Abstract
Backgrounds: Microvillus inclusion disease (MID) is a disorder with the clinical signs of intractable diarrhoea in the newborn and infancy. The typical pathological features of the disease are well known whereas the pathophysiology is still unclear.
Aim: This study was performed to define possible alterations of the cytoskeleton and exocytic as well as endocytic pathways within enterocytes in MID.
Patients: Four patients with MID were studied. Three had a congenital onset of diarrhoea and one patient had a late onset form.
Methods: Thin frozen sections of small bowel biopsies of patients were labelled by antibodies against the cytoskeleton and the brush border enzyme sucrase-isomaltase. The binding sites of the primary antibodies were visualised by immunogold particles in the electron microscope. Biopsies were labelled in organ culture to analyse the biosynthetic and endocytic pathways within the enterocytes.
Results: Labelling with antibodies against actin and villin did not differ significantly in control and patient biopsies. Biosynthetic labelling revealed normal intracellular processing and transport of the brush border enzyme sucrase-isomaltase. Secretory granules in crypt epithelial cells were positive for sucrase-isomaltase, differing in its labelling density between patients. Patient biopsies showed microvillus inclusion bodies which endocytosed cationised ferritin within five minutes after uptake as well as ovalbumin after incubation for 10 minutes. These microvillus inclusion bodies correspond to early endosomes because they lack lysosome associated membrane proteins. Late endosomes and lysosomes containing sucrase-isomaltase did not reveal microvillus-like structures.
Conclusion: Microvillus inclusion bodies in MID originate from autophagocytosis of the apical membrane of enterocytes with engulfing of microvilli.
Keywords: microvillus inclusion bodies, autophagocytosis, early endosomes
Microvillus inclusion disease (MID) is a disorder within the syndrome of intractable diarrhoea of infancy.1 The main pathological features of the disease include villus atrophy and accumulation of periodic acid-Schiff (PAS) positive material within the apical cytoplasm of enterocytes at the light microscopic level.1,2 Electron microscopic criteria are pathognomonic consisting of an increased amount of secretory granules preferentially in crypt epithelial cells and the presence of microvillus inclusion bodies (MIBs) which are most frequently found in villus enterocytes.3,4 The outcome of patients is still poor, with parenteral nutrition and bowel transplantation the only therapeutic options.1,2,5 More efficient treatments can only be developed when the pathogenetic mechanism of MID has been understood.
Since Davidson described this disease in 1978, the molecular defect as well as the pathophysiology have remained unclear.1 A founder effect of a candidate gene has been attributed to the Navajo population.6 It was suggested that MID represents a defect of the biosynthetic pathway.7,8 An increased number of secretory granules as well as an abnormal accumulation of PAS positive material within the apical cytosol of crypt epithelial cells indicated a block in the normal transport of granules from the Golgi complex to the apical membrane. Because acid phosphatase was not localised within MIBs and their membranes appear to be that of the microvillus, an autophagocytotic nature of these bodies was withdrawn.1 It was further proposed that components of the cytoskeleton such as actin, myosin, and vinculin are involved in the pathogenesis of MID.9–11
The purpose of this study was to address the pathogenetic concepts of MID, applying functional assays for analysing the intracellular pathway of enterocytes, as well as a quantitative method to determine the density of villin and actin within microvilli of MID enterocytes. Using duodenal biopsies from MID patients, we performed immunoelectron microscopic studies and metabolic labelling in organ culture to analyse the biosynthetic and endocytic transport pathway within enterocytes of this disease.
METHODS
Patients
Four patients with MID were studied (table 1 ▶). Two (case Nos 1 and 2) were brother and sister of consanguine parents. Three had a congenital onset of diarrhoea and one patient had a late onset form of the disease. All suffered from intractable diarrhoea and required total parenteral nutrition.
Table 1.
Clinical data
| Case No | Sex | Onset | Age at biopsy | Present age | Clinical outcome | Investigation |
| 1 | M | Congenital | 5 weeks and 11 months | 17 months | On TPN, growth on 50% percentile | IEM, BOC, OCT |
| 2 | F | Congenital | 2 weeks | 9 months | On TPN | IEM |
| 3 | M | Congenital | 3 weeks | 20 months | On waiting list for transplantation | IEM |
| 4 | F | Late onset | 3 and 7 months | 16 months | On TPN | IEM |
IEM, Immunogold electron microscopy; BOC, biosynthetic labelling of saccharase isomaltase in organ culture; OCT, organ culture with the endocytic tracers ovalbumin and ferritin; TPN, total parenteral nutrition.
