Abstract
Background: Restraint stress induces permeability changes in the rat small intestine but little is known of the ultrastructural events leading to defects of the paracellular sealing or of the short term evolution of these alterations.
Methods: In the present study, we performed transmission electron microscopy in the terminal ileum perfused with lanthanum after two hours of immobilisation stress and in non-stressed control rats. Moreover, immunohistochemistry of the tight junction (TJ) associated proteins, occludin and zonula occludens 1 (ZO-1), was carried out together with western blot analysis of the transmembrane protein occludin. TJ morphology was also assessed after a 22 hour recovery period.
Results: Immobilisation stress induced a significant increase in epithelial permeability to the lanthanum tracer (p<0.005) which recovered completely after 22 hours. Compared with unstressed controls, in stressed rats no differences were found on freeze fracture analysis. The TJ related immunofluorescence signals of occludin and of ZO-1 were irregularly distributed in stressed rats after two hours but returned to a normal pattern at 24 hours although with minor intensity. No quantitative alterations in occludin were detectable in stressed rats by immunoblot whereas a perinuclear concentration of occludin was observed by immunolocalisation.
Conclusions: Immobilisation stress induced an increase in TJ permeability in the rat terminal ileum. These changes were mainly due to modifications and redistribution of the TJ transmembrane protein occludin and of the plaque protein ZO-1 whereas protein synthesis, at least that of occludin, was not affected by stress.
Keywords: occludin, ZO-1, tight junctions, intestinal permeability, immobilisation stress
Stress has a variety of effects on gastrointestinal function, including changes in gut motility, secretion, and absorption. It has been demonstrated in ex vivo preparations that cold restraint stress induces ion secretion and an increase in the paracellular fluxes of 3H mannitol and 51Cr ethylenediaminetetraacetic acid (EDTA) without histological alterations in the jejunum of a highly stress susceptible rat strain, the Wistar-Kyoto strain.1,2 As a result of the stress induced permeability changes, the same authors also demonstrated enhanced intercellular uptake of macromolecules3 hypothesising that this mechanism may contribute to the clinically described flare ups in patients with inflammatory bowel disease after stressful events.
Tight junctions (TJ) are located at the most apical part of lateral membranes of epithelial cells and are thought to be involved in barrier functions creating a primary barrier to the diffusion of solutes through the paracellular pathway. In the past years, various TJ associated proteins have been identified, including three transmembrane proteins or protein families (occludin, claudins 1–16, and junction adhesion molecule) and intracytoplasmic proteins (zonula occludens 1 (ZO-1) and 2 (ZO-2)), members of the membrane associated guanylate kinase (MAGUK) protein family. Junctional proteins without known functions include ZO-3, cingulin, p130, 7H6, symplekin, and ZA-1TJ (for more information see4–8). The most intensively investigated proteins are occludin and ZO-1 but studies concerning these proteins are mostly limited to in vitro experiments on different cell lines whereas their function or regulation in the intact organism are not adequately known.
Therefore, the present study was designed to evaluate in vivo the effect of stress on: (a) ileal TJ permeability to the electrondense marker lanthanum nitrate, (b) appearance of TJ freeze fracture replicas, (c) immunofluorescence of two TJ associated proteins, occludin and ZO-1, and (d) protein expression of occludin.
METHODS
Animals
Male Sprague-Dawley rats weighing 200–225 g were purchased from Charles River (Calco, Italy). The animals were kept under controlled environmental conditions with reference to light, temperature, and humidity, with free access to tap water and standard laboratory chow. The animals were handled daily for 10 days to minimise the stress of handling. The experimental protocols were approved by the Veterinary and Health Committees of the University of Messina.
Materials
Lanthanum nitrate (molecular weight 433.02), glutaraldehyde (25%), osmium tetroxide (4%), and cacodylate buffer were from Società Italiana Chimici (Rome, Italy). EM bed-812 resin was purchased from Electron Microscopy Sciences (Bio-Optica, Milan, Italy). Common laboratory reagents were obtained from Sigma Chemical Company (Milan, Italy). Diethylether and chloralium hydrate were purchased from Carlo Erba (Milan, Italy).
Experimental protocol
Immobilisation stress was induced in 16 non-fasted animals by fixation for two hours of the four extremities with an adhesive tape under brief ether anaesthesia. As faecal pellet output is known to increase under stress conditions,9 faeces were collected to document the stress effect during the two hour stress period. Sixteen rats served as “ether controls” and were only briefly anaesthetised with ether and thereafter allowed to move freely in their cages over the following two hours. Eight rats (controls) were sacrificed after a two hour observation period that included only a brief initial handling of the animals. All experiments were carried out between 8.00 and 10.00 am to minimise any effect of the circadian rhythm.
