Abstract
Background and aim: The aim of this study was to systematically analyse the pattern of regeneration in human acute pancreatitis by testing whether pancreatic stellate cells, their myofibroblastic offspring, and pancreatic ductules are involved in the regenerative process.
Patients and methods: Between January 1994 and November 2000, 24 necrosectomy specimens containing vital tissue were obtained for pathological examination. Formalin fixed tissue samples were routinely processed and immunostained for cytokeratins 7 and 19, smooth muscle actin, desmin, Ki-67, and CD68. Pancreatic tissue from organ donors served as normal controls.
Results: Necrosectomy specimens were obtained between 11 and 41 days after the onset of symptoms. In vital areas of necrosectomy samples, spherical hypercellular spheres consisting of loose vascular connective tissue occurred, in part showing duct-like profiles which sprouted from remnant exocrine tissue almost perpendicular to the periphery of the spheres. In normal tissue, only a few stellate cells and myofibroblasts were present around ducts and ductules. In contrast, numerous stellate cells and myofibroblasts were detected in the hypercellular regenerative spheres after acute pancreatitis, both being situated within the loose tissue and forming compact periductular sheaths. Stellate cells/myofibroblasts and ductule cells exhibited increased proliferative activity.
Conclusions: Pancreatic stellate cells and their activated myofibroblastic offspring may participate in regeneration after acute necrotising pancreatitis in humans. Time course studies are needed to further strengthen this regeneration concept.
Keywords: acute pancreatitis, pancreatic regeneration, stellate cells, proliferating ductules
Acute pancreatitis encompasses a whole spectrum of inflammatory lesions in the pancreas. In accordance with a recent proposition, these lesions are being described using the terms, severe acute pancreatitis, mild acute pancreatitis, acute fluid collections, pancreatic necrosis, and acute pseudocysts.1 The aetiology and pathogenesis of necrosis and haemorrhage as hallmarks of severe acute pancreatitis have been studied in detail, and several models of pathogenic pathways have recently been developed.2–5 The end result of an acute attack of acute pancreatitis is a well characterised type of pancreatic and peripancreatic tissue breakdown ranging from interstitial oedema and low grade multifocal necrosis of the pancreas (mild acute pancreatitis) to massive haemorrhagic necrosis.1,6–8 In patients who survive, at least some of these lesions are generally considered to be reversible, in contrast with the overall progressive character of chronic pancreatitis. If necrotic tissue is present it may become infected or forms fluid collections containing debris. These acute fluid collections may disappear spontaneously or develop into pseudocysts. What has not been specifically addressed is the question as to whether, during the time period between the initiation of necrosis and necrosectomy, repair and/or regeneration of the exocrine apparatus occurs. Theoretically, regeneration of pancreatic exocrine tissue destroyed by acute pancreatitis requires replacement of both epithelial cell populations and cells forming the matrix, allowing an ordered regrowth of lost epithelia.
Recently, stellate cells representing the homologue of the respective cells occurring in the liver have been identified in the pancreas.9 Pancreatic stellate cells have been shown to play a significant pathogenic role in fibrogenesis and in particular in mechanisms involved in fibrosis occurring in chronic pancreatitis.10 In contrast, it has not been established whether pancreatic stellate cells are involved in remodelling and regenerative mechanisms ensuing after acute necrotising pancreatitis in humans.
In the present investigation in patients with acute necrotising pancreatitis, we systematically studied the roles of two cell systems holding a key position in the maintenance of pancreatic tissue homeostasis in regeneration after severe pancreatitis: ductular complexes and pancreatic stellate cells.
PATIENTS AND METHODS
Patients
Between January 1994 and November 2000, 42 necrosectomy specimens from patients with acute necrotising pancreatitis were obtained for pathological examination. Of these specimens, 18 consisted of completely necrotic tissue exclusively. The remaining 24 specimens contained vital tissue structures and were used for the present investigation. These samples were obtained from eight female and 16 male patients (median age 59 years; range 28–71; 95% confidence interval (CI) 50–61.3) between 11 and 41 days after the onset of symptoms. The extent of necrosis was assessed preoperatively as previously described.11 The pertinent data are summarised in table 1 ▶. Pancreatic tissue from organ donors served as controls.