In each child, the diagnosis was established by light and electron microscopy of proximal small intestinal mucosal biopsy specimens demonstrating the characteristic features: pathological PAS staining of enterocytes, microvillus atrophy, MIBs in villus enterocytes, and a large amount of granules at the apical region of crypt epithelial cells.
Preparation of duodenal biopsies
Fresh duodenal biopsies from two patients were incubated with ovalbumin (OVA, fraction V; Sigma, St Louis, Missouri, USA) and cationised ferritin (Sigma) for 5, 10, and 20 minutes at room temperature before preparation for immunoelectron microscopy and conventional electron microscopy. OVA was dissolved in 0.15 M sterile saline at a concentration of 100 mg/ml (360 mosmol/kg). Cationised ferritin was used at a concentration of 0.5 mg/ml diluted with phosphate buffered saline. The samples were shaken at 37°C during incubation. For conventional electron microscopy, biopsies were fixed in 3% glutaraldehyde in 0.1 mol/l sodium cacodylate buffer (pH 7.2) at room temperature for 60 minutes, postfixed in OsO4, dehydrated in graded ethanol solutions, and embedded in Epon 812.
Antibodies
The primary antibodies used in this study were as follows: monoclonal mouse antibody against human actin (dilution 1:100; CLT 9001; Cedarlane, Ontario, Canada), polyclonal rabbit antibody against human villin (dilution 1:30), monoclonal mouse and polyclonal rabbit antibodies against sucrase-isomaltase (SI) (dilution 1:50 and 1:1.000), monoclonal mouse anti-lysosome associated membrane protein 1 (LAMP-1) antibody (dilution 1:30; Pharmingen, Hamburg, Germany), and monoclonal mouse antibody against OVA (dilution 1:10; Sigma).
The binding sites of the primary antibodies were visualised by electron microscopy using gold conjugated goat sera against rabbit and mouse IgG conjugated by gold particles with a diameter of 6 and 12 nm (dilution 1:10 and 1:50; Dianova, Hamburg, Germany).
Immunolabelling of frozen sections for immunoelectron microscopy
Duodenal biopsies from all patients were frozen in liquid nitrogen after fixation in 5% paraformaldehyde in 50 mmol/l HEPES and cryoprotection by polyvinylpyrrolidone/sucrose. Sectioning and labelling of ultrathin frozen sections (50 nm) using the technique of Tokuyasu were performed as described previously.12,13 Small tissue specimens were sectioned with an ultracryomicrotome electron microscope FCS (Leica, Bensheim, Germany) at −110°C. Thawed sections were incubated for 45 minutes with antibodies at room temperature. After labelling with immunogold, the grids were contrasted with uranyl acetate and embedded in 2% methylcellulose. Examination was performed in a Philips 400 electron microscope (Kassel, Germany).
Quantitative evaluation of labelling densities for actin and villin
Quantitation of actin and villin within enterocytes was assessed by counting the numbers of gold particles per area of sectioned microvilli. Randomised electron microscopy pictures of each patient were taken from the apical part of enterocytes at a magnification of 25 000. The relative number of gold particles per μm2 area labelling microvilli were determined by point counting, as described elsewhere.12,14 Statistical analysis was performed using the Student's t test.
Biosynthetic labelling
Biopsy specimens from one patient were cultured and labelled as described previously.15 Three small pieces of the biopsy were labelled in organ culture with 35S methionine for 30 minutes, four hours, and 18 hours. Triton X-100 detergent extracts of the labelled samples were immunoprecipitated with monoclonal antibodies directed against the brush border protein SI. Immunoprecipitates were finally analysed by sodium dodecyl sulphate-polyacrylamide gel electrophoresis with or without treatment with endoglycosidase H.
RESULTS
Ultrastructural localisation of actin and villin in MID enterocytes
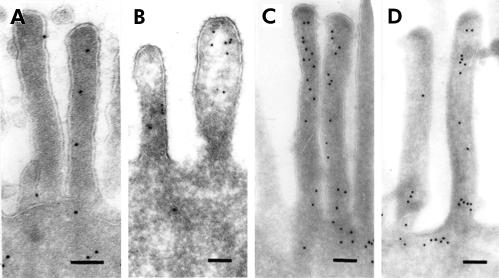
Labelling of thin frozen sections with anti-villin and anti-actin antibodies showed gold particles within microvilli of enterocytes varying in number between the microvilli of single enterocytes from control and diseased biopsies (fig 1 ▶). Binding sites for actin were also detected within the terminal web and to a lesser extent at the basolateral membrane of enterocytes. According to the position of the enterocytes on the crypt-villus axis, no difference in staining of actin and villin was found.
