Abstract
Background: Hepatitis C virus (HCV) infection is a significant problem in the management of haemodialysis patients. A high prevalence of HCV infection in haemodialysis patients has been reported. Risk factors such as the number of blood transfusions or duration on haemodialysis have been identified.
Aim: To determine the prevalence of HCV by antibody testing and HCV-RNA determination by polymerase chain reaction (PCR) in haemodialysis patients. Furthermore, liver function tests were performed and epidemiological data were obtained to determine risk factors for HCV in this cohort of patients.
Results: A total of 2796 patients from 43 dialysis centres were enrolled. The overall prevalence of HCV (HCV antibody and/or HCV-RNA positivity) was 7.0% (195 patients). Antibody positivity occurred in 171 patients (6.1%). Viraemia was detectable in 111 patients (4.0%). Twenty four of 111 HCV RNA positive patients (21.6%) were negative for HCV antibodies. Thus 0.8% of the entire study population was HCV positive but could not be diagnosed by routine HCV antibody testing. Major risk factors identified by a standard questionnaire in 1717 of 2796 patients were the number of blood transfusions individuals had received and duration of dialysis, the latter including patients who received no blood transfusions. Sequencing of the 5`untranslated region of the genome showed a dominant genotype 1 (77.6%) within the cohort. Further reverse transcription-PCR of the NS5b and core region were performed to document phylogenetic analysis. Comparing nucleic acid sequences detected by PCR, no homogeneity was found and thus nosocomial transmission was excluded.
Conclusions: HCV is common in German haemodialysis patients but screening for HCV antibodies alone does not exclude infection with HCV.
Keywords: hepatitis C, prevalence, haemodialysis, risk factors, viraemia
Patients on chronic haemodialysis treatment have been identified by serological testing with second and third generation immunosorbent assays (ELISA) as a high risk group for hepatitis C virus (HCV) infection.1–12 Hepatitis C is the most common cause of chronic viral liver disease in haemodialysis patients.13 Due to parenteral transmission of the virus, HCV contaminated blood transfusion was identified as the main risk factor for viral transmission before the availability of reliable HCV screening of blood products in 1990.13–17 The extensive use of recombinant erythropoietin to correct renal anaemia in haemodialysis patients resulted in a significant reduction in blood transfusions. However, previous studies have shown that de novo infections in single haemodialysis units may still occur in the absence of other parenteral risk factors.18–24 Furthermore, some reports demonstrated that the duration of haemodialysis is an independent predictor of HCV infection in chronic haemodialysis patients.20,24 Thus nosocomial spread of hepatitis C between patients within a haemodialysis unit was suggested.20–26 Most epidemiological studies in haemodialysis patients have been performed using serological testing of hepatitis C antibodies only.3,5,20,21,27,28 In recent years, HCV viraemia (HCV-RNA) has been routinely detected by polymerase chain reaction (PCR).29,30 In 1993, Bukh and colleagues31 were the first to describe the fact that HCV viraemia can occur without detection of HCV antibodies. This has been confirmed by several authors in small patient populations.32–35 Thus serological testing alone is inconclusive for screening of HCV.31–35 Several prevalence studies of hepatitis C have been undertaken. There is a wide range in HCV antibody positivity and HCV viraemia within the studies, ranging from 1% up to 91%. The geographical region of the study population, methods used for detection of hepatitis C (first, second, third generation ELISA, or HCV-RNA), as well as the various cohorts of patients investigated led to varied results.1,36,37 In some studies, coinfection with other hepatotropic viruses changed the prevalence of hepatitis C in haemodialysis patients.38
Thus the magnitude of hepatitis C transmission within haemodialysis units is still unclear and therefore general recommendations for prevention have not been developed.37,39 The Centre of Disease Control has made no recommendations for controlling hepatitis C in haemodialysis units.38 However, the natural course of hepatitis C in haemodialysis patients is not well understood. It seems to differ from that in other HCV patients.40 Liver function tests are close to or near normal in many cases.41,42 But the mortality of HCV infected haemodialysis patients seems to be enhanced compared with HCV negative haemodialysis patients in preliminary studies.43 Thus patients with HCV on chronic haemodialysis are at increased risk of death, which suggests that the focus should be directed more to identification and prevention of hepatitis C infection in haemodialysis patients.
