Abstract
Background: Nucleoside analogues such as lamivudine for chronic hepatitis B have an excellent safety profile while patients are on therapy but reactivation flares occur in 19–50% of patients after stopping therapy, some of whom develop liver decompensation.
Aims: To describe and report three cases who developed fatal hepatitis B reactivation after stopping nucleoside analogue therapy.
Subjects and results: Three patients are described who developed hepatitis B reactivation and liver decompensation after stopping therapy. One of the three patients was participating in a famciclovir trial and the other two were receiving lamivudine therapy for active hepatitis B infection. All three patients had documented hepatitis B flares, and all had hepatitis B virus DNA detected at that time. All patients developed decompensated liver disease despite one patient having had a prior liver biopsy showing absence of cirrhosis. Reintroduction of lamivudine therapy failed to halt progression of liver decompensation even after hepatitis B virus DNA had been demonstrated to be absent. Sequencing for lamivudine resistant mutants in two cases where serum was available failed to show evidence of mutations associated with lamivudine resistance.
Conclusion: Hepatitis B virus reactivation, leading to decompensation and death, are possible complications of treatment withdrawal and patients should be monitored closely if therapy is ceased.
Keywords: hepatitis B, lamivudine, reactivation, nucleoside analogues
The beneficial effects of lamivudine have been clearly established in a number of clinical trials.1,2 Famciclovir, another nucleoside analogue, appears to be less efficacious than lamivudine3 but has a similar profile of viral suppression and safety. Lamivudine appears to have a good safety profile to date.4 However, withdrawal of lamivudine, similar to other nucleoside analogues, results in rebound of hepatitis B virus (HBV) DNA in almost all patients,5–7 with an accompanying “flare” in transaminases in 19–50% of patients,5,6,8 and associated hepatic decompensation in a small number of patients.8,9 Consequently, the timing of elective lamivudine discontinuation is uncertain with the exception of patients who undergo hepatitis B e antigen (HBeAg) seroconversion.10 We describe and report three fatal cases of hepatic failure secondary to hepatitis B reactivation following withdrawal of nucleoside analogues.
CASE REPORTS
Case No 1: LLH, male, aged 36 years
Mr LLH, a Chinese man with HBeAg positive chronic hepatitis B infection on regular follow up since 1991, underwent a 12 month trial of famciclovir from 1996 to 1997. His baseline HBV DNA at trial entry was 34 mEq/ml (Chiron, Chiron Corp, California, USA) and alanine transaminase (ALT) level was 125 IU/l (normal <70). Although he was randomised to active famciclovir during the study period, his ALT remained abnormal, hepatitis B surface antigen (HBsAg) and HBeAg remained positive, but HBV DNA decreased to the range 2.5–9.6 mEq/ml. Famciclovir was stopped in December 1997, at the end of the study period, and liver biopsy at that time showed fatty change with mild fibrosis. On review at 3, 7, and 11 weeks post-famciclovir, LLH showed a progressive increase in HBV DNA from 26, 74, to 407 mEq/ml (Chiron), and ALT increased from 98 to 144 IU/l over this period of time.
He was then lost to follow up but presented with jaundice and epigastric discomfort 3.5 weeks after his last visit (14.5 weeks post-famciclovir). At this time, he was found to be jaundiced and unwell. Liver biochemistry showed total bilirubin 243 μM (normal <30), ALT 2539 IU/l (normal <70), aspartate aminotransferase (AST) 1905 IU/l (normal <60), and prothrombin time 29.7 seconds (normal <14.5). Testing excluded coinfection with hepatitis A, C, and delta agent, and HBV DNA was found to be 72.61 pg/ml by Abbott assay (Abbott Genostics, Abbott Laboratories, Chicago, Illinois, USA). This approximates to 1452 mEq/ml (Chiron assay) based on a conversion formula.11
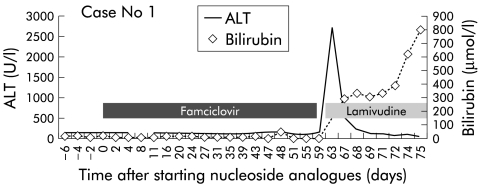
A computed tomography (CT) scan of the abdomen showed a small liver and gross ascites. A diagnosis of decompensated hepatitis B secondary to hepatitis B reactivation following cessation of famciclovir was made, and lamivudine therapy was instituted. After four weeks of lamivudine therapy, HBV DNA became undetectable (Chiron) but his clinical condition deteriorated gradually. He developed grade II encephalopathy, rising bilirubin (peak 418 μM), and prolonged prothrombin time (peak 63.9 seconds) over a six week period (see fig 1 ▶). During this time, he was assessed and listed for liver transplantation. However, LLH discharged himself against medical advice six weeks after admission but was rehospitalised five days later in grade III encephalopathy with a bilirubin of 656 μM and prothrombin time >180 seconds. He developed a left hemiplegia and became unrousable. An intracranial bleed was confirmed on CT scan and he died two days after admission, almost 16 weeks post-famciclovir.