Figure 1.
Thin frozen sections of control (A, C) and patient (B, D) small bowel biopsies labelled by antibodies against villin (A, B) and actin (C, D). Labelling densities did not differ between the microvilli of patient and control samples by qualitative means. Bar=0.1 μm.
Quantification of actin and villin labelling revealed no significant differences between patient and control biopsies. Labelling density for the actin antibody within the microvilli revealed 35.4–49.8 gold particles/μm2 in the biopsies of three patients compared with 38.5 gold particles/μm2 in three control biopsies (table 2 ▶). Villin labelling density within the microvilli showed 33.6 (patient No 2) and 19.8 (patient No 4) gold particles/μm2 in patient biopsies in comparison with 44.7 particles/μm2 in four control biopsies (table 3 ▶). The Student's t test for patients versus control showed t=0.7 for actin labelling and t=0.8 for villin labelling.
Table 2.
Quantification of labelling density for actin
| Control | Patient 1 | Patient 2 | Patient 3 | |
| No of EM pictures | 36 | 27 | 31 | 27 |
| Gold particles/μm2 | 38.5 (18.0) | 35.4 (16.1) | 49.8 (19.8) | 21.7 (8.4) |
EM, electron microscopy.
Table 3.
Quantitation of labelling density for villin
| Control | Patient 2 | Patient 4 | |
| No of EM pictures | 41 | 19 | 20 |
| Gold particles/μm2 | 44.7 (17.5) | 33.6 (27.3) | 19.83 (12.0) |
EM, electron microscopy.
Endocytic pathway in MID enterocytes
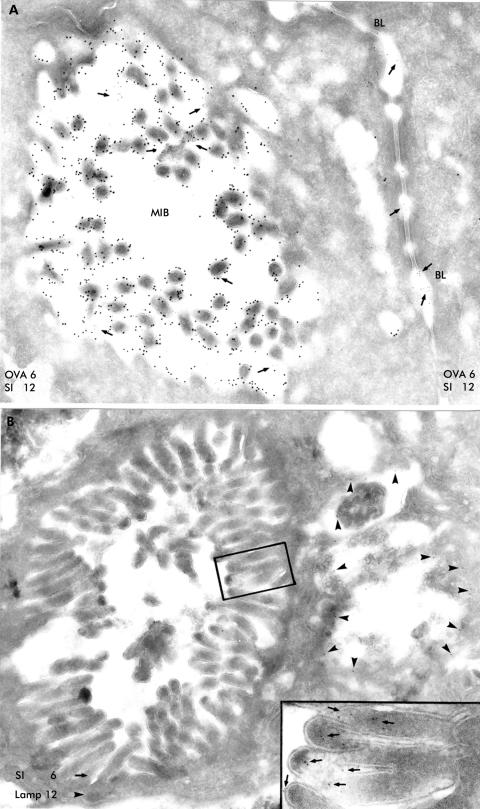
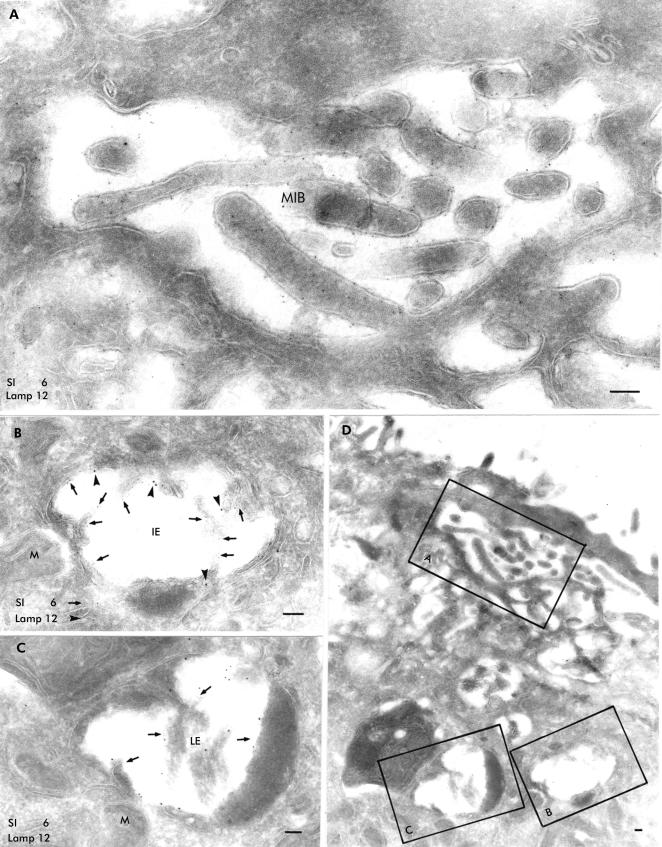
Double labelling experiments with antibodies against SI and LAMP showed that MIBs were positive for SI (fig 2A ▶) but negative for LAMP (fig 2B ▶). Villus enterocytes presented SI positive vacuoles whose morphology differed. Vacuolar bodies with complex internal structures, including single or groups of SI positive brush border microvilli, were observed at the apical membrane and were not labelled by the LAMP antibody (fig 3A ▶). In addition, there were differences in appearance between those which contained SI positive microvillus-like projections within the lumen without LAMP labelling and those which showed weak staining for SI and many LAMP molecules on the outer membrane (fig 3B ▶). Furthermore, some vacuoles were strongly positive for LAMP but revealed only weak SI staining (fig 3C ▶). These distinct structures were simultaneously noted in single villus enterocytes whose apical surface presented with few markedly shortened and disorganised microvilli (fig 3D ▶).