The aim of the present study was to assess in a cross sectional study the prevalence of hepatitis C measured serologically by HCV antibody testing and detection of HCV viraemia by PCR in a large cohort of German chronic haemodialysis patients. In this context, the prevalence of antibody negative viraemic hepatitis C patients should also be evaluated. Also, risk factors for transmission of the virus were determined.
PATIENTS AND METHODS
Study design and patient selection
The study was performed in haemodialysis units of the Patienten-Heim-Versorgung, an organisation of haemodialysis units all over Germany. A total of 3042 patients from 43 haemodialysis units were enrolled in this cross sectional trial between October 1996 and March 1997; 2796 of 3042 patients gave informed consent and thus 92% of the whole patient population were investigated. The remaining 246 patients could not been tested for the following reasons: vacation, hospital stay, death before investigation, and informed consent withdrawn (<1%). The study protocol was approved in 1996 by the ethics committee of the medical faculty of the Christian-Albrechts-University, Kiel. All patients underwent chronic haemodialysis treatment for end stage renal disease during the study period. The number of patients in the haemodialysis units varied from 17 to 177 patients. In 1717 of 2796 patients, epidemiological data were available by questionnaire (61.41%):
sex and age,
duration on haemodialysis in months,
number of blood transfusions (none, 1–5, 6–15, more than 15),
known risk factors, such as intravenous drug abuse, immunosuppression, haemophilia,
known chronic liver disease.
In the present study, men (n=917; 53%) were more often on haemodialysis than women (n=800; 47%). Mean age was 61 years (range 19–92) (men 59 years, women 63 years). Mean duration on haemodialysis treatment was 54 months (52 in men and 57 in women). Haemodialysis was performed routinely 2–3 times weekly in the patient population.
Blood (serum and plasma) (16 ml) was obtained from each patient before haemodialysis started. Blood was centrifuged immediately at the unit, plasma and sera separated, and stored in aliquots at −80°C. All samples were subsequently subjected to liver function tests: alanine aminotransferase (U/l), aspartate aminotransferase (U/l), gammaglutamyl transpeptidase (U/l), and bilirubin concentration (mg/dl) in the central laboratory of the First Department of Medicine, Kiel. Anti-HCV antibody was measured by a third generation commercial ELISA (Enzymun-Test Anti-HCV; Boehringer Mannheim, Germany). The third generation assay detects antibodies for three viral antigens (c22-3, c200, and NS5). HCV-RNA testing was performed using reverse transcription (RT)-PCR (Cobas Amplicor Monitor; Roche Brenchburg, New Jersey, USA) with a detection limit of 100 genomes/ml. All positive samples for HCV-RNA were tested twice with different aliquots. Samples positive for HCV-RNA and negative for HCV antibodies were tested again for HCV antibodies using HCV version 3.0 (detecting viral antigens c200, c100-3, and NS5; Abbott Axsym System, Wiesbaden, Germany). The percentage of measurements for each laboratory parameter in the 2796 patients was 98.5% for liver function tests, 99.3% for HCV-RNA, and 99.6% for HCV antibody testing. Missing aliquots or destruction of aliquots accounted for the discrepancy between patients investigated and laboratory measurements performed. In HCV-RNA positive patients, genotyping by sequencing of the 5`untranslated region was performed to determine the genotype of the virus. Furthermore, subsequent RT-PCR of the NS5b and core region for phylogenetic analysis of HCV-RNA positive patients from a single centre was performed to determine the genotype and detect possible patient to patient transmission.
All tests were carried out and interpreted strictly in accordance with the manufacturer's instructions. For PCR testing, strict segregation of personnel and equipment into pre- and post-PCR laboratory buildings was established to avoid the possibility of post-PCR contamination.
Epidemiological data are presented as mean and percentage of the mean. Further statistical analysis of risk factors for HCV infection (age, duration on haemodialysis, and number of blood transfusions) was performed by multivariate analysis and Fisher's exact test with a p value <0.05.