Four stressed animals, four ether controls, and four controls were anaesthetised two hours after the start of the experiments by intraperitoneal injection of chloralium hydrate (400 mg/kg). Four stressed rats and four ether controls were sacrificed after a 22 hour recovery period after stress application. The abdomen was opened by a midline incision. The abdominal aorta was freed from surrounding tissue, cannulated, and proximally ligated directly below the diaphragm. The small intestine was flushed with warm saline via the canula for one minute after having transected the vena porta in order to avoid tissue damage due to pressure overload. Subsequently, via the same canula, the small intestine was fixed by perfusion (2 ml/min) with fixative containing 2.5% glutaraldehyde and 4% lanthanum nitrate in 0.1 M cacodylate buffer, pH 7.4. (For details of the preparation of the lanthanum solution see Fries and colleagues10 and Lora and colleagues11). The terminal ileum was removed, cut into 2 mm2 pieces, and left in 2.5% glutaraldehyde for two hours. For further preparation see below.
Four animals per group (stress, ether control, control) were also sacrificed two hours after the start of the experiment and ileal tissue specimens were harvested for freeze fracture studies (n=3 stressed rats and controls), histology, immunohistochemistry, and western blot analysis (n=4 for all three groups). The stomach of these animals was opened along the greater curvature and examined for macroscopic lesions. For sample preparation see below. The remaining animals (four stress, four ether controls) were killed at 24 hours for histology, immunohistochemistry, and western blot.
Optical microscopy
After fixation for one week at room temperature in Dietrich solution (14.25% ethanol, 1.85% formaldehyde, 1% acetic acid), samples were dehydrated in graded ethanol and embedded in paraplast (Sherwood Medical, Mahwah, New Jersey, USA). Thereafter, 7 μm sections were deparaffinised with xylene, stained with alcian blue/periodic acid-Schiff and observed in a Dialux 22 microscope (Leitz, Wetzlar, Germany).
Transmission electron microscopy
After fixation, specimens were rinsed for 30 minutes in 0.1 M cacodylate buffer and post-fixed for one hour at 4°C in 1% osmium tetroxide. Subsequently, tissue samples were dehydrated by graded ethanol and embedded in EM bed-812 resin. Fragments were cut into 100 Å sections with an Ultramicrotome System 2128 (Ultratome, Bromme, Germany) ultramicrotome and examined in a Hitachi H-600 (Hitachi Ltd, Tokyo, Japan) at 50 kV at a magnification of 35 000×. From each animal an average of 250 consecutive TJ were observed by an observer unaware of the relative treatment (EM), avoiding those next to goblet cells known to have minor resistance. TJ were considered to have increased permeability where the electrondense marker penetrated into the junctional complex and electrondense material was identified on the luminal surface (see Fries and colleagues10 and Fallon and colleagues12). Results are expressed as percentage of “leaky” junctions over continent junctional complexes.
Freeze fractures
In six animals (three controls and three stress) the terminal ilea were fixed in 2% glutaraldehyde in 0.1 M cacodylate buffer for 30 minutes and preserved at 4°C in 0.1 M cacodylate buffer in the presence of 3 M sucrose to avoid crystallisation. Fracturing of the apical portion of the intestinal villi was performed at −110°C in a vacuum chamber (Balzers Instruments, Liechtenstein). Specimens were shadowed with platinum/carbon vapour with an angle of 45° followed by carbon. Replicas were cleaned with commercial bleach, mounted on copper grids, and observed in a Hitachi H-600 at 50 kV at a magnification of 100 000×. The average strand number and depth were recorded for each sample, observing at least 10 valuable freeze fractures. Fractures next to goblet cells were not considered.
Immunohistochemistry
Indirect immunofluorescence staining was performed on 7 μm thick sections of unfixed tissue specimens. Sections were cut with a cryostat at −30°C, transferred onto clean glass slides, and dried overnight at room temperature. Sections were permeabilised with acetone at −20°C for 10 minutes and rehydrated with phosphate buffered saline (PBS, 150 mM NaCl, 20 mM sodium phosphate, pH 7.2) at room temperature for 45 minutes. Sections were incubated with rabbit polyclonal C terminal antioccludin antibody (150 AA) (Zymed Laboratories, San Francisco, California, USA; 1:500 in PBS, v/v) or with rabbit polyclonal anti-ZO-1 antibody raised against a 69 kDa fusion protein, corresponding to amino acids 463–1109 of human cDNA (Zymed Laboratories, San Francisco, California, USA; dilution 1:500 in PBS, v/v), in a humidified chamber for one hour at 37°C. Sections were washed with PBS and incubated with secondary antibody (Texas red or fluorescein isothiocyanate conjugated goat antirabbit antibody; both 1:100 in PBS, v/v) for one hour at 37°C. Sections were washed as before, and for nuclear staining 4`6-diamidino-2-phenylindole (DAPI; Hoechst, Frankfurt; Germany) 0.5 μg/ml in PBS was added (three minutes). Subsequently, sections were rinsed in PBS, mounted in PBS-glycerol (PBS containing 50% glycerol and 2.5 diazabicycle [2.2.2.] octane), and an average number of 20 sections per animal and tissue specimen were observed in a blinded fashion with a confocal laser microscope (LSM 510, Zeiss, Jena, Germany).