Table 1.
Characteristics of the 24 patients with vital tissue in the necrosectomy specimen
| Sex (F/M) | 8/16 |
| Median age (y)* | 59 (28–71; 50–61.3) |
| APACHE II score* | 10.5 (2–21; 8.2–13) |
| C reactive protein (mg/l)* | 324 (91–456; 181–379) |
| Day of surgery* | 23.5 (11–41; 16–31) |
| Biliary aetiology | 8/24 (33%) |
| Alcohol overindulgence | 11/24 (46%) |
| Other or unknown aetiology | 5/24 (21%) |
| Extent of necrosis more than 50% of the gland | 14/24 (58%) |
| Pancreatic infection | 22/24 (92%) |
*Values are median (range; 95% confidence interval).
APACHE, acute physiology and chronic health evaluation.
Histology and immunohistochemistry
Randomly chosen samples were fixed in 4% neutral buffered formalin and embedded in paraffin. Standard morphological evaluation was based on haematoxylin and eosin stained sections. For immunohistochemistry, deparaffinised sections were rehydrated through a graded series of ethanol and brought into Tris buffered saline. Depending on the antibody used, sections were subjected to trypsin digestion (Difco, Detroit, Michigan, USA) or to heat induced epitope retrieval in 10 mM citrate buffer, pH 6.0, either in a microwave oven or in a pressure cooker. Primary antibodies were directed against cytokeratin (CK)-7 (clone OV-TL 12/30; Dako Diagnostics AG, Zug, Switzerland; working concentration 2 7mu;g/ml; trypsin pretreatment), CK-19 (clone RCK108; Dako; 0.8 7mu;g/ml; trypsin), desmin (clone D33; Dako; 5 7mu;g/ml; microwave), α smooth muscle actin (SMA, clone 1A4 ; Sigma, St Louis, Missouri, USA; dilution 1:600; no pretreatment), CD68 (clone PG-M1; Dako; 2.5 7mu;g/ml; microwave), and Ki-67 (clone MIB1; Dako; 1 7mu;g/ml; pressure cooker). After the primary antibody a biotinylated goat-antimouse Ig antibody (Dako) was applied, followed by streptavidin-biotin complex/alkaline phosphatase (Dako). Sections were developed in new fuchsin-naphtol AS-BI (Sigma), counterstained with haematoxylin, and mounted. For double immunohistochemistry, stainings for the respective antibodies employed an avidin-biotin complex/horseradish peroxidase system (Vector, Burlingame, California, USA) and 3,3-diaminobenzidine as chromogen, and a streptavidin-biotin complex/alkaline phosphatase system as outlined above, respectively. For immunohistochemistry, positive control sections were processed simultaneously. For estimation of the proliferation index (in per cent; labelled nuclei/all nuclei counted×100) of stellate cells and ductule cells, in acute pancreatitis and in normal controls, Ki-67/SMA and Ki-67/CK-7 double immunostains were used. In these preparation, five areas were randomly chosen, and in each area 300 nucleated cells of interest were counted and analysed for the presence of labelled nuclei.
Statistics
All clinical data were collected prospectively and entered into a statistical package program (SPSS Statistical Software, Chicago, Illinois, USA) on a personal computer. Data were analysed using Fisher’s exact test or the Mann Whitney U test where appropriate.
RESULTS
Normal pancreatic tissue: qualitative analysis
Control tissue was structurally normal. Reactivity for CK-19 was markedly present in epithelia of large and small ducts, and less so in ductules, whereas a marked positivity for CK-7 was detected in both ductal and ductular epithelial cells. SMA was strongly expressed in vascular muscle cells serving as an internal control. SMA reactive spindle and stellate cells were present around pancreatic ducts and, in smaller numbers, around ductules, mainly at their points of exit from acini. Few of these cells were observed around acinar cells close to ductules. Few desmin positive stellate and spindle cells were present around ducts and ductules (data not shown).