RESULTS
Of the 2786 patients tested for hepatitis C virus antibodies by third generation ELISA, 171 were positive (6.1%). All positive samples were confirmed by an independent third generation HCV antibody ELISA. HCV-RNA measured by RT-PCR with a detection limit of 100 genomes/ml was detected in 111 of 2777 patients (4.0%). All positive HCV-RNA samples were tested twice using different aliquots. In 24 of 111 hepatitis C viraemic patients (21.6%), the antibodies tested negative with two different third generation ELISA. Thus the overall prevalence of hepatitis C (HCV antibody positivity and/or HCV-RNA positivity) in the 2796 haemodialysis patients investigated was 7.0% (195 of 2796 patients). The prevalence data are given in table 1 ▶.
Table 1.
Prevalence of hepatitis C virus (HCV) antibodies and HCV-RNA in 2796 haemodialysis patients
| Parameter | n (%) |
| HCV antibody and/or HCV-RNA positive | 195 (7.0) |
| HCV antibody positive | 171 (6.1) |
| HCV-RNA positive | 111 (4.0) |
| HCV-RNA positive; HCV antibody negative | 24 (0.8) |
| HCV-RNA and HCV antibody negative | 2591 (93) |
In the underlying haemodialysis patients, hepatitis C viraemia was detected in 64.9% of infected patients. Subgroup analysis showed no difference in age, sex, or time on haemodialysis for patients who cured hepatitis C spontaneously or those patients who were suffering from chronic hepatitis C infection. There was a wide range of HCV antibody and HCV-RNA prevalence in the 43 haemodialysis units investigated. In three of 43 haemodialysis units with a total of 137 patients, none was found to be positive for HCV antibodies. The highest prevalence for HCV antibodies was 22.5% in a single centre with 43 patients. Similar to the results for HCV antibodies in eight haemodialysis units with 455 patients, no viraemic hepatitis C patient was seen. The highest prevalence was 13.3% and was observed in a single unit with 50 haemodialysis patients.
One hundred and three of 111 hepatitis C viraemic patients were investigated for genotyping using RT-PCR. Genotype 1b was predominant (67 patients; 65%) followed by genotype 1a (13 patients; 12.6%). The distribution of genotypes is given in fig 1 ▶. In 16 samples with positive HCV-RNA, genotyping by RT-PCR was unsuccessful. Few patients showed genotype 2, 3, or 4 and one patient had a non-classified genotype. Thus genotype 1 was found in more than 75% of infected cases. Furthermore, partial sequencing of the 5`untranslated region, NS5b, and core region in patients infected with the same genotype in a single centre failed to show the same quasispecies and thus no patient to patient transmission was demonstrated. Although the rare genotype 2b was detected in two patients from one haemodialysis unit, comparison of the sequences also failed to detect homogeneity.
Figure 1.
Distribution of the different genotypes (1, 2, 3, 4), unsuccessful determinations, or unclassified results in 103 of 111 hepatitis C viraemic patients.
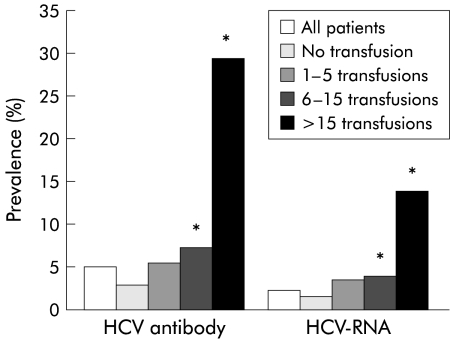
In 1717 of 2796 patients (61.4%), including 195 patients with positive hepatitis C results, epidemiological data were obtained and thus risk factors were determined. Figure 2 ▶ demonstrates that there was a slight increase over time in the number of hepatitis C antibodies and HCV-RNA. Interestingly, there was a high prevalence of hepatitis C antibodies (7%) and HCV-RNA (3.8%) in the first year of haemodialysis treatment. Thus pre-haemodialysis status as a risk factor for acquisition of hepatitis C may be underestimated. As hepatitis C is transmitted parenterally, patients with blood transfusions, especially those transfused before testing of blood products for hepatitis C, are at high risk for HCV infection. The prevalence data for HCV antibodies and HCV-RNA in relation to blood transfusions are given in fig 3 ▶.
Figure 2.
