertino Diana, Todaro Matilde, et al. Control of target cell survival in thyroid autoimmunity by T helper cytokines via regulation of apoptotic proteins. Nature Immunology 2000;1:483–8. [DOI] [PubMed] [Google Scholar]
- 39.Sunyer T, Monastirsky B, Codina J, et al. Studies on nucleotide and receptor regulation of Gi proteins: effects of pertussis toxin. Mol Endocrinol 1989;3:1115–24. [DOI] [PubMed] [Google Scholar]
- 40.Brady MT, Mahon BP, Mills KH. Pertussis infection and vaccination induces Th1 cells. Immunol Today 1998;19:534. [DOI] [PubMed] [Google Scholar]
- 41.Mahon BP, Ryan MS, Griffin F, et al. Interleukin-12 is produced by macrophages in response to live or killed Bordetella pertussis and enhances the efficacy of an acellular pertussis vaccine by promoting induction of Th1 cells. Infect Immun 1996;64:5295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Munoz JJ, Bernard CC, Mackay IR. Elicitation of experimental allergic encephalomyelitis (EAE) in mice with the aid of pertussigen. Cell Immunol 1984;83:92–100. [DOI] [PubMed] [Google Scholar]
- 43.Silver PB, Chan CC, Wiggert B, et al. The requirement for pertussis to induce EAU is strain-dependent: B10.RIII, but not B10.A mice, develop EAU and Th1 responses to IRBP without pertussis treatment. Invest Ophthalmol Vis Sci 1999;40:2898–905. [PubMed] [Google Scholar]
- 44.He J, Gurunathan S, Iwasaki A, et al. Primary role for Gi protein signaling in the regulation of interleukin 12 production and the induction of T helper cell type 1 responses. J Exp Med 2000;191:1605–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dohi T, Fujihashi K, Rennert PD, et al. Hapten-induced colitis is associated with colonic patch hypertrophy and T helper cell 2-type responses. 1999;189:1169–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mizoguchi A, Mizoguchi E, Chiba C, et al. Role of appendix in the development of inflammatory bowel disease in TCR-alpha mutant mice. J Exp Med 1996;184:707–15. [DOI] [PMC free article] [PubMed] [Google Scholar]