Abstract
Background and aims: The success of Helicobacter pylori eradication regimens depends on gastric pH, inflammation, and mucus thickness. Our aim was to investigate the effects of acid secretion, inflammation, and mucolysis on gastric antibiotic transfer.
Subjects and methods: A total of 134 anaesthetised rats were given metronidazole, amoxicillin, or clarithromycin intravenously and gastric contents were aspirated via an indwelling cannula. Acid secretion was controlled by either omeprazole or pentagastrin while gastritis was induced by infection with H pylori or dosing with iodoacetamide. Mucolysis was achieved by instilling pronase into the gastric lumen.
Results: Metronidazole transfer increased with acid secretion and fell with omeprazole, independently of gastric pH. Clarithromycin was also transferred with acid but was then rapidly degraded. Omeprazole prevented this degradation, raising gastric luminal concentrations. Omeprazole did not alter amoxicillin transfer. Gastritis induced by H pylori did not alter gastric transfer of metronidazole and amoxicillin but that of clarithromycin was increased by 23%. However, gastritis induced by iodoacetamide reduced clarithromycin transfer without any effect on metronidazole or amoxicillin transfer. Pronase treatment increased amoxicillin transfer fourfold and metronidazole by 66% but reduced clarithromycin transfer by 35%.
Conclusions: Metronidazole and clarithromycin are predominantly transferred with gastric acid rather than by an acid trapping mechanism. Pronase increases the appearance of amoxicillin and metronidazole in gastric secretions.
Keywords: acid secretion, gastritis, mucus thickness, gastric transfer, antibiotics, rats
The development of Helicobacter pylori treatment regimens has been largely empirical. There are however several marked discrepancies between in vitro and in vivo efficacy, indicating the importance of gastric distribution of antibiotics. The efficacy of delivery of the antibiotic to the gastric mucus layer where H pylori resides has been surprisingly little studied. As H pylori is becoming more resistant to standard antibiotics,1 there is a need to discover the fundamental mechanisms of gastric antibiotic delivery in order to help the design of novel agents.2 Clinical trials using amoxicillin, metronidazole, and clarithromycin have indicated the key importance of raising intragastric pH by means of proton pump inhibitors.3 Studies using less potent regimens have also indicated that eradication rates in patients with an inflamed stomach are greater than in those with minimal inflammation.4–6 This suggests that penetration of some antibiotics might be facilitated by inflammation just as transport of amoxicillin into cerebrospinal fluid is enhanced in meningitis.7 Gastric mucus, whose function it is to protect the gastric epithelium from injurious substances, may also provide a barrier to antibiotic penetration, and mucolytic agents have been used with good effect to enhance eradication rates.8,9 The aim of this study was to examine the role of acid secretion and gastric luminal pH on the gastric transfer rate of the three most commonly used anti-H pylori antibiotics, metronidazole, amoxicillin, and clarithromycin using a new anaesthetised rat model of gastric antibiotic transport. This model allows independent control of both acid secretion and intragastric pH. The effect of gastritis on gastric antibiotic transfer rate was also investigated after inducing gastritis by either infecting rats with H pylori or addition of iodoacetamide to the drinking water. Finally, gastric mucus was disrupted with pronase and the effect on gastric antibiotic secretion rate determined.
MATERIALS AND METHODS
All experiments were performed in accordance with a UK Home Office Project Licence, using a total of 134 male Wistar rats weighing 250–370 g. Rats were fasted on a wire bottomed cage for 24 hours with free access to water. Anaesthesia was induced with a 2.7 ml/kg intraperitoneal dose of a 1:1:2 mixture of Hypnorm (fentanyl/fluanisone), midazolam, and H2O. Vital signs were monitored using an intracarotid blood pressure transducer connected to a computer recording system. Hydration was maintained with an infusion of 0.9% saline via a tail vein. At laparotomy, a cannula cuffed with two “O” rings was inserted through a duodenal incision into the gastric antrum via the pylorus. The stomach was washed with 0.9% saline until the aspirate was free of debris and then 1.5 ml of saline was instilled. After a 30 minute equilibration period to allow gastric blood flow to stabilise, a bolus intravenous dose of antibiotic was given at the start of the two hour sampling period, followed by a continuous infusion. The following doses were administered: metronidazole 9 mg/kg bolus, 3.6 mg/kg/h infusion; amoxicillin 11 mg/kg bolus, 20 mg/kg/h infusion; and clarithromycin 26 mg/kg bolus, 7.3 mg/kg/h infusion.