Figure 2.
Thin frozen sections of a bowel biopsy obtained from a patient with microvillus inclusion disease. The biopsy was incubated with ovalbumin (OVA) for 10 minutes before processing for immunoelectron microscopy. (A) This section was simultaneously labelled by a monoclonal antibody against OVA and 6 nm large immunogold particles as well as a polyclonal antibody against sucrase-isomaltase (SI) and 12 nm large immunogold particles. Endocytosed OVA (arrows) was present within the microvillus inclusion body (MIB) in a villus enterocyte and the paracellular space. (B) Double labelling with the SI antibody indicated by 6 nm large immunogold particles and the lysosome associated membrane protein (LAMP) antibody indicated by 12 nm large immunogold particles revealed that MIBs in a villus epithelial cell were negative for LAMP. The inset shows an enlarged detail of the microvilli from the MIB with visualisation of the SI binding sites by 6 nm large gold particles (arrows). LAMP positive organelles are situated beside the MIB (on the right side of the figure); arrowheads indicate 12 nm large gold particles of LAMP binding sites. BL, basolateral membrane.
Figure 3.
Ultrastructural localisation of sucrase-isomaltase (SI) and lysosome associated membrane protein (LAMP) on a thin frozen section from a patient small bowel biopsy. Binding sites for SI are indicated by 6 nm large gold particles and those of LAMP by 12 nm large gold particles. (A) Microvillus inclusion body (MIB) situated close to the apical membrane (see overview in (D)) contained microvilli labelled by the SI antibody (6 nm large gold particles); there was no significant LAMP labelling within MIB. (B) Intermediate endosome located below the apical region (see overview in (D)) contained SI (6 nm large gold particles, arrows) on microvilli-like projections and few LAMP molecules (12 nm large gold particles, arrowheads). (C) Late endosome within the intermediate endosome (B) and an autophagic lysosome showed many LAMP molecules (12 nm large gold particles) but only a few SI binding sites (6 nm large gold particles) without any detectable microvilli. (D) Overview from this villus enterocyte; the insets reveal the location of (A), (B), and (C). The apical membrane showed only few and shortened microvilli. IE, early endosome; LE, late endosome; M, mitochondrium; bars=0.1 μm.