Tissue preparation for western blot analysis
Samples were homogenised in buffer (150 mM NaCl, 2 mM EDTA, containing 1% sodium dodecyl sulphate (SDS)) added with 10 μl/ml of the following solution: 1 mM phenylmethylsulphonyl fluoride, 10 mg/ml pepstatin A, 2 μg/ml aprotinin, 0.1 mM leupeptin, and 10 μl/ml benzamidine. Extracts were then centrifuged (10 000 g for five minutes at 4°C) to remove cellular particles. After centrifugation the supernatants were mixed 1:1 with SDS sample buffer and boiled for three minutes prior to SDS-polyacrylamide gel electrophoresis (SDS-PAGE). Protein concentrations of extracts were evaluated spectrophotometrically to load identical amounts per well.
Gel electrophoresis and immunoblotting
SDS-PAGE (12% polyacrylamide) was carried out on Bio-Rad Minigels and were stained with Coomassie brillant blue R-250 (0.25% in 10% acetic acid, 50% methanol).
For immunoblotting, proteins were electrophoretically transferred from gels onto nitrocellulose (Schleicher and Schuell, 0.1 μm pore size), blocked with 10% non-fat milk in Tris buffered saline (TBS, 20 mM Tris-HCl, pH 7.5, 0.5% NaCl), incubated over three hours in TBS-milk containing antioccludin antibody (1:1000), followed by antirabbit IgG conjugated with alkaline phosphatase (Promega, 1:5000 in TBS milk), and developed with alkaline phosphatase colour reagent (bromochloroindolyl phosphate and nitroblue tetrazolium substrate). MW-SDS-BLUE (Sigma) was used as a reference standard for approximate molecular sizes in gels and blots (180 kDa, α2-macroglobulin; 116 kDa, β-galactosidase; 84 kDa, fructose-6-phosphate kinase; 56 kDa, pyruvate kinase; 48 kDa, fumarase; 36 kDa, lactic dehydrogenase; 26 kDa, triosophosphate isomerase).
Statistics
Results are given as mean (SEM) values. ANOVA and the Mann-Whitney U test were used when appropriate. A p value <0.05 was considered significant.
RESULTS
Faecal pellet output during the two hour immobilisation period was significantly higher than in non-stressed controls and ether controls, indicating effective stress induction (controls 0.42 (0.20) g; ether controls 0.72 (0.32) g; stress 7.61 (1.29) g; p<0.025) but gastric stress ulcers were never found. Light microscopy with alcian blue/periodic acid-Schiff stain revealed no alterations in the mucosa of the terminal ileum in stressed rats (data not shown) whereas the colon epithelium, as already described by Castagliuolo and colleagues,13 showed a reduction in goblet cells in the upper third of the crypt compartments and vacuolisation of goblet cells, also at the crypt bottom (fig 1 ▶).