Carotid artery blood samples (0.3 ml) were taken at 15 minute intervals for two hours; plasma was separated by centrifugation and snap frozen in liquid N2. At 15 minute intervals the gastric contents were aspirated by syringe and the volume recorded. Net gastric secretion volume was calculated by subtracting the volume instilled (1.5 ml) from the volume aspirated. pH was measured with a glass electrode before the sample was immediately snap frozen in liquid N2 and later transferred to a −80°C freezer. After two hours, the rat was sacrificed by neck dislocation, the stomach excised and portions were preserved in 10% formalin, H pylori culture transport medium, and for mucus thickness measurements in liquid N2. Mucosal sections were categorised as to the degree of inflammation (none, mild, moderate, or severe), as is conventional in assessing human gastritis10 by a single expert gastrointestinal pathologist. Sections were stained with toluidine blue for identification of H pylori.
Acid secretion/suppression experiments
Two dosing regimens of intravenous omeprazole were used to suppress gastric acid secretion. A single bolus dose (10 μmol/kg) was used for the metronidazole experiments. For the amoxicillin and clarithromycin experiments, 20 μmol/kg omeprazole was administered initially and again at 45 minutes into the sampling period, a regimen which gave similar but more consistent acid suppression. In stimulated acid secretion experiments, intravenous bolus doses of pentagastrin (25 μg/kg) were given every 15 minutes. Where intragastric pH was controlled (table 1 ▶) the following buffers were instilled instead of saline: pH 2.7: glycine/HCl buffer, 300 mosmol/kg; pH 6.0: 2-(N-morpholino) ethanesulphonic acid buffer at pH 6.0, 300 mosmol/kg.
Table 1.
Gastric metronidazole transfer: effect of acid secretion and luminal pH
| Intravenous drug | Omeprazole | Pentagastrin | Omeprazole | Pentagastrin |
| Gastric luminal solution | 0.9% saline | 0.9% saline | Glycine/HCl buffer | MES buffer |
| n | 8 | 7 | 8 | 8 |
| Median gastric aspirate pH (range) | 5.74 (4.39–6.30)*** | 2.54 (2.32–2.79)†† | 2.77 (2.69–2.82) | 6.00 (5.81–6.03) |
| Gastric aspirate Cmax (mg/l) | 14.2 (2.7)‡‡ | 50.8 (8.5) | 14.8 (1.8)‡‡ | 66.0 (28.0) |
| Gastric clearance (l/min) | 87 (14) ‡‡ | 352 (68) | 97 (11) ‡‡ | 429 (172) |
| Gastric transfer fraction (%) | 7.1 (1.3) ‡‡ | 24.5 (5.0) | 7.2 (0.8) ‡‡ | 27.8 (10.2) |
Unless otherwise stated, values are mean (SD).
***p<0.001 versus pentagastrin+saline and omeprazole+glycine/HCl buffer.
††p≤0.01 versus all other groups.
‡‡ p<0.01 versus pentagastrin+saline and pentagastrin+MES.
MES, 2-(N-morpholino) ethanesulphonic acid.
Induction of gastritis
Four H pylori strains were prepared for dosing into 32 rats weighing 180–220 g: two Hel73 toxigenic strains11 (rat and mouse passaged variants), the Sydney strain, and a fresh clinical isolate. Using the method of Li and colleagues,11 rats were gavaged with 2 ml of H pylori in suspension (5×108 cfu/ml of each strain) at midday and at 4pm for two days. One rat died early during surgery and no data were obtained; seven rats that had neither surgery nor antibiotics were sacrificed at 12 weeks and their stomachs sampled for H pylori culture and histology.
Chemical gastritis was induced by adding 0.1% w/v iodoacetamide (Sigma, Poole, UK) to the water supply of a further group of rats for one week prior to surgery. This reduced food and fluid intake but all animals survived the study period.
Gastric mucolysis experiments
Gastric mucus dissolution was achieved using pronase, a non-specific protease active at neutral pH.12 All pronase treated rats were also given intravenous omeprazole. The stomach was washed with saline initially, and then 1.5 ml of a 20 mg/ml solution of pronase (Sigma) in pH 7.4 phosphate buffered saline (osmolality 312 mosmol/kg) was instilled instead of saline for the 30 minute equilibration period and the two hour experiment. The dose of pronase (440 tyrosine units/kg) was chosen to achieve a concentration similar to that used in human trials.8,9 Rat stomachs were prepared for mucus thickness measurements using the method of Jordan and colleagues.13 Mucus continuity was expressed as a percentage of the mucosal surface covered by mucus.