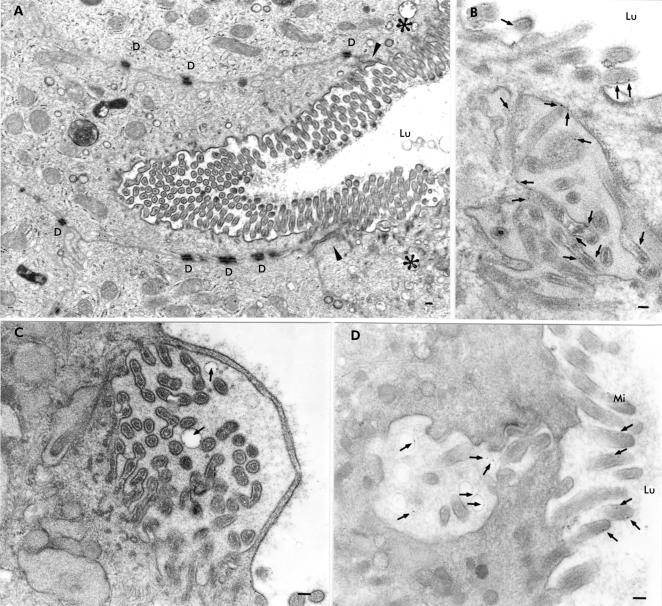
Patient biopsies disclosed villus enterocytes whose brush border was engulfed inwards (fig 4A ▶) and whose microvilli were covered by a thin cytosolic strand (fig 4C ▶). Biopsies incubated with cationised ferritin for five minutes after taking biopsies and embedded in Epon revealed ferritin localised to microvillus membranes (fig 4B ▶) and vesicular membranes within MIBs close to the apical membrane of the enterocytes (fig 4C, D ▶). Specimens incubated with OVA for 10 minutes showed OVA containing MIBs which were labelled by anti-SI antibodies (fig 2A ▶).
Figure 4.
Epon sections of small bowel biopsies from patients with microvillus inclusion disease demonstrating autophagocytosis of the apical membrane of the villus enterocyte. The biopsies shown in (B) and (C) were incubated with cationised ferritin (arrows) for five minutes. (A) Engulfing of the apical membrane deeply inwards in the enterocyte. Arrowheads indicate the zone between the apical and basolateral membranes and the asterisks the apical region of the neighbouring enterocytes. (B) Cationised ferritin appears to be attached to the membranes of microvilli contained within a microvillus inclusion body which was located in the vicinity of the apical membrane. (C) Infolded microvilli covered by a thin cytosolic strand. The structure contains two vesicles with cationised ferritin particles on their membranes (arrows). (D) Cationised ferritin (arrows) is not only detectable on microvilli but also within vesicles of an invagination of the apical membrane. D, desmosome; Lu, intestinal lumen; Mi, microvilli; bars=0.1 μm.
Biosynthetic pathway in MID enterocytes
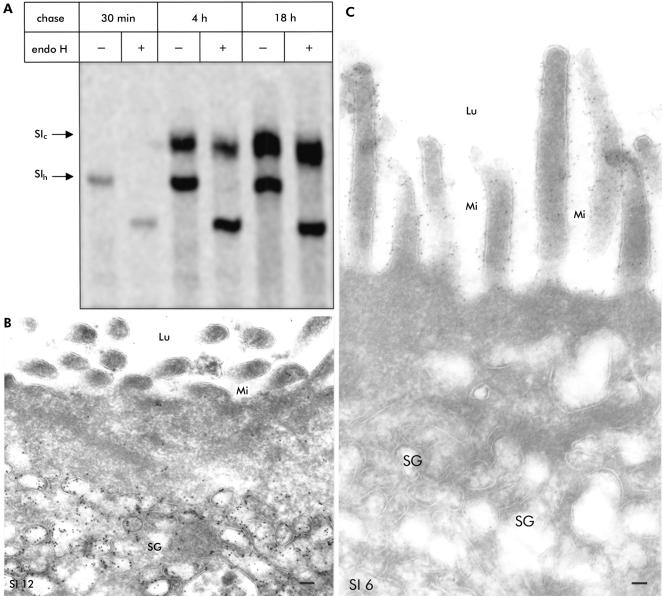
Biosynthetic labelling of the patient's biopsy sample in organ culture revealed normal intracellular processing and transport of the brush border protein SI compared with its counterpart in control biopsy samples. This was demonstrated by the normal conversion rates of the endo H sensitive mannose rich precursor forms of these proteins (SIh) to the endo H resistant complex glycosylated and mature species (SIC) (fig 5A ▶).
Figure 5.
Exocytic pathway within enterocytes of patients with microvillus inclusion disease. (A) Biosynthetic labelling of the patient's biopsy sample in organ culture: endo H resistant glycoprotein which corresponds to complex glycosylated mature SIc is detected after four hours. SIh is the endo H sensitive mannose rich precursor form. (B) Thin frozen section of a diseased small bowel labelled with the sucrase-isomaltase (SI) antibody and 12 nm large immunogold particles. The secretory granules in the apical region of this crypt epithelial cell were strongly positive for SI. In contrast, the apical membrane does not contain SI. (C) Crypt epithelial cell with strong SI labelling on the apical membrane indicated by 6 nm large immunogold particles. In contrast, there was no SI staining of the secretory granules within the apical region of this enterocyte. Lu, intestinal lumen; Mi, microvilli; SG, secretory granules; bar=0.1 μm.