Figure 1.
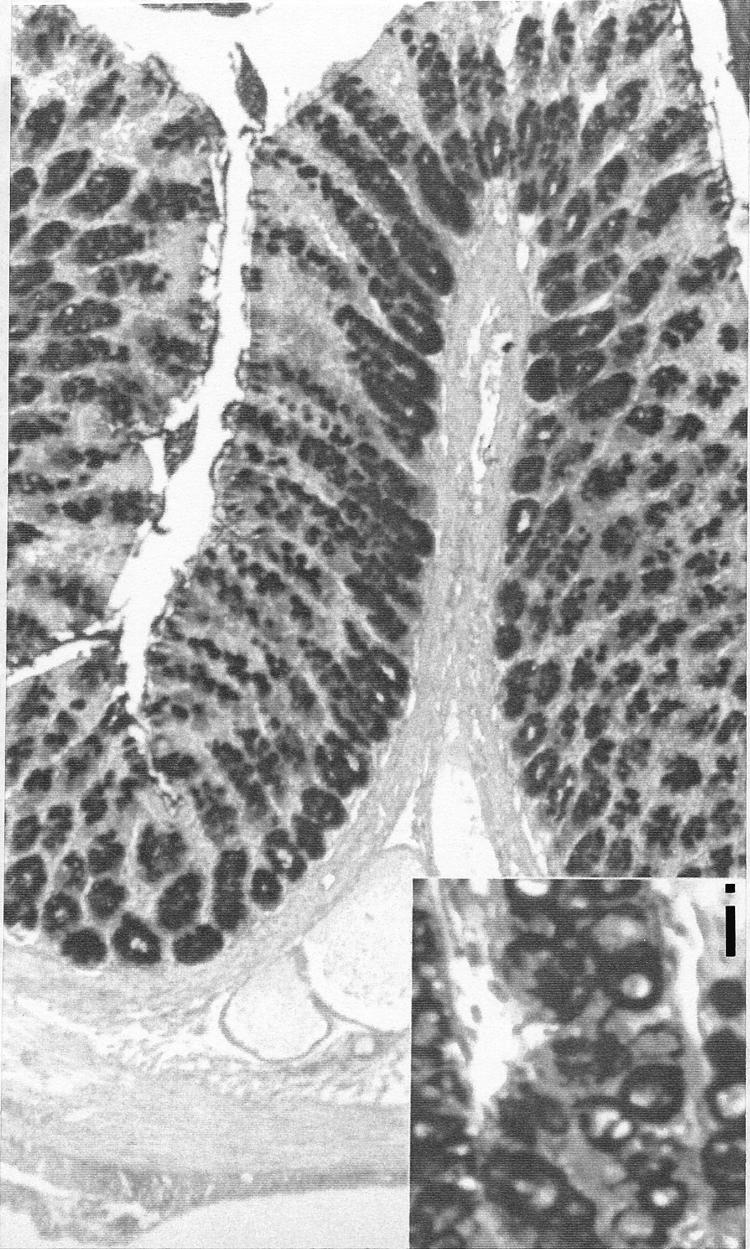
Alcian blue/periodic acid-Schiff staining of the colon after two hours of immobilisation stress showing a reduction in goblet cell density in the upper third of the crypt compartments with evident goblet cell vacuolisation (i: insertion 500×) also at the crypt bottom (magnification 100×).
Lanthanum perfusion (figs 2, 3 ▶ ▶)
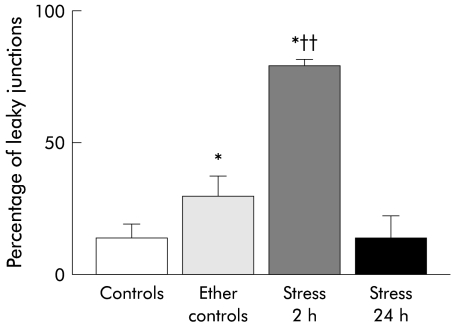
Figure 2.
Percentage of “leaky” junctions of the terminal ileum in control rats, ether controls, after two hours of immobilisation stress, and after a recovery period of 22 hours post-stress. Four animals per group. *p<0.03 or less versus controls; ††p<0.002 versus ether controls (Mann-Whitney U test). Data are mean (SEM).
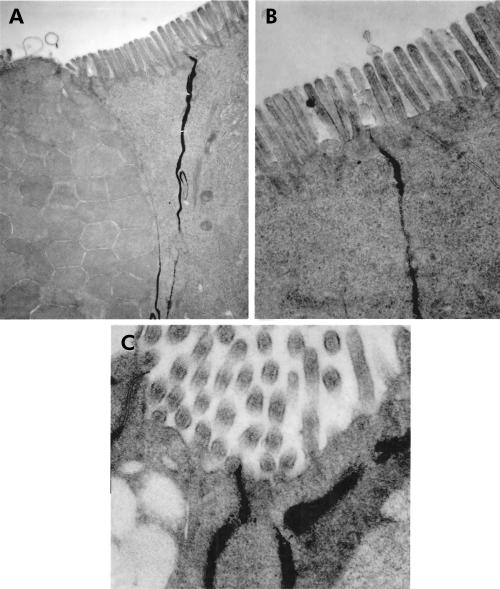
Figure 3.
Electron microscopy after perfusion with lanthanum nitrate after two hours of immobilisation stress (C) with evident penetration of the marker within the junctional complex, whereas in non-stressed controls (A) and in stressed rats after a recovery period of 22 hours (B) the marker is withheld below the zonula occludens (A: 19 500×; B: 38 500×; C: 55 000×).
An average number of 250 TJ per animal and tissue specimen were counted. In the terminal ileum the percentage of open TJ—that is, TJ where the lanthanum penetrates within the junctional complex—was significantly higher in animals stressed for two hours compared with ether controls (p<0.005). The percentage of open TJ of the ilea of ether controls was significantly higher compared with controls (p<0.05), indicating an important stress due to ether anaesthesia (controls 10 (4)%; ether controls 35 (8)%; stress-two hours 77 (2)%). No ultrastructural alterations in enterocytes were detectable (for example, mitochondria, endoplasmic reticulum, etc). Twenty four hours after the start of the experiment ileal permeability returned to control levels in stressed rats (stress-24 hours 6 (2)).