Antibiotic analysis
Concentrations of metronidazole, amoxicillin, and clarithromycin in plasma and gastric aspirate samples were determined using modified narrow bore high performance liquid chromatography techniques based on methods previously reported.14–16 Aliquots of 50 μl plasma or 200–500 μl gastric aspirate were used for analysis. Controls were prepared by spiking plasma and 0.9% saline with stock antibiotic solutions. The presence of pronase in the sample had no effect on the quantification of any of the antibiotics. The limit of quantification was 0.015 mg/l for metronidazole, 0.04 mg/l for amoxicillin, and 0.10 mg/l for clarithromycin. The precision of the assays (defined as the relative standard deviation for repeated measurements of the same concentration) over the range of concentrations studied was ±1.4–13.1%, and deviation from linearity was <1%.
Calculations and statistics
Gastric antibiotic clearance (in ml/min) for the 120 minute experiments was calculated as:
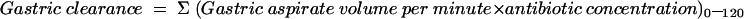 |
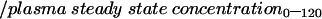 |
Gastric transfer fraction was calculated by dividing gastric clearance by plasma clearance. Both parameters enable a description of the relative transfer of antibiotics across the gastric mucosa under different experimental conditions by correcting for intersubject variation in plasma antibiotic concentration.17
Sample statistics were calculated using SPSS 8.0 software (SPSS Inc., Chicago, USA). The Mann-Whitney U test was used to compare the results of the two groups; the Kruskal-Wallis H-test was used to compare three or more groups. A p value <0.05 was regarded as statistically significant.
RESULTS
Vital signs of the rats remained within normal limits throughout the experiments.
Histology
Control rats had histologically normal gastric mucosa following the 2.5 hour experiment. H pylori dosed rats all developed mild to moderate mixed inflammatory cell infiltration of the gastric mucosa, with mainly sparse colonisation by H pylori. None showed any macroscopic mucosal ulceration. Marked vascular engorgement was a striking histological feature in iodoacetamide dosed rats. Iodoacetamide caused a similar degree of inflammatory cell infiltrate as H pylori gastritis. Pronase treated gastric mucosa was macroscopically normal but showed marked histological changes. The superficial epithelial layer was fissured with shedding of some apical cell layers. In some sections vascular engorgement, as seen in iodoacetamide treated rats, was observed. There was however no abnormal inflammatory cell infiltration of the pronase treated rat stomachs.
Mucus thickness
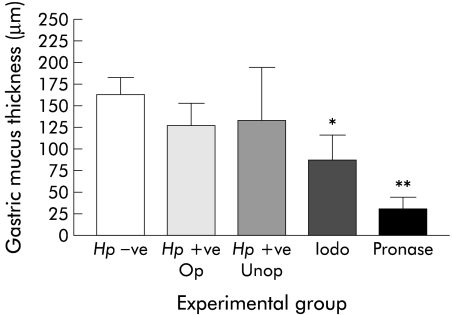
Gastric mucus thickness in H pylori infected rats and controls was similar, whether or not surgery was performed (fig 1 ▶). Mean mucus thickness was 87 (29) μm in iodoacetamide treated rats compared with 162 (21) μm in controls (p<0.05). Coverage of gastric mucus in control rats was 100% whereas in iodoacetamide rats there were areas of denuded gastric mucosa with only 88% coverage overall. Pronase markedly thinned gastric mucus creating large gaps; overall, coverage was reduced to 60%. Mean mucus thickness was reduced to 30 (13) μm (p<0.001 v controls).
Figure 1.
Effect of Helicobacter pylori, iodoacetamide (iodo), and pronase on gastric mucus thickness. Hp−ve, H pylori negative control (no surgery); Hp+ve Op, H pylori infected (had surgery); Hp+ve Unop, H pylori infected control (no surgery).
H pylori culture
H pylori was cultured from the gastric mucosa in 86% of infected controls but no positive cultures were obtained from the stomachs of rats that received antibiotics which showed similar degrees of histological gastritis and a similar density of colonising H pylori.