Immunoelectron microscopy showed preferentially in patient No 1 as well as in patient No 3 that a minority of crypt epithelial cells contained many SI positive granules (fig 5 ▶) in addition to a majority of crypt epithelial cells with granules that were completely negative for SI (fig 5C ▶). Crypt epithelial cells with SI positive secretory granules (fig 5B ▶) as well as enterocytes containing MIBs (fig 3D ▶) did not reveal SI on the apical membrane. Most enterocytes showed dense labelling for SI at the brush border in all patients, and no significant difference between patients and control biopsies in labelling density was found. Other intracellular structures such as the endoplasmic reticulum and Golgi stacks were labelled by only a few gold particles. The apically located vesicles were negative for cationised ferritin in the biopsies incubated with this ligand for 5–10 minutes (not shown).
DISCUSSION
Electron microscopic studies in MID revealed unique MIBs within enterocytes that do not occur in any other intestinal disorder.1,2,4,16 An additional characteristic feature of MID is the marked increase in electron dense vesicles in the apical cytosol of crypt epithelial cells.
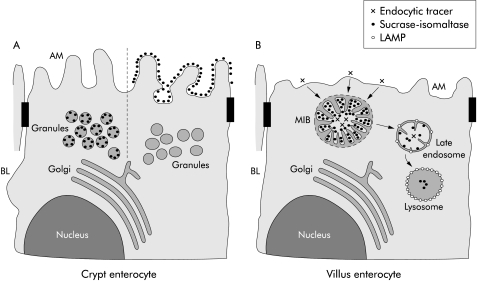
Because of the large amount of secretory granules in MID, a transport defect within enterocytes seems likely.3,7,8 Philips et al have already shown that the direct and indirect constitutive pathways of enterocytes are intact in MID.16 These findings were confirmed by our results demonstrating normal intracellular processing and transport of the brush border protein SI. Most of the secretory granules in the apical region of a minority of crypt epithelial cells were significantly labelled by the anti-SI antibody. This is in contrast with Philips et al who found SI at the apical membrane and in the MIBs but not in secretory granules.16 We suggest in our model of MID (fig 6A ▶) that the transport granules deliver SI (and other brush border proteins) to the apical membrane but to a different extent in different patients, most likely depending on which secretory protein is induced. This is supported by the observation that (induced) enterocytes with SI positive secretory granules do not contain SI on the apical membrane in contrast with (uninduced) enterocytes with SI negative secretory granules and SI positivity of the brush border. While the presence of immunoreactive SI in the apical membrane supports the concept of an intact biosynthetic pathway in MID, the high number of SI positive granules within the apical region of crypt epithelial cells may be indicative of delayed fusion of these granules within the apical membrane.
Figure 6.
A model of the exocytic and endocytic pathways in crypt and villus microvillus inclusion disease (MID) epithelial cells. (A) Secretory granules, which are present in crypt epithelial cells, are labelled by the sucrase-isomaltase (SI) antibody with variable intensity. Crypt epithelial cells whose secretory granules do not contain SI show SI labelling on the apical membrane (AM). In contrast, crypt epithelial cells with no SI labelling on microvilli reveal secretory granules with SI staining. (B) Villus enterocytes with detectable microvillus inclusion bodies (MIBs) are characterised by a few and shortened microvilli. Microvilli within MIBs are labelled by the SI antibody but are negative for lysosome associated membrane protein (LAMP) indicating their early endosomal nature. Late endosomes of MID enterocytes, which are identified by LAMP positivity, reveal few microvilli-like projections and SI labelling. Lysosomes, which are strongly positive for LAMP, contain a low amount of SI without any microvilli-like structures. BL, basolateral membrane.