Figure 1.
Alanine transaminase and bilirubin levels in patient No 1.
Case No 2: CSY, male, 66 years
Mr CSY, a 66 year old Chinese man with known chronic hepatitis B cirrhosis, was started on lamivudine in March 1998, and one month later his HBV DNA was negative and ALT was normal. After 14 months of continuous lamivudine, a trial of lamivudine discontinuation was instituted. On review, his HBV DNA was 143 mEq/ml (Chiron) (normal <0.7) four weeks after stopping lamivudine. Seven weeks after stopping lamivudine, he was admitted to hospital for hepatitis B reactivation and hepatic decompensation. At that time, ALT was 2140 IU/l (normal<40), total bilirubin was 308 μM (normal>30), and prothrombin time was 29.5 seconds (control 12). Coinfection with hepatitis A, C, and delta agent were excluded. He had not been taking any concurrent medication or Chinese herbal remedies.
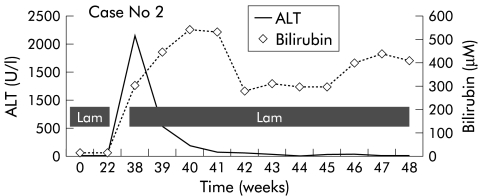
Lamivudine 150 mg daily was immediately restarted and HBV DNA (Chiron) became negative three weeks after restarting lamivudine. However, he continued to deteriorate and developed ascites and ankle oedema. Subsequently, he progressed to grade II encephalopathy with worsening ascites and peripheral oedema. His ALT normalised but bilirubin (281 μM) and prothrombin time (30.4 seconds) worsened (see fig 2 ▶). He was then listed for liver transplantation. However, before a suitable donor became available, he developed sepsis and grade IV encephalopathy that required ICU management. He then developed hepatorenal syndrome complicated by sepsis that led to his demise 19 weeks after discontinuing lamivudine and 11 weeks after restarting lamivudine.
Figure 2.
Alanine transaminase and bilirubin levels in patient No 2.
Case No 3: TTG, male, aged 37 years
Mr TTG, a 37 year old Chinese man, was diagnosed with decompensated chronic hepatitis B liver disease in November 1998, having presented with jaundice, ascites, and hepatic encephalopathy. Investigations showed that bilirubin was 268 μM, ALT 878 U/l, AST 430 U/l, albumin 30 g/l, prothrombin time 41.8 seconds, HBV DNA 7.65 mEq/ml (Chiron), HBeAg was negative, and a shrunken cirrhotic liver with ascites was found on ultrasound. Lamivudine 100 mg daily was started. His condition gradually recovered and 12 months later the patient was noted to be well with no evidence of ascites or hepatic decompensation. At that time his liver panel and prothrombin time had normalised, albumin had increased to 43 g/dl, and HBV DNA was negative.