Gastric secretion
This was higher in pentagastrin dosed rats compared with those receiving high dose omeprazole (3.3 (1.6) ml/kg/h v 2.0 (1.2) ml/kg/h; p=0.019). Neither H pylori infection nor iodoacetamide gastritis significantly affected gastric juice secretion volume. Gastric secretion was much greater in pronase treated rats compared with controls (8.3 (1.6) ml/kg/h v 2.0 (1.2) ml/kg/h; p<0.001).
Metronidazole transfer
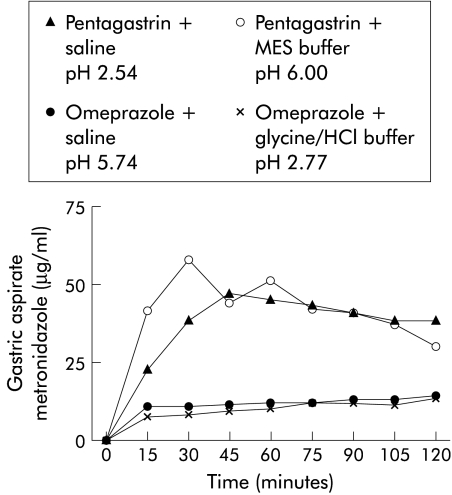
During pentagastrin dosing, gastric aspirate metronidazole concentrations were markedly higher than those seen at very similar gastric pH levels during omeprazole dosing (fig 2 ▶, table 1 ▶). Neither H pylori nor iodoacetamide gastritis affected the gastric metronidazole transfer rate but mucus digestion with pronase produced a 66% increase in gastric metronidazole transfer rate (p=0.005 v control) (table 2 ▶).
Figure 2.
Effect of acid secretion and luminal pH on gastric metronidazole transfer. MES, 2-(N-morpholino) ethanesulphonic acid.
Table 2.
Gastric metronidazole transfer: effect of Helicobacter pylori, iodoacetamide, and pronase
| Intravenous drug | Omeprazole | Omeprazole | Omeprazole | Omeprazole |
| Gastric luminal solution | 0.9% saline | 0.9% saline | 0.9% saline | Pronase |
| Preparation | Normal | H pylori infected | Iodoacetamide | Normal |
| n | 8 | 8 | 8 | 8 |
| Gastric aspirate Cmax (mg/l) | 14.2 (2.7) | 13.9 (3.4) | 12.2 (3.7) | 20.5 (6.6)* |
| Gastric clearance (l/min) | 87 (14) | 84 (19) | 89 (28) | 145 (41)* |
| Gastric transfer fraction (%) | 7.1 (1.3) | 5.4 (1.4)* | 7.2 (2.1) | 9.9 (2.5)* |
Values are mean (SD).
*p<0.05 versus control.
Amoxicillin transfer
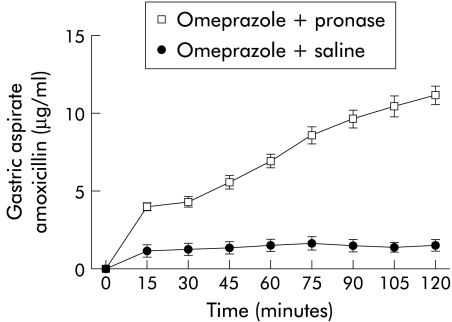
There were no significant effects of acid secretion/suppression, iodoacetamide, or H pylori induced gastritis on gastric amoxicillin transfer. Pronase caused a fourfold increase in the gastric clearance of amoxicillin (p<0.001; table 3 ▶) with rising gastric aspirate amoxicillin concentrations as the experiment progressed (fig 3 ▶).
Table 3.
Gastric amoxicillin transfer: effect of acid secretion, Helicobacter pylori, iodoacetamide, and pronase
| Intravenous drug | Omeprazole | Pentagastrin | Omeprazole | Omeprazole | Omeprazole |
| Gastric luminal solution | 0.9% saline | 0.9% saline | 0.9% saline | 0.9% saline | Pronase |
| Preparation | Normal | Normal | H pylori infected | Iodoacetamide | Normal |
| n | 8 | 8 | 8 | 8 | 9 |
| Median gastric aspirate pH (range) | 6.09 (5.53–6.44) | 2.27 (2.11–3.41)*** | 6.23 (6.01–6.39) | 6.21 (5.99–6.38) | 6.45 (6.33–6.57)*** |
| Gastric aspirate Cmax (mg/l) | 1.9 (1.3) | 1.84 (1.13) | 2.79 (0.84) | 1.21 (0.40) | 11.4 (1.9) *** |
| Gastric clearance (l/min) | 2.9 (1.9) | 3.3 (2.5) | 2.3 (0.6) | 1.3 (0.4) | 12.0 (3.8) *** |
| Gastric transfer fraction (%) | 0.15 (0.11) | 0.12 (0.09) | 0.15 (0.05) | 0.10 (0.04) | 0.86 (0.14) *** |
Values are mean (SD), except for pH.