Freeze fractures (fig 4 ▶)
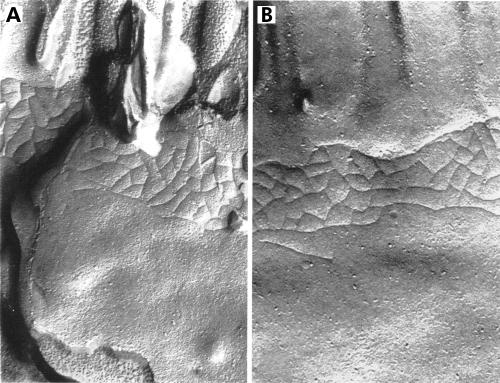
Figure 4.
Freeze fracture replicas of the terminal ileum of controls (A) and after two hours of immobilisation stress (B) showing normal anastomosing fibrils in both groups (magnification A and B: 70 000×).
There were no differences in strand counts (controls 3.43 (0.16); stress 3.39 (0.14); NS) or strand depth (controls 225 (11) nm; stress 220 (12) nm; NS) in the terminal ilea between stressed rats and non-stressed ether control animals. Because of the normality of fibrils in stressed animals, freeze fractures were not performed in stressed rats after 24 hours.
Immunohistochemistry (figs 5–7 ▶ ▶ ▶)
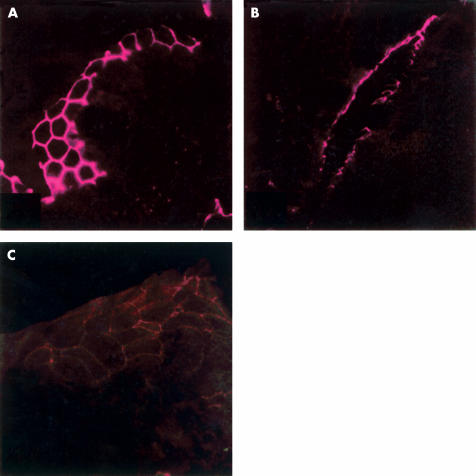
Figure 5.
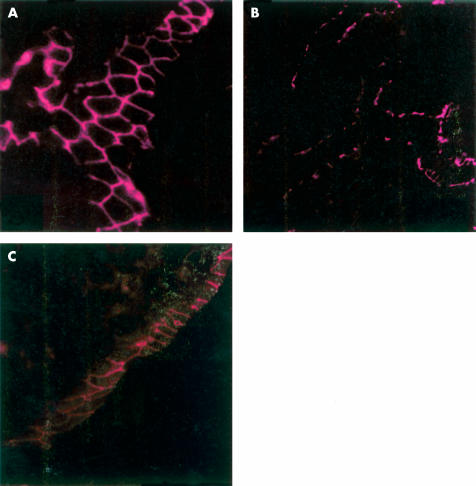
Indirect immunofluorescence of occludin in the terminal ileum of control rats (A), after two hours of immobilisation stress (B), and in stressed rats after 22 hours (C). On transverse sections in controls and stressed animals sacrificed 22 hours after immobilisation there was a linear distribution with the typical reticular pattern of occludin. In animals sacrificed immediately after two hours of immobilisation stress (B), the occludin immunofluorescence signal appeared disrupted and irregularly distributed (magnification A and C: 1500×; B: 750×).
Figure 6.
Indirect immunofluorescence of zonula occludens 1 (ZO-1) in the terminal ileum of control rats (A), after two hours of immobilisation stress (B), and in stressed rats after 22 hours (C). On transverse sections in controls and in stressed animals sacrificed 22 hours after immobilisation there was a linear distribution with the typical reticular pattern of occludin. In animals sacrificed immediately after two hours of immobilisation stress (B), the ZO-1 immunofluorescence signal appeared disrupted and irregularly distributed (magnification A and C: 1500×; B: 750×)
Figure 7.
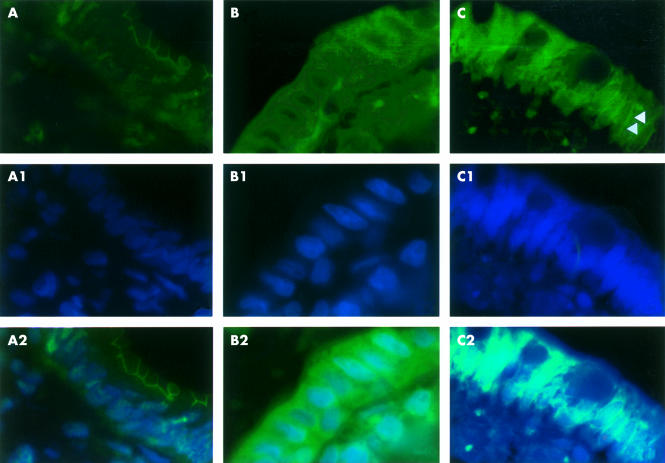
Immunolocalisation of occludin in the ileal epithelium of control rats (A). The signal of occludin results mainly in the upper cytoplasmic compartment showing a linear honeycomb-like distribution in association with the cellular surface; nuclear staining (A1) with 4`6-diamidino-2-phenylindole reveals a normal distribution of nuclei in the basal compartments of enterocytes; double staining of occludin and nuclei (A2). Ileal epithelium after two hours of immobilisation stress (B); compared with controls occludin immunofluorescence is found in all cytoplasmic compartments with a spotty distribution, the nuclei with evident nucleoli appear enlarged (B1) compared with controls and occupy mainly the central part of enterocytes; double staining of occludin and nuclei after two hours of immobilisation stress (B2). In the ileal epithelium after 22 hours recovery post-stress, immunofluorescence of occludin (C) appears concentrated around the nuclei of enterocytes (arrowheads); nuclear staining reveals still enlarged nuclei together with dissolution of nucleoli (C1); double staining of occludin and nuclei (C2). Magnification 1000×, all photographs.