Acute pancreatitis: qualitative analysis
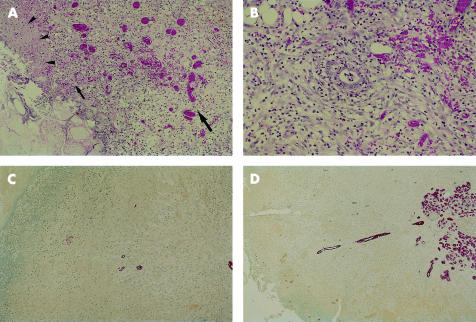
Analysis of necrosectomy samples obtained at different time points after the onset of clinical symptoms showed that some (see below) contained spherical structures of preserved tissue. These vascularised structures, which were clearly demarcated from necrotic tissue (fig 1A ▶), were interpreted to represent a regenerative response, and were therefore termed regenerative spheres. Their histological characteristics are described in detail in figs 1–3 ▶ ▶ ▶.
Figure 1.
(A) Border of a hypercellular regenerative sphere. Note the distinct zonation of this lesion. In direct contact with an area of complete necrosis (bottom left hand corner) there is a thin layer of fibrin rich exudate (zone 1: arrowheads) sharply separating the vital regenerative spheres from the surrounding necrosis, followed by a hypovascular zone rich in leucocytic infiltrates (zone 2: small arrow). More inside, a highly vascular granulation tissue of steadily increasing maturity is seen (zone 3: large arrow) (haematoxylin and eosin stain, ×120). (B) Zone 3 of a hypercellular regenerative sphere. Within this zone of granulation tissue a small duct-like profile is seen (centre of the figure). Note that this structure is encircled by a mantle of spindle cells (haematoxylin and eosin stain, ×200). (C) Peripheral parts of a hypercellular regenerative sphere; the necrosis interface is represented at the top left hand corner. Small ductules are scattered within the loose and matrix-rich tissue. The peripheral most part of such a ductule is seen in the form of small epithelial cell cluster (red) whereas more centrally placed parts exhibit more mature cells (cytokeratin 7 (CK-7) immunostain, ×80). (D) In a larger regenerative sphere, a cytokeratin positive ductule takes its origin from a residual pancreatic lobule, sprouting from here to the periphery of the hypercellular regenerative sphere forming long and ordered structures (CK-7 immunostain, ×80).
Figure 2.
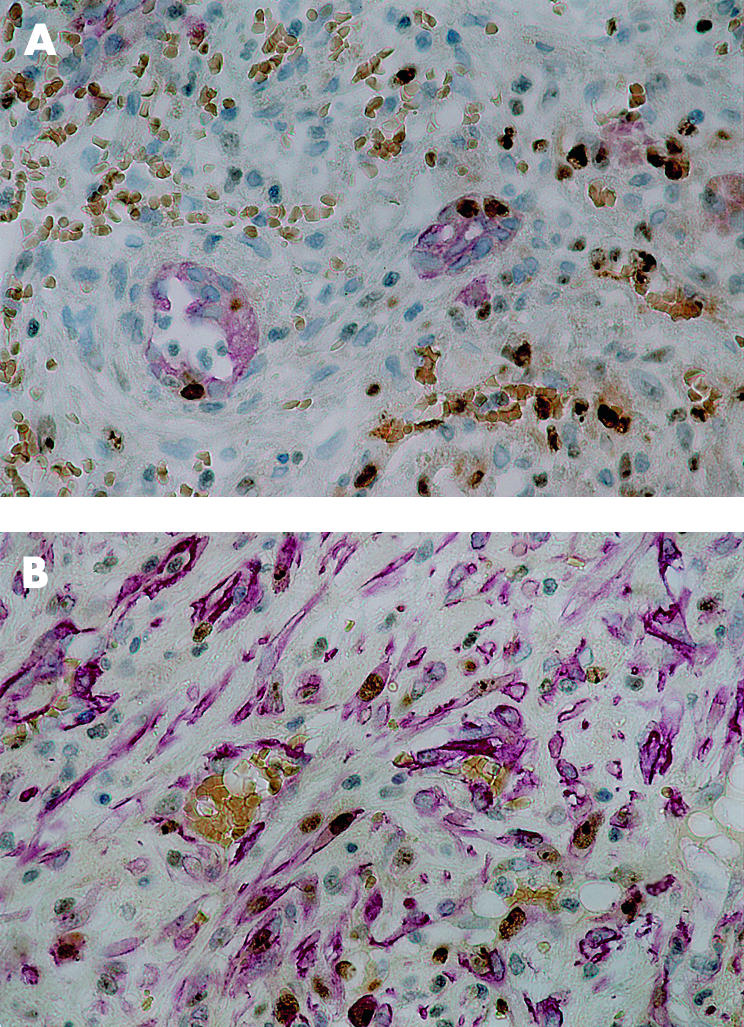
(A) Proliferative activity in ductules. Epithelia of two ductular profiles are red (cytokeratin 7 (CK-7)) whereas nuclei of proliferating cells are in brown (Ki-67). Note that the ductular profiles contain labelled nuclei. Proliferating cells are also visualised in the connective tissue surrounding the ductules (CK-7/Ki-67 double immunostain, ×400). (B) Proliferative activity in stellate cells/myofibroblasts. α Smooth muscle actin (SMA) expressing stellate cells are in red, nuclei of proliferating cells (Ki-67) are in brown. Several stellate cells contain labelled nuclei indicating proliferation (SMA/Ki-67 double immunostain, ×400)
Figure 3.