*** p<0.001 versus control.
Figure 3.
Effect of pronase on gastric amoxicillin transfer.
Clarithromycin transfer
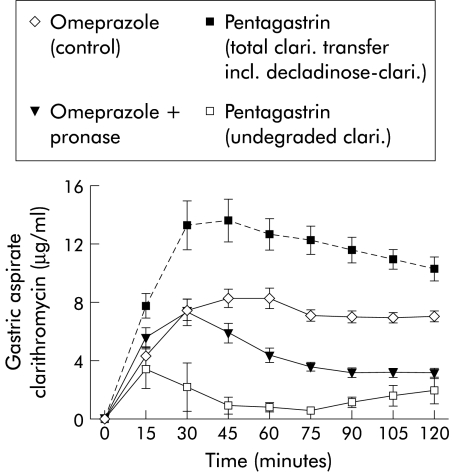
Gastritis induced by H pylori caused a 23% increase in gastric clarithromycin clearance compared with controls (table 4 ▶). Gastritis induced by iodoacetamide caused a small but significant reduction in the gastric transfer fraction of clarithromycin compared with controls; gastric clearance was also reduced but just failed to reach statistical significance (p=0.06). Gastric clarithromycin transfer was reduced by 35% in pronase treated rats (fig 4 ▶). Pentagastrin dosing resulted in a significantly lower gastric clearance and gastric transfer fraction of clarithromycin compared with omeprazole dosed rats. However, subsequent analysis of an unknown compound in the gastric aspirate samples revealed that clarithromycin had been broken down into the inactive acid degradation product, decladinose-clarithromycin. Taking this degradation into account, gastric clearance and gastric transfer fraction of clarithromycin during pentagastrin dosing were 222 μl/min and 3.92%, respectively, significantly greater (p<0.001) than that seen during omeprazole dosing.
Table 4.
Gastric clarithromycin transfer: effect of acid secretion, Helicobacter pylori, iodoacetamide, and pronase
| Intravenous drug | Omeprazole | Pentagastrin | Omeprazole | Omeprazole | Omeprazole |
| Gastric luminal solution | 0.9% saline | 0.9% saline | 0.9% saline | 0.9% saline | Pronase |
| Preparation | Normal | Normal | H pylori infected | Iodoacetamide | Normal |
| n | 8 | 8 | 8 | 7 | 7 |
| Median gastric aspirate pH (range) | 6.13 (5.89–6.35) | 2.58 (2.30–2.85)**** | 6.19 (6.15–6.46) | 5.83 (5.58–6.34) | 6.60 (6.56–6.72)**** |
| Gastric aspirate Cmax (mg/l) | 8.7 (1.8) | 4.4 (4.5)* | 10.3 (1.6) | 6.7 (2.2) | 7.3 (2.4) |
| Gastric clearance (l/min) | 128 (31) | 30 (33)*** | 158 (22)* | 95 (29)** | 82 (18)*** |
| Gastric transfer fraction (%) | 2.28 (0.25) | 0.55 (0.63)**** | 2.09 (0.22) | 1.72 (0.41)*** | 1.33 (0.35)**** |
Values are mean (SD), except for pH.
*p<0.05, **p=0.06 , ***p<0.01, ****p<0.001 versus control.
Figure 4.
Effect of acid secretion and pronase on gastric clarithromycin (clari.) transfer.
DISCUSSION
The anaesthetised rat model provides an opportunity to investigate the mechanisms of gastric antibiotic transfer by minimising intersubject variability (age, sex, weight, strain). In contrast with human studies, no pyloric loss or bile contamination of gastric juice occurs17 and the gastric transport of novel compounds can readily be evaluated. Pentagastrin or omeprazole dosing gave gastric pH values similar to those found in humans during basal acid output and omeprazole pre-dosing.17 Rat plasma antibiotic concentrations were comparable with those found in human pharmacokinetic studies.