Because acid phosphatase, a lysosomal marker, was not found in MIBs, it was postulated that MIBs do not originate from autophagocytosis of the apical membrane.1 It was further suggested that inclusion bodies develop due to a block within the exocytic pathway with retention of transport granules within the cytosol. Smaller granules were thought to develop into MIBs or large vesicular bodies.7 Our findings obtained under organ culture conditions demonstrate that cationised ferritin and OVA are possibly endocytosed as ligands from the apical surface of MID enterocytes within 5–10 minutes and are then localised within MIBs. This indicates that MIBs originate from autophagocytosis of engulfing brush border membranes. Induction of autophagocytosis and formation of MIB-like structures in epithelial cells by drugs depolymerising microtubules17,18 and actin10 support the concept that a defect in the cytoskeleton of enterocytes is involved in the generation of MIBs. MIB-like structures termed vacuolar apical compartment are produced in MDCK cells kept under low calcium conditions which prevents the establishment of cell-cell contact.19 There are many reports on epithelial cells which form organelles with an intracellular lumen.20 Thus intracellular lumen has been described in many epithelial cells, including cancer cells with increased development under culture conditions. It is interesting that dysfunction of the cytoskeleton is discussed in all of these instances.
Because MIBs do not contain LAMPs which are present in late endosomes, lysosomes, and to a lesser extent in early endosomes,21 they are considered to be early endosomes which is in accordance with their lack of acid phosphatase.1 Early endosomes constitute the first endocytic compartment through which ligands internalised from the cell surface are recycled or pass en route to late endosomes and lysosomes.22 Ligands appear in these organelles which are less acidic than late endosomes and lysosomes, within five minutes after its uptake.23 We have recently shown that enterocytes endocytose luminal OVA into early and late endosomes and transport this ligand to the basolateral membrane and the paracellular space within 5–10 minutes after its oral administration.13 We assume that the morphology of microvilli within MIBs disappears during further progression and degradation within late endosomes and lysosomes which are the organelles with the strongest presence of acid phosphatase and LAMP.
While this study served to disclose the pathogenetic mechanism of MIB formation in MID, the basic molecular defect of MID remains obscure. Our quantitative results do not confirm any structural abnormalities of actin and villin in MID enterocytes although a functional defect of these proteins cannot be excluded by the applied experimental approach. Nevertheless, ample evidence indicates that the molecular defect of MID is localised within the cytoskeleton of the MID enterocyte. Components of the cytoskeleton such as actin and tubulin are not only involved in endocytic24 but also in secretory processes25,26 of (polarised) epithelial cells. Further studies are necessary to define the basic molecular genetic defect of the cytoskeleton in MID.
In summary, our results refute a defect in the biosynthetic pathway of MID enterocytes. Our data suggest that MID is a disorder caused by increased autophagocytosis of the apical membrane of enterocytes with the formation of MIBs by engulfing of microvilli.
Acknowledgments
The authors thank Cordula Westermann for excellent technical assistance; Dr A Zweibaum (INSERM U178, Villejuif Cedex, France) for the polyclonal antibody against sucrase-isomaltase; and Dr D Louvard (Curie Institute, Paris, France) for the polyclonal antibody against human villin. The study was supported by the German Research Foundation (DFG-Zi 294/6-1).
Abbreviations
MID, microvillus inclusion disease
SI, sucrase-isomaltase
LAMP, lysosome associated membrane protein
OVA, ovalbumin
PAS, periodic acid-Schiff
MIB, microvillus inclusion body
REFERENCES
- 1.Davidson G, Cutz E, Hamilton J, et al. Familial enteropathy: A syndrome of protracted diarrhea from birth, failure to thrive, and hypoplastic villous atrophy. Gastroenterology 1978;75:783–90. [PubMed] [Google Scholar]
- 2.Phillips A, Schmitz J. Familial microvillus atrophy: A clinico-pathological survey of 23 cases. J Pediatr Gastroenterol Nutr 1992;14:380–96. [PubMed] [Google Scholar]
- 3.Phillips A, Szafransky M, Man LY, et al. Periodic acid-Schiff staining abnormality in microvillus atrophy: Photometric and ultrastructural studies. J Pediatr Gastroenterol Nutr 2000;30:34–42. [DOI] [PubMed] [Google Scholar]
- 4.Phillips A, Jenkins P, Raafat F, et al. Congenital microvillus atrophy: Specific diagnostic features. Arch Dis Child 1985;60:135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Couper RTL, Berzen A, Berall G, et al. Clinical response to the longacting somatostatin analogue SMS 201-995 in a child with congenital microvillus atrophy. Gut 1989;30:1020–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pohl JF, Mitchel DS, Trevelline EE, et al. A cluster of microvillous inclusion disease in the Navajo population. J Pediatr 1999;134:103–6. [DOI] [PubMed] [Google Scholar]
- 7.Cutz E, Rhoads M, Drumm B, et al. Microvillus inclusion disease: An inherited defect of brush-border assembly and differentiation. N Engl J Med 1989;320:646–51. [DOI] [PubMed] [Google Scholar]
- 8.Ameen NA, Salas P. Microvillus inclusion disease: a genetic defect affecting apical membrane protein traffic in intestinal epithelium. Traffic 2000;1:76–83. [DOI] [PubMed] [Google Scholar]
- 9.Carruthers L, Philips A, Dourmashkin R, et al. Biochemical abnormality in brush border membrane protein of a patient with congenital microvillus atrophy. J Pediatr Gastroenterol Nutr 1985;4:902–7. [DOI] [PubMed] [Google Scholar]
- 10.Carruthers L, Dourmashkin R, Philips A. Disorders of the cytoskeleton of the enterocyte. Clin Gastroenterol 1986;15:105–20. [PubMed] [Google Scholar]
- 11.Cutz E, Thorner P, Sherman P. Microvillus inclusion disease—generalized defect of brush border membranes. Lab Invest 1988;58:3P. [Google Scholar]
- 12.Griffiths G. Fine structure immunocytochemistry. Heidelberg: Springer, 1993.