(A) Loose regenerating tissue of a hypercellular regenerative sphere contains several desmin reactive cells representing elongated forms of pancreatic stellate cells (desmin immunostain, ×400). (B) Zones 1 and 2 of a regenerative sphere. Several α smooth muscle actin (SMA) reactive myofibroblasts are seen with a burst-like orientation towards the interface with necrosis. One myofibroblast follows an immature blood vessel (SMA immunostain, ×120). (C) SMA reactive myofibroblasts situated in peripheral zones of regenerative spheres exhibit cytoplasmic projections, in part producing a stellate morphology (SMA immunostain, ×400). (D) A terminal (peripheral) segment of a pilot ductule (red) presents in the form of epithelial cell clusters. Note that myofibroblasts (brown) are in close contact with ductular cells (CK-7 and SMA double immunostain, ×400). (E) Pilot ductule (cytokeratin 7 (CK-7) immunostain; red). A periductular mantle-like spindle cell sheath is detectable, encircled by a vascular arcade (×200). (F) Proximal and therefore more mature segments of pilot ductules are encircled by a compact sheath of myofibroblasts The centre shows a pilot ductule with irregular arrangement of epithelial cells. The spindle cells forming the now compact periductular sheath are markedly positive for SMA, these cells representing myofibroblasts (double immunostain: CK-7, red; SMA, brown) ×400).
Already in conventionally stained sections the ductules (fig 1B ▶) were seen to sometimes almost reach the periphery of the regenerative spheres. Some of the regenerative spheres contained a single or a few artery branches apparently feeding the vascular channels forming the capillary network of the more peripherally located granulation tissue.
The presence and distinct orientation of ductules was confirmed by immunohistochemical staining. In both CK-7 and CK-19 preparations, the immunophenotype and structure of these profiles clearly corresponded to pancreatic ductules in variable phases of differentiation, and probably formed the tip of an advancing ductular profile (fig 1C, D ▶). As, together with artery branches, the ductular outgrowths appeared to determine the overall growth pattern and layout of regenerative spheres, we termed these structures pilot ductules. Around pilot ductules the arrangement of spindle cells was more compact. Based on dual immunostaining (CK-7/Ki-67 and SMA/Ki-67), proliferative activity was seen in ductules and stellate cells (fig 2A, B ▶).
In summary, pilot ductules sprouting from lobular remnants to the periphery of regenerative spheres were shown to grow out in close association with a population of activated pancreatic stellate cells forming a distinct periductular sheath with a characteristic maturation gradient reflecting a temporal order in the development of these structures.