The fluorescence signals of the transmembrane protein occludin (fig 5 ▶) and of the cytoplasmic plaque associated protein ZO-1 (fig 6 ▶) showed a uniform and linear distribution localised at the apical portion of cell to cell contact of the enterocytes in control rats showing the typical reticular pattern in horizontal sections of the intestinal epithelium which was also conserved in ether controls. After two hours of immobilisation stress, the signals of both proteins (occludin and ZO-1) were disrupted and irregularly distributed at the outer enterocyte periphery. The immunofluorescence signals of occludin and ZO-1 reappeared with their typical honeycomb-like pattern, but with minor intensity, 22 hours after stress.
In sections where double staining was performed (fig 7 ▶), immunolocalisation of occludin in the ileal epithelium of control rats was confined mainly to the upper cytoplasmic compartment showing its linear honeycomb-like distribution. Nuclear staining with DAPI revealed a normal distribution of nuclei in the basal compartments of enterocytes. Compared with controls, after two hours of immobilisation stress occludin immunofluorescence was found in all cytoplasmic compartments with a spotty distribution whereas nuclei appeared enlarged, with evident nucleoli, occupying mainly the central part of enterocytes. After 22 hours of recovery post-stress, immunofluorescence of occludin appeared concentrated around the cell nuclei. Nuclear staining revealed still enlarged nuclei together with dissolution of the nucleoli.
Immunoblot of occludin (fig 8 ▶)
Figure 8.
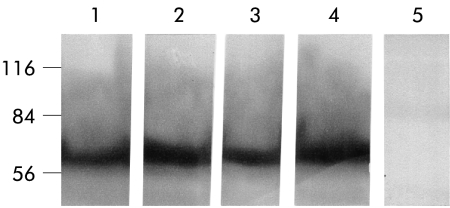
Immunoblot of occludin of the terminal ileum of controls (lane 1), ether controls (lane 2), after two hours of immobilisation stress (lane 3), and in stressed rats after a 22 hour recovery period (lane 4); lane 5 is a negative control to test antibody specificity. There was no evidence of quantitative or qualitative alterations in occludin in the four experimental groups.
In control ilea (lane 1, controls; lane 2, ether controls) occludin located between 60 and 80 kDa showed no quantitative differences. No alterations were observed after two hours of immobilisation stress (lane 3) or 24 hours after the start of the experiments (lane 4), suggesting that the observed permeability alterations were not due to a reduction in transmembrane protein occludin.
DISCUSSION
In the present study a two hour period of immobilisation of the four extremities induced an increase in faecal pellet output and a reduction in goblet cell count in the upper compartments of the colonic mucosa together with vacuolisation of crypt bottom goblet cells, indicating an effective stressful event. Our results demonstrate for the first time the functional and immunohistochemical alterations that the small intestinal TJ and two of their principal associated proteins undergo in vivo during two hours of immobilisation stress. As much as 77% of the observed TJ in the terminal ileum were found to have been passed through, as assessed by the electrondense marker lanthanum nitrate, compared with 10% under non-stress conditions. These changes were associated with an alteration in the immunofluorescence signals of both junctional proteins investigated (occludin and ZO-1). Cytoplasmic occludin showed an irregular distribution whereas enterocyte nuclei were no longer confined to the basal cell compartments and appeared enlarged. Interestingly, the short ether anaesthesia had already caused significant loss of TJ permselectivity regarding the electrondense marker lanthanum (35%) without immunohistochemical alterations, underlining the susceptibility of the intestinal barrier to stressful events. TJ permselectivity changes disappeared after a 22 hour recovery period under non-stress conditions and the typical honeycomb pattern of occludin and ZO-1 was restored, although with reduced intensity in comparison with controls. Cytoplasmic occludin distribution showed a perinuclear concentration. At this time point, the nuclei showed a reduction in size compared with the immediate post-stress appearance together with dissolution of their nucleoli. These changes were not associated with changes in occludin concentrations, as demonstrated by western blot analysis, but with reorganisation of pre-existent protein at the junctional border and in the perinuclear area. Cell migration from the crypt to the tip of the villi, one of the major repair mechanisms of the intestinal epithelium, is generally believed to take 72 hours. It is unlikely therefore that such a mechanism took place in the present study.