Influence of duration of haemodialysis on percentage prevalence of hepatitis C virus antibodies (HCV-AK) and HCV-RNA in haemodialysis patients.
Figure 3.
Influence of number of blood transfusions on percentage prevalence of hepatitis C virus (HCV) antibodies and HCV viraemia (HCV-RNA) in haemodialysis patients. *p<0.05 compared with patients who were not transfused or received 1–5 transfusions.
HCV antibodies were found in nearly 30% and hepatitis C viraemia in 14% of patients with multiple transfusions. More then five blood transfusions was found to be an independent risk factor for hepatitis C infection (p<0.05). Thus duration of haemodialysis and number of blood transfusions were risk factors for hepatitis C. As duration of haemodialysis is often related to the number of blood transfusions given, it is unclear if time on haemodialysis is an independent factor. Thus the subgroup of patients who did not receive blood transfusions was analysed for their risk of hepatitis C in relation to duration of haemodialysis (fig 4 ▶). The same rise in HCV antibody prevalence and HCV-RNA prevalence was observed as for patients who had blood transfusions. This demonstrates that time on haemodialysis is an independent risk factor for developing hepatitis C infection (p<0.05 for a duration of more than 10 years on haemodialysis). There were no significant differences in age, duration of haemodialysis, or elevation in liver function tests in the 111 viraemic hepatitis C patients compared with the whole study population. Furthermore, the same was observed in the 24 antibody negative viraemic hepatitis C patients when compared with the epidemiological data of the 1717 patients. As 21.6% of viraemic hepatitis C patients were negative for HCV antibodies, and liver function tests remained normal in most cases of HCV infection, 0.8% (24 of 2796 patients) of our viraemic hepatitis C patients would have been undiagnosed by routine screening.
Figure 4.
Influence of duration on haemodialysis treatment on percentage prevalence of hepatitis C virus (HCV) antibodies and HCV-RNA in haemodialysis patients who were not previously transfused. *p<0.05 compared with patients on haemodialysis for less then 10 years.
DISCUSSION
It is well known that haemodialysis patients are at high risk for hepatitis C infection. But there is a wide range in prevalence rates in different regions of the world, ranging from 1% in the UK to more than 90% in Eastern Europe.1,2,4,6–7,13–15,27 HCV treatment with interferon is not as successful in haemodialysis patients as in the general population, and there is no approval for the drug in end stage renal disease. Ribavirin treatment is contraindicated due to its long half life and renal elimination. Thus our patients had not yet been treated for HCV. In the present cross sectional study in a large cohort of haemodialysis patients, a high prevalence of 7.0% was confirmed. Prevalence data for hepatitis C in the general population of Germany suggest that the rate is between 0.42% and 0.84%.44 Thus haemodialysis patients in Germany have a 8–16-fold increase in risk.
There are many difficulties in designing hepatitis C prevalence studies. A representative cohort of haemodialysis patients is necessary. In general, the published studies enrolled at least 500 patients.8,13,17,19,27,38 Only few studies were performed in more than 1000 patients.16,26,35 The prevalence of HCV antibodies in the present study was 0–22.5% and for HCV-RNA 0–13.3%. Thus our data suggest that results obtained in 100–400 patients may under or overestimate HCV prevalence. As 2796 patients were enrolled in the present study, the calculated prevalence data for hepatitis C in haemodialysis patients are reliable.
The methods used for detecting hepatitis C can lead to differences in prevalence data. In the early 1990s, HCV antibody testing with only first or second generation ELISA assays were performed.3,5,11,12,20 Today, third generation ELISA assays have the highest sensitivity and specificity.4,26,36,45 Also, viraemia of hepatitis C can now be detected by HCV-RNA measurement.29 There are different techniques for detection of viral RNA.18,19 RT-PCR has the highest sensitivity with a detection limit of 100 genomes/ml. Thus prevalence data for hepatitis C in haemodialysis patients should be obtained using third generation ELISA assays for detection of HCV antibodies and a highly sensitive RT-PCR for HCV-RNA detection. We found hepatitis C viraemia in 4.0% of haemodialysis patients. Compared with ELISA for HCV antibodies, viraemia occurred in 64.9% of the infected patients. Thus viraemia was lower than estimated in the general HCV population where 80% is suggested.1,46
The present study confirmed preliminary data that seroconversion to HCV antibodies does not occur in all haemodialysis patients.31–35 Twenty four patients with viraemic hepatitis C where discovered in our cohort of 2796 haemodialysis patients (0.8%) who did not develop HCV antibodies. None of the patients was coinfected with human immunodeficiency virus. Other factors influencing immunosuppression (that is, chemotherapy, immunosupression due to prior transplantation) were not available. These patients were not detected by routine screening of liver function tests or HCV antibody testing. This is of clinical interest as the route of transmission in haemodialysis patients still remains unclear.