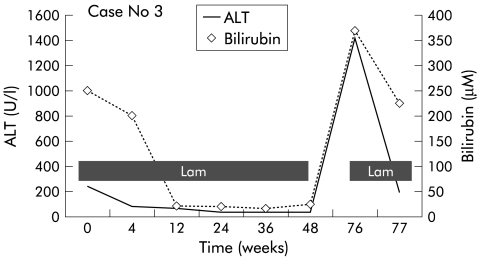
However, he then defaulted follow up, and was admitted six months later with jaundice and grade IV hepatic encephalopathy, having stopped lamivudine approximately three months earlier. He was intubated and transferred to the intensive care unit for active resuscitation. Investigations on admission showed that bilirubin was 347 μM, ALT 836 U/l, AST 492 U/l, albumin 27 g/l, gamma glutamyl transferase 65 U/l, and prothrombin time 76.1 seconds; HBeAg was positive and HBV DNA was 20 400 mEq/ml (Chiron) (see fig 3 ▶). CT of the brain showed cerebral oedema. The clinical impression was acute exacerbation of hepatitis B following lamivudine withdrawal and return of HBeAg. Lamivudine was restarted at a dose of 300 mg daily. However, despite maximum support, he died of liver failure and septicaemia shortly thereafter.
Figure 3.
Alanine transaminase and bilirubin levels in patient No 3.
HBV sequencing and detection of lamivudine resistant mutations
Saved serum was available from patient Nos 1 and 3 at the time of HBV DNA rebound following lamivudine cessation. DNA was extracted from 100 μl of serum using QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany). Serum extracted DNA (5 μl) was used as the polymerase chain reaction template to amplify an approximately 750 base pair region defined by nucleotide 253 and nucleotide 1006 of the HBV genome using HotStarTaq polymerase (Qiagen). This region corresponds to all published mutations associated with lamivudine resistance. Annealing was carried out at 50°C for one minute, and 35 cycles were used. Forward primer F253 (5‘-GAC TCG TGG TGG ACT TCT CTC AA-3‘) and reverse primer R1006 (5‘-CCC ACA ATT CTT TGA CAT ACT TTC C-3‘) were used. The forward and reverse sequences were aligned using the Seqman program (DNAstar).
Results of the sequencing showed that patient Nos 1 and 3 had no evidence of mutations associated with lamivudine resistance. Potential mutations that were examined included L428V/I, L430M, V511I, F514L, L528M, A529T, L535I, A548V, M552V(YVDD), M552I (YIDD), A5548V, V/L/M555I, S561T, S567A, and A570T.
DISCUSSION
In all cases, there was good documentation of increased HBV DNA together with increased ALT after discontinuing famciclovir or lamivudine. The close temporal relationship of deterioration in liver biochemistry and liver function to nucleoside analogue discontinuation (the longest was three months after discontinuation) makes this the most compelling reason for their liver decompensation, after coinfection with other hepatotrophic viruses were excluded.
Patients with cirrhosis are thought to have a higher risk of hepatic decompensation following a transaminase flare during a hepatitis B reactivation event,12 but in case No 1 there was no evidence of cirrhosis on liver biopsy, which suggests that all patients with hepatitis B may be at risk of this event on drug withdrawal. Reported cases of transaminase flares following lamivudine withdrawal occurred in patients with abnormal liver function tests,6 and less so in those that had mild abnormalities in liver function.13 In the only reported case of death following nucleoside analogue withdrawal (famciclovir) in a patient with hepatitis B, the patient had underlying cirrhosis.14
In two of the cases reported here, lamivudine was unable to prevent the progressive deterioration in hepatic function despite reduction of HBV DNA below the detectable limit (indicating that the virus was lamivudine responsive). In cases of severe liver decompensation, the effects of lamivudine in reducing viral load may not have been rapid enough (approximately 3–4 weeks required to decrease HBV DNA by >90%13) to reverse liver decompensation. Hence lamivudine may not be able to rescue such patients. No lamivudine resistant mutants were discovered during sequencing of serum, indicating that this was not a cause of the failure to respond clinically to lamivudine.
When can nucleoside analogues be safely stopped? It appears that patients who develop HBeAg seroconversion during lamivudine therapy10 can be safely withdrawn. In the setting of anti-hepatitis B e antigen positive chronic hepatitis B, the timing of cessation of nucleosides and duration of therapy is uncertain. In the 41 cases where lamivudine was stopped under controlled conditions and close monitoring,8 17.5% of patients developed hepatitis flares and 5% developed incipient liver failure. Our three fatal cases are likely to reflect the most extreme type of adverse event associated with the general use of lamivudine in hepatitis B patients where close monitoring of patients may not occur or be possible. Under these circumstances, patients may cease lamivudine of their own accord or with the consent of their physician. Physicians intending to treat patients with chronic hepatitis B using nucleoside analogues need to warn patients that stopping treatment may have serious consequences, particularly if patients have cirrhosis or remain HBeAg positive. Patients stopping therapy should be closely monitored for evidence of reactivation flares, and lamivudine should be reinstituted before significant liver decompensation occurs.