- 13.Zimmer KP, Büning J, Weber P, et al. Modulation of antigen trafficking to MHC class II-positive late endosomes of enterocytes. Gastroenterology 2000;118:128–37. [DOI] [PubMed] [Google Scholar]
- 14.Zimmer KP, Matsuda I, Matsuura T, et al. Ultrastructural, immunocytochemical and stereological investigation of hepatocytes in a patient with the mutation of the ornithine transcarbamylase gene. Eur J Cell Biol 1995;67:73–83. [PubMed] [Google Scholar]
- 15.Naim HY, Sterchi EE, Lentze MJ. Biosynthesis and maturation of lactase-phlorizin hydrolase in the human small intestinal epithelial cells. Biochem J 1987;241:427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phillips A, Fransen J, Hauri HP, et al. The constitutive pathway in microvillus atrophy. J Pediatr Gastroenterol Nutr 1993;17:239–46. [DOI] [PubMed] [Google Scholar]
- 17.Stromhaug PE, Berg TO, Fengsrud M, et al. Purification and characterization of autophagosomes from rat hepatocytes. Biochem J 1998;335:217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pavelka M, Gangl A. Effects of colchicine on the intestinal transport of endogenous lipid. Gastroenterology 1983;84:544–55. [PubMed] [Google Scholar]
- 19.Vega-Salas DE, Salas PJI, Rodriguez-Boulan E. Exocytosis of vacuolar apical compartment (VAC): A cell-cell contact controlled mechanism for the establishment of the apical plasma membrane domain in epithelial cells. J Cell Biol 1988;107:1717–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Remy L. The intracellular lumen: origin, role and implications of a cytoplasmic neostructure. Biol Cell 1986;56:97–106. [DOI] [PubMed] [Google Scholar]
- 21.Peters PJ, Neefjes JJ, Oorschot V, et al. Segregation of MHC class II molecules from MHC class I molecules in the Golgi complex for transport to lysosomal compartments. Nature 1991;349:669–76. [DOI] [PubMed] [Google Scholar]
- 22.Helenius A, Mellman I, Wall D, et al. Endosomes. Trends Biochem Sci 1983;8:245–50. [Google Scholar]
- 23.Schmid S, Fuchs R, Mâle P, et al. Two distinct subpopulations of endosomes involved in membrane recycling and transport to lysosomes. Cell 1988;52:73–83. [DOI] [PubMed] [Google Scholar]
- 24.Apodaca G. Endocytic traffic in polarized epithelial cells: role of the actin and microtubule cytoskeleton. Traffic 2001;2:149–59. [DOI] [PubMed] [Google Scholar]
- 25.Valentijn K, Valentijn JA, Jamieson JD. Role of actin in regulated exocytosis and compensatory membrane retrieval: insights from an old acquaintance. Biochem Biophys Res Commun 1999;266:652–61. [DOI] [PubMed] [Google Scholar]
- 26.Hamm-Alvarez SF, Sheetz MP. Microtubule-dependent vesicle transport: modulation of channel and transporter activity in liver and kidney. Physiol Rev 1998;78:1109–29. [DOI] [PubMed] [Google Scholar]