We confirmed that administration of blood products is the main risk factor for developing hepatitis C.1,7,13,15,16,26 But duration of haemodialysis in patients with or without blood transfusions is also an independent risk factor.15,16,20,24 Thus patient to patient transmission during haemodialysis has been suggested.18,19,21–23 RT-PCR of the hepatitis C virus allows the sequencing of the viral genome. Thus in addition to genotyping, detection of quasispecies with high homogeneity in the genome is also possible. In the underlying 111 viraemic patients, no direct patient to patient transmission was observed. As this study was conducted as a single point prevalence study, a negative result does not exclude nosocomial transmission of the virus over time. Patient to patient transmission was prospectively proved in several incidence studies in haemodialysis patients.21–23
The intensive use of recombinant erythropoietin for control of renal anaemia in the last 10 years has led to reduced blood transfusions. Thus one would suggest lower prevalence data in patients with a duration of haemodialysis of less than five years. Patients in the present study showed a high prevalence of hepatitis C in their first year of haemodialysis with a prevalence of HCV antibodies of 7% and positive HCV-RNA of 3.8%. Thus the pre-haemodialysis status is also of interest. This has not been studied previously. As patients with end stage renal disease who had previously received a renal transplant were included in this study, it may help explain the high prevalence in the first year of haemodialysis, as those patients may have been infected during their first period on haemodialysis.
In conclusion, patients on maintenance haemodialysis treatment are at high risk for hepatitis C infection. HCV-RNA measurement for hepatitis C infection should be carried out as HCV in 0.8% of the study population would not have been detected by measuring HCV antibodies alone. Measurements of liver function remained normal in the majority of hepatitis C patients and was a non-specific marker as elevation does not correlate with viral liver disease. There are still no strict recommendations for HCV management in haemodialysis patients.37,39 As HCV-RNA measurement by RT-PCR is expensive, PCR in pooled sera of 50 haemodialysis patients, who are known to be HCV negative, might be useful. The detection limit of 100 genomes/ml would be raised to 5000 genomes/ml which is a low virus load. This has been shown to be effective in blood donations47 and has been used in an incidence study for hepatitis C in haemodialysis patients.26 This procedure is inexpensive and highly sensitive for detection of hepatitis C infection. As routes of transmission are still unclear, detection of all infected patients is mandatory for HCV prophylaxis in haemodialysis patients.
Acknowledgments
The present study was supported by a grant from the Patienten-Heim-Versorgung (PHV), Bad Homburg, Germany.
We are grateful to all physicians of the haemodialysis centres who participated in this study: P Arnold, P Dieker, H Schneider in Siegen; ME Bohling in Jever; HJ Buff, F Lauruhn in Herford; H Damrath, K Ehrler in Sangershausen; J Engelmann, J George in Groβenhain; A Weber, H Finn in Altenburg; M Euchenhofer, H Würz in Esslingen; W Schroer in Lippstadt; J Grünberg in Minden; I Grünwald, U Hövelborn in Herrenberg/Sindelfingen; M Hacker, P Harms in Bad Oeynhausen; D Hummel, M Köber in Waiblingen; G Meister in Salzgitter; T Klein in Limburg; HJ Knieβ in Detmold; E Knödler, W Zimmermann in Gelsenkirchen; H Stradtmann in Bad Wildungen; E Kothe, U Schirrmeister, W Krüger in Bad Harzburg; W Nagel, T Kiefer in Dürrlewang; M Oppitz in Halberstadt; H Plache, R Valentin, A Plöger in Bielefeld; M Puhm, U Wagner, G Scholl in Reutlingen; P Rawer, in Wetzlar; O Richter, R Behnisch in Dresden; K Sauer, J Nehrkorn in Wernigerode; P Schilken, M Vischedyk, P Fowler in Paderborn; H Schneider, R Teigelkötter in Gütersloh; HW Schneider, J Meinshausen, T Kirschner, M Fromme, M Traub, C Machleidt in Stuttgart; U Häbel in Hildesheim; G Seyffart, R Scholz in Bad Homburg; G Weikert in Flensburg; G Loose in Kiel; W Niedermayer in Kiel; V Wizemann, K Mueller in Gieβen/Alsfeld; R Götz in Bad Windsheim; U Knödle, K Teuffel in Leonberg; and S Schütterle, H Lange in Marburg-Cappel.