Two other animal models of gastric antibiotic secretion have been reported. The Ussing chamber model uses rat gastric mucosa placed in a Lucite chamber bathed by solutions on the mucosal and serosal sides.18 In this model high serosal antibiotic concentrations were necessary, the drug diffusion path was different from in vivo as there was no intact gastric blood supply, and mucosal acid secretion was not proven to occur. The recently described explanted embryonic human stomach model of gastric antibiotic secretion used human gastric tissue and could also be infected with H pylori but its great complexity limits its usefulness for studies on gastric pharmacokinetics.19
During normal gastric acid secretion conditions, weak bases such as metronidazole might passively diffuse from gastric capillaries across the gastric mucosa, become ionised by the low pH of gastric juice, and then become “trapped” in the gastric lumen in accordance with the pH partition hypothesis.17,20 An alternative possibility is that, in addition to passive diffusion, weak bases might accumulate in the acid secretory apparatus of parietal cells and subsequently be secreted into the gastric lumen with gastric acid. According to the latter hypothesis, transport of weak bases into the gastric lumen should be independent of luminal pH. “Acid trapping” may still occur, but will take place within the secretory apparatus, rather than within the lumen. By controlling gastric acid secretion with either omeprazole or pentagastrin, and gastric luminal pH with iso-osmotic pH buffers, this model was able to distinguish between these possibilities. Gastric metronidazole transfer rates observed during pentagastrin treatment, with a median gastric luminal pH held at either 2.54 or 6.00 with buffers, were very similar. Moreover, omeprazole markedly reduced the gastric metronidazole secretion rate both at gastric pH 2.77 and at pH 5.74. Therefore, it is the rate of acid secretion and not luminal pH that determines gastric metronidazole secretion, implying that metronidazole is predominantly transferred with gastric acid. These findings might at first appear paradoxical as in clinical studies coadministration of omeprazole substantially enhanced the effectiveness of regimens containing metronidazole with amoxicillin or clarithromycin. This may be due to enhancement of the stability of clarithromycin and amoxicillin. However, it should also be noted that even during acid suppression in humans, gastric aspirate metronidazole Cmax is still approximately twice the MIC90. Furthermore, the raised luminal pH ensures that metronidazole (pKa=2.52) is totally unionised, lipophilic, and therefore available for passive diffusion across the lipid outer membrane of H pylori.
At the gastric pH (approximately pH 6) achieved following administration of omeprazole, amoxicillin exists as a hydrophilic zwitterion with a logD (a measure of lipophilicity as a function of pH 17) of −1.6, and therefore would be predicted to transfer poorly across lipid membranes. Our studies confirm this prediction; we were able to detect only very low concentrations of amoxicillin (less than 2 mg/l in the normal stomach) in human gastric aspirates. Omeprazole potentiates the antibacterial effect of amoxicillin by reducing the volume of gastric secretion and hence effectively increasing the drug concentration.17 Omeprazole also improves the stability of amoxicillin and the susceptibility of H pylori to this antibiotic.21 Studies of gastric transport of amoxicillin in humans are problematic due to contamination of gastric aspirate samples with amoxicillin-rich bile and the difficulty in detecting low concentrations of amoxicillin. We were able to demonstrate a very low gastric amoxicillin transfer rate in the rat consistent with that seen in humans. As bile was excluded in the rat model, we can conclude that in spite of its hydrophilic nature, amoxicillin can penetrate the gastric epithelium.
There was an apparent fourfold reduction in gastric clarithromycin clearance and transfer fraction during pentagastrin stimulated acid secretion. The net result of omeprazole dosing was that clarithromycin became concentrated in the gastric aspirate compared with plasma. In human phagocytes, macrolides are concentrated in a protonated form in acidic lysosomes and to a lesser extent in the cell cytosol.22 It would therefore be expected that clarithromycin should be concentrated in the gastric mucosal acid secretory apparatus, as observed with metronidazole. An explanation for this discrepancy was suggested by the discovery of biologically inactive decladinose-clarithromycin, an acid breakdown product of clarithromycin, only in the gastric aspirate of pentagastrin dosed rats. The reduction in gastric transfer rate during omeprazole therapy is outweighed by the increase in clarithromycin stability seen at a higher pH (fig 4 ▶, manuscript in preparation).