The present results differ from those of a previous study by our group where we examined small intestinal TJ in a rat model of chronic distal colitis.10 We found, together with similar functional results—that is, loss of permselectivity of the TJ to the lanthanum tracer—an alteration of only occludin together with a quantitative reduction in protein content.14
Our new data suggest that stress leads to the same functional changes but to different structural alterations in TJ. Previous in vivo studies investigating TJ permeability in different organs (pancreas or liver) reported more or less pronounced structural alterations in TJ associated proteins, invariably in the presence of increased paracellular permeability. In cerulein induced experimental pancreatitis, ZO-1 and f-actin showed progressive disruption within 30–60 minutes after cerulein hyperstimulation, together with an increase in paracellular permeability to horseradish peroxidase.12 In bile duct ligated rats, ZO-1 and occludin showed immunohistochemical alterations within two days, with an increase in ZO-1 protein levels and a decrease in occludin.15
Occludin is a transmembrane phosphoprotein located at the TJ with a molecular weight, depending on the degree of phosphorylation, between 62 and 82 kDa.16,17 It binds with its cytoplasmic C terminal domain to the plaque protein ZO-1. In the present study the latter protein was also altered by immobilisation stress. The junctional strands observed on freeze fracture replicas are considered to be constituted, at least in part, by the transmembranous portion of occludin. As in our former report on colitis induced permeability changes, stress did not affect strand organisation on freeze fracture analysis, despite the occludin alterations observed by immunohistochemistry. An explanation for this apparent contrast between immunohistochemistry and freeze fractures may be that occludin is not the only integral TJ protein found to be localised in association with the strands visualised by freeze etching.18 In fact, the recently identified integral membrane proteins, the claudins, seem to be mainly responsible for TJ strand formation.8,19 Moreover, studies in Madin-Darby canine kidney cells expressing a mutant occludin have shown disruption of the continuous junctional distribution of occludin with a normal appearance of freeze fracture replicas.20 In a very recent study carried out on the intestinal mucosa of patients with inflammatory bowel disease, both transmembrane proteins, claudin-1 and occludin, were investigated showing no variation of the former together with a reduction of the latter.21 Finally, the antibody we used in the present study was directed against the C terminal plaque oriented portion of occludin and not against the transmembrane loops of the protein. Thus a conserved appearance of anastomosing fibrils on freeze etching does not necessarily imply conservation of occludin.
ZO-1, a member of the MAGUK homologues, seems to be responsible for accumulation of occludin at the site of cell-cell contact22 and appears to constitute a link between occludin and the actin cytoskeleton.23
Taking into consideration the results of our studies and of the above mentioned experiments carried out in different organs, it seems likely that one of the intracellular key events is contraction of actin. This may also be an explanation for our present findings on the functional alterations induced by ether anaesthesia. This short stress period may have induced contraction of the actin cytoskeleton increasing the distance between adjacent enterocytes. By prolonging the stressful event through immobilisation, molecular dislocations occurred, as evidenced by immunohistochemistry. Comparing our data with results obtained in other organs with different noxious stimuli, organ specific response differences may exist with regard to TJ related protein expression. Bile duct ligation is associated with a pressure increase in the biliary tree whereas cerulein acts via membrane receptors leading to a rise in intracellular Ca2+. The exact pathway of stress induced TJ alterations is poorly known. Intraperitoneal administered corticotropin releasing hormone (CRH) mimics the effect of stress on TJ whereas atropine, α-helical CRH, and the mast cell stabiliser doxantrazole abolish stress or CRH induced alterations. These data suggest involvement of cholinergic nerves, CRH receptors, and activation of mast cells.1,2,24 As permeability changes are not an expression of a unique alteration, future experimental studies on the paracellular barrier should include analysis of TJ associated proteins.
In conclusion, our results show for the first time that stress induced ileal permeability changes are associated, on a cellular level, with fragmentation of the immunofluorescence signal of ZO-1 and occludin, without a reduction in protein content, but with an altered intracytoplasmic distribution of the latter protein. We have demonstrated that stress induces functional and morphological alterations and that, after 24 hours, reorganisation of pre-existing protein occurs indicating intracellular repair mechanisms.
Acknowledgments
This work was supported by ministerial grants (MURST 60%) (WF, NF) (GCS cofinanziamento MURST 9906275238-009).