We are grateful to Mrs Eike Juergen, medical assistant, for her excellent work. Thanks to Jörg Petersen and D Zuckerman for critical reading of the manuscript.
Abbreviations
HCV, hepatitis C virus
PCR, polymerase chain reaction
RT-PCR, reverse transcription-polymerase chain reaction
REFERENCES
- 1.Alter MJ. Epidemiology of hepatitis C in the West. Semin Liver Dis 1995;15:5–14. [DOI] [PubMed] [Google Scholar]
- 2.Chan TM, Lok ASF, Cheng IKPC, et al. Prevalence of hepatitis C virus infection in hemodialysis patients: A longitudinal study comparing the results of RNA and antibody assays. Hepatology 1993;17:135–9. [PubMed] [Google Scholar]
- 3.Chaveau P, Courouce A, Le Marrec N, et al. Antibodies to hepatitis C virus by second-generation test in hemodialysed patients. Kidney Int 1993;43(suppl):149–52. [PubMed] [Google Scholar]
- 4.Courouce AM, Bouchardeau F, Chauveau P, et al. Hepatitis C virus infection in haemodialysed patients: HCV RNA and anti-HCV antibodies (third generation assays). Nephrol Dial Transplant 1995;10:234–9. [PubMed] [Google Scholar]
- 5.Esteban JH, Esteban R, Viladomiu L, et al. Hepatitis C virus antibodies among risk groups in Spain. Lancet 1989;ii:294–7. [DOI] [PubMed] [Google Scholar]
- 6.McIntyre PG, McCruden EAB, Dow BC, et al. HCV infection in renal dialysis patients in Glasgow. Nephrol Dial Transplant 1994;9:291–5. [PubMed] [Google Scholar]
- 7.Medin C, Allander T, Roll M, et al. Seroconversion to hepatitis C virus in dialysis patients. A retrospective and prospective study. Nephron 1993;65:40–5. [DOI] [PubMed] [Google Scholar]
- 8.Morikawa T, Nakata K, Hamasaki K, et al. Prevalence and characterization of hepatitis C virus in hemodialysis patients. Intern Med 1999;38:626–31. [DOI] [PubMed] [Google Scholar]
- 9.Niu M, Coleman P, Alter MJ. Multicenter study of hepatitis C virus infection in chronic hemodialysis patients and hemodialysis center staff members. Am J Kidney Dis 1993;22:568–73. [DOI] [PubMed] [Google Scholar]
- 10.Oguchi H, Miyasaka M, Tokunaga S, et al. Hepatitis C infection in eleven Japanese hemodialysis untis. Clin Nephrol 1992;38:49–52. [PubMed] [Google Scholar]
- 11.Schlipköter U, Gladziwa U, Chomalkov K, et al. Prevalence of hepatitis C virus infections in dialysis patients and their contacts using a second generation enzyme-linked immunosorbent assay. Med Microbiol Immunol 1992;181:173–80. [DOI] [PubMed] [Google Scholar]
- 12.Zeldis JB, Depner TA, Kuramoto IK, et al. Prevalence of hepatitis C virus antibodies among hemodialysis patients. Ann Intern Med 1990;112:958–60. [DOI] [PubMed] [Google Scholar]
- 13.Knudsen F, Wantzin P, Rasmussen K, et al. Hepatitis C in dialysis patients: Relationship to blood transfusions, dialysis and liver disease. Kidney Int 1993;43:1353–6. [DOI] [PubMed] [Google Scholar]
- 14.Dentico P, Buongiorno R, Volpe A, et al. Prevalence and incidence of HCV in hemodialysis patients: Study of risk factors. Clin Nephrol 1992;38:49–52. [PubMed] [Google Scholar]
- 15.Jadoul M, Cornu C, van Ypersele de Strihou C, and UCL Collaboratory Group. Incidence and risk factors for hepatitis C seroconversion in hemodialysis: A prospective study. Kidney Int 1993;44:1322–6. [DOI] [PubMed] [Google Scholar]