Acute pancreatitis: quantitative analysis
For quantitative analysis of histological and immunohistochemical findings, the following features were assessed separately in all samples: hypercellular regenerative spheres; zonation of regenerative spheres; presence of feeding arteries in regenerative spheres; presence of CK-7 and CK-19 reactive ductules; density of ductules in central and peripheral parts of regenerative spheres; presence of desmin reactive pancreatic stellate cells; presence of loosely arranged SMA reactive myofibroblasts; and presence of thick periductular myofibroblast sheaths.
Regenerative spheres were observed in each of the 24 necrosectomy specimens examined in the study. Of the 24 cases of acute pancreatitis analysed histologically, 18 (75%) showed one or more feeding arteries. A characteristic zonation with formation of a gradient of ductule containing granulation tissue was present in 100% of regenerative spheres. CK-7 and CK-19 reactive ductules of varying maturity were detectable in 12/24 (50%) regenerative spheres, and in 8/12 (66.6%) spheres typical pilot ductules reaching from the centre of regenerative spheres to the periphery were observed. In 100% of regenerative spheres, increased numbers of pancreatic stellate cells and myofibroblasts were in evidence, being located both in a central and peripheral position of regenerative spheres in 21/24 (88%) samples. Pilot ductules with a thick and ordered ductulocentric sheath of SMA reactive cells were present in 9/12 samples where ductules were found (75%).
For samples revealing pilot ductules, the earliest time point where such structures were in evidence was 17 days after the start of symptoms, the time periods ranging from 11 days to 41 days.
For stellate cells/myofibroblasts, the mean Ki-67 proliferation index was 22.2% (range 8–35%; normal controls 0.26%, range 0–0.6%). Increased proliferative activity was also observed in ductule cells, mainly at the periphery of regenerative spheres and the tip area of ductules (mean proliferation index 3.6%, range 1.9–5.8%; normal controls 0.13%, range 0–0.4%).
There was no correlation between aetiology, sex, C reactive protein value, APACHE II score, or extent of pancreatic stellate cell activation.
DISCUSSION
In this study we have shown that regeneration after acute necrotising pancreatitis in humans evolves in a distinct and highly ordered fashion and seems to be independent of the type of pancreatic injury causing the acute disease. The regenerative process involves pancreatic stellate cells, their differentiated myofibroblastic offspring, and pancreatic ductules originating from remnant lobules, suggesting that stellate cells/myofibroblasts and pilot ductules represent a structural and functional unit growing in parallel.
Recent evidence suggests that pancreatic stellate cells represent a key cell type for pancreatic fibrogenesis and remodelling, but their role in acute pancreatitis has not yet been clarified. Vitamin A storing cells in the pancreas were originally observed in 1982 in mice fed an excess of this vitamin12 but these cells were first described in the human pancreas eight years later.13 A potential role of this cell system in pancreatic fibrogenesis was suggested via isolation of myofibroblast-like cells from the human pancreas,14 and it has been demonstrated that vitamin A storing cells from pancreas can in fact differentiate in primary culture into myofibroblasts producing extracellular matrix proteins.15 Similar to rat periportal, but not pericentral, hepatic stellate cells,16 pancreatic stellate cells can express desmin whereas myofibroblasts derived from these cells are typically reactive for SMA.17,18 In contrast with the liver, where most but not all of the stellate cells are located within the perisinusoidal space of Disse (the so-called littoral compartment), stellate cells in the pancreas appear to be mainly situated in a periacinar location.14 Their preference for this tissue space is of interest insofar as it has been shown that hepatic stellate cells are also located close to ductules in portal tracts of the liver, thus forming an extralittoral or extrasinusoidal compartment.19