H pylori colonisation of rat gastric mucosa was sparse and caused relatively mild acute inflammation. H pylori resides in humans for many years whereas infection in rats was only two months old so that gastric atrophy and effects on acid secretion may not have had time to develop. Perhaps not surprisingly, no difference in gastric mucus thickness or luminal pH achieved during omeprazole dosing was demonstrated; even in chronically infected humans, mucus thickness is not significantly affected except in older subjects.23
There was a significant increase in gastric clarithromycin clearance in H pylori infected rats compared with the control group. One explanation for this increase might be that clarithromycin is actively taken up by polymorphonuclear leucocytes which act as potential delivery agents for the drug to inflamed tissues as clarithromycin is rapidly lost from leucocytes in culture when extracellular clarithromycin is removed.22 Clarithromycin is the most highly protein bound of the three antibiotics, and it is interesting that H pylori extracts cause albumin leakage from the rat gastric mucosal microcirculation.24
Compared with H pylori infection, iodoacetamide gastritis was associated with more severe histological changes. There was vascular engorgement and mucus thinning, not seen with H pylori. The rats reduced food and fluid intake and lost weight. These complex possibly conflicting effects suggest that iodoacetamide was not a useful model of H pylori gastritis. Iodoacetamide did not cause any significant changes in gastric antibiotic clearance. Gastric clarithromycin transfer fraction was reduced, perhaps as an artefact of low body weight in iodoacetamide dosed rats.
The apparent benefit of pronase in clinical studies could be caused by either disruption of the normal habitat of H pylori, increasing its susceptibility to antibiotics,25 or by affecting antibiotic transfer. Our studies show that the latter mechanism is important. Pronase caused pronounced degradation of the gastric mucus layer and also considerable signs of epithelial cell disruption. There was a marked increase in gastric secretion volume, possibly by inducing copious protective mucus secretion. Although we did not measure blood contamination from the damaged mucosal surface, this could have contributed to the rise in gastric amoxicillin level, as plasma has a high concentration of amoxicillin relative to gastric aspirate. It may partially explain the reduction in aspirate clarithromycin level, as clarithromycin is usually concentrated in gastric juice, which may have been diluted with plasma containing a lower concentration of clarithromycin. The progressive rise in amoxicillin transfer rate during the experiment suggests amoxicillin traverses the mucosa via the paracellular route due to tight junction disruption, or that mucus is a considerable barrier to amoxicillin transfer that is gradually digested away during the experiment. The progressive reduction in gastric clarithromycin transfer rate during the pronase experiments strongly suggests that mucus is not a significant barrier to gastric clarithromycin secretion but that an active process requiring a healthy epithelium has been gradually degraded by pronase. The safety and efficacy of mucolytics with antibiotics is worthy of future research in human clinical trials.
Acknowledgments
The authors are grateful for the support and encouragement of Christer Cederberg, Michael Wrangstadt, and colleagues at Astra-Zeneca. The study was funded by an Astra Research Fellowship. We would also like to thank Andrew Goddard, Darren Fowkes, Dave Reffin, Ian Jansen, Robert Logan, James Bebb, Jo Whetstone, Joanne Bower, and Gill Turner for technical assistance given to the work.
REFERENCES
- 1.De Koster E, Devaster J-M, Vandenborre C, et al. A nine years surveillance of Hp resistance to macrolides and imidazoles. Gastroenterology 1999;116(4 part 2):A145. [Google Scholar]
- 2.Goddard AF. Getting to the route of Helicobacter pylori treatment. J Antimicrob Chemother 1998;42:1–3. [PubMed] [Google Scholar]
- 3.Lind T, Megraud F, Unge P, et al. The MACH2 study: role of omeprazole in eradication of Helicobacter pylori with 1-week triple therapies. Gastroenterology 1999;116:248–53. [DOI] [PubMed] [Google Scholar]
- 4.Spiller RC. Is there any difference in Helicobacter pylori eradication rates in patients with active peptic ulcer, inactive peptic ulcer and functional dyspepsia? Eur J Gastro Hepatol 1999;11(suppl 2):S25–8. [DOI] [PubMed] [Google Scholar]