- 16.Di Lallo D, Micelli M, Petrosillo N, et al. Risk factors of hepatitis C virus infection in patients on hemodialysis: a multivariate analysis based on a dialysis register in Central Italy. Eur J Epidemiol 1999;15:11–14. [DOI] [PubMed] [Google Scholar]
- 17.Sandhu J, Preiksaitis JK, Campbell PM, et al. Hepatitis C prevalence and risk factors in the northern Alberta dialysis population. Am J Epidemiol 1999;150:58–66. [DOI] [PubMed] [Google Scholar]
- 18.Fabrizi F, Martin P, Dixit V, et al. Acquisition of hepatitis C virus in hemodialysis patients: a prospective study by branched DNA signal amplification assay. Am J Kidney Dis 1998;31:647–54. [DOI] [PubMed] [Google Scholar]
- 19.Fabrizi F, Martin P, Dixit V, et al. Detection of de novo hepatitis C virus infection by polymerase chain reaction in hemodialysis patients. Am J Nephrol 1999;19:383–8. [DOI] [PubMed] [Google Scholar]
- 20.Hardy NM, Sandroni S, Danielson S, et al. Antibody to hepatitis C virus increases with time on hemodialysis. Clin Nephrol 1992;38:44–8. [PubMed] [Google Scholar]
- 21.Irish DN, Blake C, Christophers J, et al. Identification of hepatitis C seroconversion resulting from nosocomial transmission on a haemodialysis unit: implications for infection control and laboratory screening. J Med Virol 1999;59:135–40. [PubMed] [Google Scholar]
- 22.Katsoulidou A, Paraskevis D, Kalapothaki V, et al. Molecular epidemiology of a hepatitis C outbreak in a haemodialysis unit. Multicentre haemodialysis cohort study on viral hepatitis. Nephrol Dial Transplant 1999;14:1188–94. [DOI] [PubMed] [Google Scholar]
- 23.Mizuno M, Higuchi T, Kanmatsuse K, et al. Genetic and serological evidence for multiple instances of unrecognized transmission of hepatitis C virus in hemodialysis patients. J Clin Microbiol 1998;36:2926–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okuda K, Hayashi H, Kobayashi S, et al. Mode of hepatitis C infection not associated with blood transfusion among chronic hemodialysis patients. J Hepatol 1995;23:28–31. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi M, Tanaka E, Oguchi H, et al. Prospective follow-up study of hepatitis C virus infection in patients undergoing maintenance haemodialysis: comparison among haemodialysis units. J Gastroenterol Hepatol 1998;13:604–9. [DOI] [PubMed] [Google Scholar]
- 26.Salama G, Rostaing L, Sandres K, et al. Hepatitis C virus infection in French hemodialysis units: A multicenter study. J Med Virol 2000;61:44–51. [PubMed] [Google Scholar]
- 27.Covic A, Luminita I, Apetrei C, et al. Hepatitis virus infection in haemodialysis patients from Moldavia. Nephrol Dial Transplant 1999;14:40–5. [DOI] [PubMed] [Google Scholar]
- 28.Fabrizi F, Bacchini G, Pontoriero G, et al. Antibodies to hepatitis C virus (HCV) and transamniase concentration in chronic hemodialysis patients: A study with second generation assay. Nephrol Dial Transplant 1993;8:744–7. [DOI] [PubMed] [Google Scholar]
- 29.Gretch D, de la Rosa C, Carithers RL, et al. Assessment of hepatitis C viremia using amplification technologies: Correlations and clinical implications. Ann Intern Med 1995;123:321–9. [DOI] [PubMed] [Google Scholar]
- 30.de Medina M, Schiff ER. Hepatitis C: diagnostic assays. Semin Liver Dis 1995;15:33–40. [DOI] [PubMed] [Google Scholar]