It has been demonstrated that pancreatic stellate cells, similar to their hepatic counterpart, play a pathogenic role in fibrosis.20 In chronic alcoholic pancreatitis, active synthesis of collagen by stellate cells appears to co-localise with lipid peroxidation derived aldehydes,21 and exposure to ethanol or acetaldehyde led to cell activation in cultured rat pancreatic stellate cells.22 Mechanisms involved in the activation of pancreatic stellate cells have been shown to include transforming growth factor β, in part derived from activated macrophages,20,23 interleukin 1β, and tumour necrosis factor α, inducing secretion of interleukin 8, monocyte chemotactic protein 1, and RANTES,24 and platelet derived growth factors.25
It is of particular interest that pancreatic stellate cells and their activated offspring develop and grow in a non-random fashion within the regenerative spheres. On the one hand, the cells appear to evolve at the same rate as the growing regenerating tissue, forming highly oriented structures. On the other hand, they form a distinct compound structure or unit together with proliferating ductules, establishing a myofibroblastic periductular sheath. These epitheliomesenchymal sprouts are most probably recruited from pre-existing structures located in remnant lobules, and progressively extend into the growing regenerating sphere ductules thereby acting as pilot elements. A ductular reaction ensuing after acute pancreatitis, termed tubular complexes, has previously been observed in experimental models,26 and these complexes were derived from altered acinar cells proliferating 4–7 days after initiation of pancreatitis. In addition, it has been shown that pancreatic repair following trypsin induced necrohaemorrhagic pancreatitis involved proliferation of cells from intact acini and from tubular complexes.27
These findings suggest that pancreatic stellate cells may not only be involved in fibrogenesis but also in tissue remodelling. Thus they mimic their hepatic analogues where it has recently been shown that myofibroblasts in the rat liver reflect the degree of hepatic remodelling rather than cirrhosis inasmuch as the myofibroblast volume fraction inversely reflects hepatocyte volume bimodality,28 suggesting that ductular complexes and stellate cells act as pacemakers in tissue remodelling.
The mechanisms operational in the phenomena observed in the present study are not known.
In conclusion, the results of this study suggest that pancreatic stellate cells and their activated myofibroblastic offspring may participate in regeneration after acute necrotising pancreatitis. Time course studies are needed to further strengthen this regeneration concept.
Abbreviations
SMA, α smooth muscle actin
CK, cytokeratin
REFERENCES
- 1.Bradley ELR. A clinically based classification system for acute pancreatitis. Arch Surg 1993;128:586–90. [DOI] [PubMed] [Google Scholar]
- 2.Glasbrenner B, Adler G. Pathophysiology of acute pancreatitis. Hepatogastroenterology 1993;40:517–21. [PubMed] [Google Scholar]
- 3.Rattner D. Experimental models of acute pancreatitis and their relevance to human disease. Scand J Gastroenterol Suppl 1996;219:6–9. [DOI] [PubMed] [Google Scholar]
- 4.Furue S, Hori Y, Kuwabara K, et al. Increased activity of group II phospholipase A2 in plasma in rat sodium deoxycholate induced acute pancreatitis. Gut 1997;41:826–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uhl W, Schrag HJ, Schmitter N, et al. Pathophysiological role of secretory type I and II phospholipase A2 in acute pancreatitis: an experimental study in rats. Gut 1997;40:386–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bockman DE, Büchler M, Beger HG. Ultrastructure of human acute pancreatitis. Int J Pancreatol 1986;1:141–53. [DOI] [PubMed] [Google Scholar]
- 7.Bockman DE. Morphology of the exocrine pancreas related to pancreatitis. Microsc Res Tech 1997;37:509–19. [DOI] [PubMed] [Google Scholar]
- 8.Kloppel G, Maillet B. Pathology of acute and chronic pancreatitis. Pancreas 1993;8:659–70. [DOI] [PubMed] [Google Scholar]