- 5.Labenz J, Leverkus F, Börsch G. Omeprazole plus amoxicillin for cure of Helicobacter pylori infection. Factors influencing the treatment success. Scand J Gastroenterol 1994;29:1070–5. [DOI] [PubMed] [Google Scholar]
- 6.Cutler AF, Schubert TT. Patient factors affecting Helicobacter pylori eradication with triple therapy. Am J Gastroenterol 1993;88:505–9. [PubMed] [Google Scholar]
- 7.Bakken JS, Bruun JN, Gaustad P, et al. Penetration of amoxicillin and potassium clavulanate into the cerebrospinal fluid of patients with inflamed meninges. Antimicrob Agents Chemother 1986;30:481–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akamatsu T, Gotoh A, Shimizu H, et al. Combination effect of pronase for eradication therapy against Helicobacter pylori. Gastroenterology 1998;112:A54. [Google Scholar]
- 9.Zala G, Wirth HP, Scwery St, et al. N-Acetylcyteine improves eradication of H. pylori by omeprazole/amoxicillin in cigarette smokers. Gastroenterology 1994;106:A215. [Google Scholar]
- 10.Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis: The updated Sydney system. Am J Surg Pathol 1996;20:1161–81. [DOI] [PubMed] [Google Scholar]
- 11.Li H, Kalies I, Mellgård B, et al. A rat model of chronic Helicobacter pylori infection. Studies of epithelial cell turnover and gastric ulcer healing. Scand J Gastroenterol 1998;33:370–8. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto Y, Tsuiki S, Nisizawa K, et al. Action of proteolytic enzymes on purified bovine submaxillary mucin. Ann NY Acad Sci 1963;106:233–46. [DOI] [PubMed] [Google Scholar]
- 13.Jordan N, Newton J, Pearson J, et al. A novel method for the visualization of the in situ mucus layer in rat and man. Clin Sci 1998;95:97–106. [PubMed] [Google Scholar]
- 14.Jessa MJ, Barrett DA, Shaw PN, et al. A rapid and selective high performance liquid chromatography method for the determination of metronidazole and its active metabolite in human plasma, saliva and gastric juice. J Chromatography B 1996;677:374–9. [DOI] [PubMed] [Google Scholar]
- 15.Miyazaki K, Ohtani K, Sunada K, et al. Determination of ampicillin, amoxicillin, cephalexin and cephradine in plasma by high-performance liquid chromatography using fluorescence detection. J Chromatography B 1983;276:478–82. [DOI] [PubMed] [Google Scholar]
- 16.Chu S-Y, Sennello LT, Sonders RC. Simultaneous determination of clarithromycin and 14-hydroxyclarithromycin in plasma and urine using high-performance liquid chromatography with electrochemical detection. J Chromatogr 1991;571:199–208. [DOI] [PubMed] [Google Scholar]
- 17.Goddard AF, Jessa MJ, Barrett DA, et al. Effect of omeprazole on the distribution of metronidazole, amoxycillin and clarithromycin in human gastric juice. Gastroenterology 1996;111:358–67. [DOI] [PubMed] [Google Scholar]
- 18.Goddard AF, Spiller RC. In vitro assessment of gastric mucosal transfer of anti-Helicobacter therapeutic agents. Antimicrob Agents Chemother 1997;41:1246–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lozniewski A, Duprez A, Renault C, et al. Gastric penetration of amoxicillin in a human Helicobacter pylori-infected xenograft model. Antimicob Agents Chemother 1999;43:1909–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shore PA, Brodie BB, Hogben CAM. The gastric secretion of drugs: a pH partition hypothesis. J Pharm Exp Ther 1956;119:361–9. [PubMed] [Google Scholar]
- 21.Goddard AF, Sherwood PV. Bioavailability of antimicrobials after oral and parenteral administration. In: Hunt RH, Tytgat GNJ, eds. Helicobacter pylori: Basic mechanisms to clinical cure. Dordrecht: Kluwer Academic Publishers, 1998:392–7.
- 22.Fietta A, Merlini C, Gialdroni Grassi G. Requirements for intracellular accumulation and release of clarithromycin and azithromycin by human phagocytes. J Chemother 1997;9:23–31. [DOI] [PubMed] [Google Scholar]
- 23.Newton JL, Jordan N, Pearson J, et al. The adherent gastric antral and duodenal mucus layer thins with advancing age in subjects infected with Helicobacter pylori. Gerontology 2000;46:153–7. [DOI] [PubMed] [Google Scholar]
- 24.Kalia N, Bardhan KD, Read MW, et al. Mechanisms of Helicobacter pylori-induced rat gastric mucosal microcirculatory disturbances in vivo. Dig Dis Sci 2000;45:763–72. [DOI] [PubMed] [Google Scholar]
- 25.Mégraud F, Trimoulet P, Lamouliatte H, et al. Bactericidal effect of amoxicillin on Helicobacter pylori in an in vitro model using epithelial cells. Antimicrob Agents Chemother 1991;35:869–72. [DOI] [PMC free article] [PubMed] [Google Scholar]