Abbreviations
CRH, corticotropin releasing hormone
DAPI, 4`6-diamidino-2-phenylindole
EDTA, ethylenediaminetetraacetic acid
MAGUK, membrane associated guanylate kinase
PBS, phosphate buffered saline
SDS-PAGE, sodium dodecyl sulphate-polyacrylamide gel electrophoresis
TBS, Tris buffered saline
TJ, tight junction
ZO, zonula occludens
REFERENCES
- 1.Saunders PR, Kosecka U, McKay DM, et al. Acute stressors stimulate ion secretion and increase epithelial permeability in rat intestine. Am J Physiol 1994;267:G794–9. [DOI] [PubMed] [Google Scholar]
- 2.Saunders PR, Hanssen NPM, Perdue MH. Cholinergic nerves mediate stress-induced intestinal transport abnormalities in Wistar-Kyoto rats. Am J Physiol 1997;273:G486–90. [DOI] [PubMed] [Google Scholar]
- 3.Kiliaan AJ, Saunders PR, Bijlsma PB, et al. Stress stimulates transepithelial macromolecular uptake in rat jejunum. Am J Physiol 1998;275:G1037–44. [DOI] [PubMed] [Google Scholar]
- 4.Balda MS, Matter K. Tight junctions. J Cell Sci 1998;111:541–7. [DOI] [PubMed] [Google Scholar]
- 5.Denker BM, Nigam SK. Molecular structure and assembly of the tight junction. Am J Physiol 1998;274:F1–9. [DOI] [PubMed] [Google Scholar]
- 6.Fanning AS, Mitic LL, Anderson JM. Molecular architecture of tight junctions. J Am Soc Nephrol 1999;10:1337–45. [DOI] [PubMed] [Google Scholar]
- 7.Tsukita S, Furuse M. Overcoming barriers in the study of tight junction functions: from occludin to claudin. Genes Cells 1998;3:569–73. [DOI] [PubMed] [Google Scholar]
- 8.Heiskala M, Peterson PA, Yang Y. The roles of claudin superfamiliy in paracellular transport. Traffic 2001;2:93–8 [DOI] [PubMed] [Google Scholar]
- 9.Barone FC, Deegan JF, Price WJ, et al. Cold-restraint stress increases rat fecal pellet output and colonic transit. Am J Physiol 1990;258:G329–37. [DOI] [PubMed] [Google Scholar]
- 10.Fries W, Mazzon E, Squarzoni S, et al. Experimental colitis increases small intestine permeability in the rat. Lab Invest 1999;79:49–57. [PubMed] [Google Scholar]
- 11.Lora L, Mazzon E, Martines D, et al. Hepatocyte tight-junctional permeability is increased in rat experimental colitis. Gastroenterology 1997;113:1347–54. [DOI] [PubMed] [Google Scholar]
- 12.Fallon MB, Gorelick FS, Anderson JM, et al. Effect of cerulein hyperstimulation on the paracellular barrier of rat exocrine pancreas. Gastroenterology 1995;108:1863–72. [DOI] [PubMed] [Google Scholar]
- 13.Castagliuolo I, LaMont JT, Qiu B, et al. Acute stress causes mucin release from rat colon: role of corticotropin releasing factor and mast cells. Am J Physiol 1996;271:G884–92. [DOI] [PubMed] [Google Scholar]
- 14.Mazzon E, Fries W, Longo G. The transmembrane protein occludin in DNB-colitis in the rat. Ital J Gastroenterol Hepatol 1998;30(suppl 2):A154. [Google Scholar]
- 15.Fallon MB, Brecher AR, Balda MS, et al. Altered hepatic localization and expression of occludin after common bile duct ligation. Am J Physiol 1995;269:C1057–62. [DOI] [PubMed] [Google Scholar]
- 16.Furuse M, Hirase T, Itoh M, et al. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol 1993;123:1777–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denker, BM, Nigam SK. Molecular structure and assembly of the tight junction. Am J Physiol 1998;274:F1–9. [DOI] [PubMed] [Google Scholar]
- 18.Furuse M, Fujita K, Hiiragi T, et al. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol 1998;141:1539–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furuse M, Sasaki H, Fujimoto K, et al. A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J Cell Biol 1998;143:391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balda MS, Whitney JA, Flores C, et al. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol 1996;134:1031–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ooi CJ, Rosenberg IM, Reinecker H-C, et al. Regulation of tight junction proteins in human subjects with inflammatory bowel disease. Gastroenterology 2000;118(suppl):A795. [Google Scholar]
- 22.Mitic LL, Anderson JM. Molecular architecture of tight junctions. Annu Rev Physiol 1998;60:121–42. [DOI] [PubMed] [Google Scholar]
- 23.Fanning, AS, Jameson BJ, Jesaitis LA, et al. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem 1998;273:29745–53. [DOI] [PubMed] [Google Scholar]
- 24.Santos J, Saunders PR, Hanssen NPM, et al. Corticotropin-releasing hormone mimics stress-induced colonic pathophysiology in the rat. Am J Physiol 1999;277:G391–9. [DOI] [PubMed] [Google Scholar]