- 9.Pinzani M. New kids on the block: pancreatic stellate cells enter the fibrogenesis world. Gut 1999;44:451–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wells RG, Crawford JM. Pancreatic stellate cells: the new stars of chronic pancreatitis? Gastroenterology 1998;115:491–3. [DOI] [PubMed] [Google Scholar]
- 11.Büchler MW, Gloor B, Müller CA, et al. Acute necrotizing pancreatitis: Treatment strategy according to the status of infection. Ann Surg 2000;232:619–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watari N, Hotta Y, Mabuchi Y. Morphological studies on a vitamin A-storing cell and its complex with macrophage observed in mouse pancreatic tissues following excess vitamin A administration. Okajimas Folia Anat Jpn 1982;58:837–58. [DOI] [PubMed] [Google Scholar]
- 13.Ikejiri N. The vitamin A-storing cells in the human and rat pancreas. Kurume Med J 1990;37:67–81. [DOI] [PubMed] [Google Scholar]
- 14.Saotome T, Inoue H, Fujimiya M, et al. Morphological and immunocytochemical identification of periacinar fibroblast-like cells derived from human pancreatic acini. Pancreas 1997;14:373–82. [DOI] [PubMed] [Google Scholar]
- 15.Bachem MG, Schneider E, Gross H, et al. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology 1998;115:421–32. [DOI] [PubMed] [Google Scholar]
- 16.Ballardini G, Groff P, Badiali de Giorgi L, et al. Ito cell heterogeneity: desmin-negative Ito cells in normal rat liver. Hepatology 1994;19:440–6. [PubMed] [Google Scholar]
- 17.Apte MV, Haber PS, Applegate TL, et al. Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut 1998;43:128–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gabbiani G. The cellular derivation and the life span of the myofibroblast. Pathol Res Pract 1996;192:708–11. [DOI] [PubMed] [Google Scholar]
- 19.Zimmermann A, Zimmermann H, Fellay M, et al. Cells with morphological and immunohistochemical features of hepatic stellate cells (Ito cells) form an extralittoral (extrasinusoidal) compartment in the cirrhotic rat liver. Histol Histopathol 1999;14:719–27. [DOI] [PubMed] [Google Scholar]
- 20.Haber PS, Keogh GW, Apte MV, et al. Activation of pancreatic stellate cells in human and experimental pancreatic fibrosis. Am J Pathol 1999;155:1087–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casini A, Galli A, Pignalosa P, et al. Collagen type I synthesized by pancreatic periacinar stellate cells (PSC) co-localizes with lipid peroxidation-derived aldehydes in chronic alcoholic pancreatitis. J Pathol 2000;192:81–9. [DOI] [PubMed] [Google Scholar]
- 22.Apte MV, Phillips PA, Fahmy RG, et al. Does alcohol directly stimulate pancreatic fibrogenesis? Studies with rat pancreatic stellate cells. Gastroenterology 2000;118:780–94. [DOI] [PubMed] [Google Scholar]
- 23.Schmid-Kotsas A, Gross HJ, Menke A, et al. Lipopolysaccharide-activated macrophages stimulate the synthesis of collagen type I and C-fibronectin in cultured pancreatic stellate cells. Am J Pathol 1999;155:1749–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andoh A, Takaya H, Saotome T, et al. Cytokine regulation of chemokine (IL-8, MCP-1, and RANTES) gene expression in human pancreatic periacinar myofibroblasts. Gastroenterology 2000;119:211–19. [DOI] [PubMed] [Google Scholar]
- 25.Luttenberger T, Schmid-Kotsas A, Menke A, et al. Platelet-derived growth factors stimulate proliferation and extracellular matrix synthesis of pancreatic stellate cells: implications in pathogenesis of pancreas fibrosis. Lab Invest 2000;80:47–55. [DOI] [PubMed] [Google Scholar]
- 26.Elsasser HP, Adler G, Kern HF. Time course and cellular source of pancreatic regeneration following acute pancreatitis in the rat. Pancreas 1986;1:421–9. [DOI] [PubMed] [Google Scholar]
- 27.Lechene de la Porte P, Iovanna J, Odaira C, et al. Involvement of tubular complexes in pancreatic regeneration after acute necrohemorrhagic pancreatitis. Pancreas 1991;6:298–306. [DOI] [PubMed] [Google Scholar]
- 28.Zimmermann A, Zhao D, Reichen J. Myofibroblasts in the cirrhotic rat liver reflect hepatic remodeling and correlate with fibrosis and sinusoidal capillarization. J Hepatol 1999;30:646–52. [DOI] [PubMed] [Google Scholar]