- 31.Bukh J, Wantzin P, Kroogsgard K, et al. High prevalence of hepatitis C virus (HCV) RNA in dialysis patients: Failure of commercially available antibody tests to identify a significant number of patients with HCV infection. J Infect Dis 1993;168:1343–8. [DOI] [PubMed] [Google Scholar]
- 32.Fernandez JL, del Pino N, Lef L, et al. Serum hepatitis C RNA in anti-HCV negative hemodialysis patients. Dial Transplant 1996;25:14–18. [Google Scholar]
- 33.Kuhns M, de Medina M, McNamara A, et al. Detection of hepatitis C virus RNA in hemodialysis patients. J Am Soc Nephrol 1994;4:1491–7. [DOI] [PubMed] [Google Scholar]
- 34.Schroter M, Feucht HH, Schafer P, et al. High percentage of seronegative HCV infections in hemodialysis patients: the need for PCR. Intervirology 1997;40:277–8. [DOI] [PubMed] [Google Scholar]
- 35.Seelig R, Renz M, Bottner C, et al. Hepatitis C virus infections in dialysis units: prevalence of RNA and antibodies to HCV. Ann Med 1994;26:45–52. [DOI] [PubMed] [Google Scholar]
- 36.Fabrizi F, Lunghi G, Raffaele L, et al. Serologic survey for control of hepatitis C in hemodialysis patients: Third generation assays and analysis of costs. Nephrol Dial Transplant 1997;12:298–303. [DOI] [PubMed] [Google Scholar]
- 37.Moyer LA, Alter MJ. Hepatitis C virus in the hemodialysis setting: a review with recomendations for control. Semin Dial 1994;7:124–7. [Google Scholar]
- 38.De Medina M, Ashby M, Schlüter V, et al. Prevalence of hepatitis C and G virus infection in chronic hemodialysis patients. Am J Kidney Dis 1998;31:224–6. [DOI] [PubMed] [Google Scholar]
- 39.Blumberg A, Zehnder C, Burckhardt JJ. Prevention of hepatitis C infection in haemodialysis units: A prospective study. Nephrol Dial Transplant 1995;10:230–3. [PubMed] [Google Scholar]
- 40.Simon N, Courouce AM, Lemarrec N, et al. A twelve year natural history of hepatitis C infection in hemodialysed patients. Kidney Int 1994;46:504–11. [DOI] [PubMed] [Google Scholar]
- 41.Guh JY, Lai YH, Yang CY, et al. Impact of decreased serum transaminase levels on the evaluation of viral hepatitis in hemodialysis patients. Nephron 1995;69:459–65. [DOI] [PubMed] [Google Scholar]
- 42.Fabrizi F, Lunghi G, Andrulli S, et al. Influence of serum HCV RNA upon aminotransfrerase activity in chronic dialysis patients. Nephrol Dial Transplant 1997;12:1295–300. [DOI] [PubMed] [Google Scholar]
- 43.Stehman-Breen CO, Emerson S, Gretch D, et al. Risk of death among chronic dialysis patients infected with the hepatitis C virus. Am J Kidney Dis 1998;32:629–34. [DOI] [PubMed] [Google Scholar]
- 44.Palitzsch KD, Hottenträger B, Schlottmann K, et al. Prevalence of antibodies against hepatitis C in the adult German population. Eur J Gastroenterol Hepatol 1999;11:1215–20. [DOI] [PubMed] [Google Scholar]
- 45.Fabrizi F, Locatelli F. Hepatitis C virus in the haemodialysis units: novel insights by new techniques? Nephrol Dial Transplant 1999;14:1072–5. [DOI] [PubMed] [Google Scholar]
- 46.Müller R. The natural history of hepatitis C: clinical experiences. J Hepatol 1996;24(suppl 2):52–4. [PubMed] [Google Scholar]
- 47.Roth WK, Weber M, Seifried E. Feasibility and efficacy of routine PCR screening of blood donations for hepatitis C virus, hepatitis B virus, and HIV-1 in a blood-bank setting. Lancet 1999;353:359–63. [DOI] [PubMed] [Google Scholar]