Abbreviations
ALT, alanine transaminase
AST, aspartate aminotransferase
CT, computed tomography
HBsAg, hepatitis B surface antigen
HBeAg, hepatitis B e antigen
HBV, hepatitis B virus
REFERENCES
- 1.Lai CL, Chien RN, Leung NW, et al. A one-year trial of lamivudine or chronic hepatitis B. Asia Hepatitis Lamivudine Group. N Engl J Med 1998;339:61–8. [DOI] [PubMed] [Google Scholar]
- 2.Tassopoulos NC, Volpes R, Pastore G, et al. Efficacy of lamivudine in patients with hepatitis B e-antigen-negative/hepatitis B virus DNA-positive (precore mutant) chronic hepatitis B. Lamivudine Precore Mutant Study Group. Hepatology 1999;29:889–96. [DOI] [PubMed] [Google Scholar]
- 3.Gunther S, von Breunig F, Santantonio T, et al. Absence of mutations in the YMDD/motif/B region of the hepatitis B virus polymerase in famciclovir therapy failure. J Hepatol 1999;30:101–41. [DOI] [PubMed] [Google Scholar]
- 4.Jarvis B, Faulds D. Lamivudine. A review of its therapeutic potential in chronic hepatitis B. Drugs 1999;58:101–41. [DOI] [PubMed] [Google Scholar]
- 5.Nevens F, Main J, Honkoop P, et al. Lamivudine therapy for chronic hepatitis B: a six-month randomised dose-ranging study. Gastroenterology 1997;13:1258–63. [DOI] [PubMed] [Google Scholar]
- 6.Dienstag JL, Perrillo RP, Schiff ER, et al. A preliminary trial of lamivudine for chronic hepatitis B infection. N Engl J Med 1995;333:1657–61. [DOI] [PubMed] [Google Scholar]
- 7.Honkoop P, de Man RA, Niesters HG. Quantitative assessment of hepatitis B virus DNA during a 24-week course of lamivudine therapy. Ann Intern Med 1998;128:697. [DOI] [PubMed] [Google Scholar]
- 8.Honkoop P, de Man RA, Niesters HG, et al. Acute exacerbation of chronic hepatitis B virus infection after withdrawal of lamivudine therapy. Hepatology 2000;32:635–9. [DOI] [PubMed] [Google Scholar]
- 9.Honkoop P, de Man RA, Niesters HG, et al. Hepatitis B reactivation after lamivudine. Lancet 1995;346:1156–7. [DOI] [PubMed] [Google Scholar]
- 10.Dienstag JL, Schiff ER, Mitchell M, et al. Extended lamivudine retreatment for chronic hepatitis B: maintenance of viral suppression after discontinuation of therapy. Hepatology 1999;30:1082–7. [DOI] [PubMed] [Google Scholar]
- 11.Hwang SL, Lee SD, Lu RN, et al. Comparison of three different hybidization assays in the quantitative measurement of serum hepatitis B virus DBA. J Virol Methods 1996;62:123–9. [DOI] [PubMed] [Google Scholar]
- 12.Liaw YF, Chen JJ, Chen TJ. Acute exacerbation in patients with liver cirrhosis: a clinicopathological study. Liver 1990;10:177–84. [DOI] [PubMed] [Google Scholar]
- 13.Lai CL, Ching CK, Tung AK, et al. Lamivudine is effective in suppressing hepatitis B virus DNA in Chinese hepatitis B surface antigen cariers: a placebo-controlled trial. Hepatology 1997;25:241–4. [DOI] [PubMed] [Google Scholar]
- 14.Myers RP, Chaudhary R, Fonseca K, et al. Fatal hepatic decompensation in a patient with hepatitis B cirrhosis following famciclovir withdrawal. Can J Gastroenterol 2000;14:725–7. [DOI] [PubMed] [Google Scholar]