Abstract
Background: Transient lower oesophageal sphincter relaxation (TLOSR) is the predominant mechanism of gastro-oesophageal reflux (GOR) in healthy infants but the mechanisms of GOR in infants with GOR disease (GORD) are poorly understood.
Aims: To measure the occurrence of TLOSR, GOR, and gastric emptying (GE) rate in preterm and term infants with GORD.
Patients: Thirty six infants were studied and grouped as normals or GORD based on a routine clinical assessment and confirmation of an assessment of GORD by reflux symptom charts and oesophageal pH monitoring.
Methods: A micromanometric assembly incorporating a micro pH electrode recorded oesophageal motility and pH. GE rate was determined using the 13C-octanoic acid breath test.
Results: TLOSR was the predominant mechanism of GOR, triggering 50–100% of GOR episodes (median 91.5%). Abdominothoracic straining significantly increased the occurrence of GOR in association with TLOSR. In infants with GORD, the number of TLOSRs overall was similar to normals but the proportion of TLOSRs accompanied by acid GOR was significantly higher than in normals (16.5% v 5.7%, respectively; p<0.001). Infants with GORD had a similar GE rate to normals.
Conclusions: In infant GORD, acid reflux associated TLOSRs are abnormally common and likely to be a major contributing factor to the pathophysiology of GORD. Infants with GORD do not have delayed GE.
Keywords: preterm neonate; term neonate; gastro-oesophageal reflux; gastro-oesophageal reflux disease; oesophageal motility; transient lower oesophageal sphincter relaxation, gastric emptying
Gastro-oesophageal reflux disease (GORD) is common in infants and causes irritability, frequent vomiting, apnoea, aspiration pneumonia, and failure to thrive.1 Recent studies in healthy preterm infants have shown that transient lower oesophageal sphincter relaxation (TLOSR) is the predominant mechanism of GOR.2–4 There is still debate as to whether in patients with GORD, TLOSRs occur at a similar rate to normal subjects, but are more often associated with the occurrence of acid reflux.5
Pathophysiological mechanisms of GORD have not been studied in preterm and term infants and there are no published data in these patients. Delayed gastric emptying (GE) may be present in infants6 but a previous study which correlated GE and reflux parameters in infants showed no relationship.7 Improvement of GE, in addition to increased salivary secretion, LOS tone, and oesophageal motility, is one rationale for prokinetic therapy although recent studies have been unable to demonstrate acceleration of GE with cisapride.8,9
The aim of this study was to use simultaneous pH monitoring, oesophageal manometry recording, and GE assessment to characterise the motor mechanisms responsible for GOR in premature and term infants with and without GORD.
MATERIALS AND METHODS
Subjects
The study was approved by the ethics committee of the Women’s and Children’s Hospital and informed consent was obtained before each study. Studies were performed in 36 (16 male and 20 female) infants with a mean postmenstrual age of 36±2 weeks (range 33–40). Mean infant weight was 2079 g (range 1480–2840). Sixteen infants were receiving xanthine treatment for apnoea of prematurity. Gavage (tube) feeds were of non-fortified expressed breast milk (EBM) in 22 infants and 14 were receiving infant formula (Enfalac 20 or 24 calorie/ml; Mead Johnson, Canada). Infants received bolus feeds at two (n=7), three (n=19), or four (n=10) hour intervals.
Patient grouping
The normal (control) group in this study consisted of 22 infants who were healthy for relative gestational age with no history of feeding problems and/or GOR. These infants were compared with a group of 14 symptomatic infants with GORD who had been seen from birth by one physician (their consistent medical care giver). The research team was notified in cases where an infant was experiencing feeding problems and/or reflux based on a symptomatic profile (feed problems, vomiting, irritability, xanthine resistant apnoea, weight loss) and an intention to treat using conservative therapy (feed thickeners, postural changes, antacids) and/or pharmacotherapy (cisapride, ranitidine).
All infants, in both the normal and GORD groups, were further evaluated using a GOR symptom assessment chart.9 Symptoms were recorded from the time of consent to the start of the manometric study (period of 2–7 days). The GOR chart allowed for the recording of feeding times and the frequency of (i) vomiting, (ii) apnoea, (iii) choking, and (iv) behavioural changes (that is, irritability/fussing, back arching, grimacing, gagging). Staff responsible for routine care of the infants completed the chart whenever these events were observed. Symptom frequency (total number of symptomatic events recorded divided by the number of days of charting) was determined by chart analysis.
Twenty four hour oesophageal pH monitoring in GORD patients was carried out using the Medtronic “Digitrapper” pH monitoring system (Medtronic, Salt Lake City, Utah, USA) and the pH probe (Medtronic 24 ME, diameter 1.5 mm) was located 3 cm above the LOS as previously described 10. The reflux index (% time pH <4) was determined using the “Esophagram” analysis program.
The presence or absence of ongoing symptoms was evaluated three months after discharge by completion of Orenstein’s “I-GERQ” reflux questionnaire.11
Manometric technique
Patterns of oesophageal motility were recorded with a micromanometric feeding assembly (2 mm od) which incorporated a sleeve sensor for LOS pressure measurement.2–4 The core channel of the assembly was used for gavage feeding. Oesophageal pH was monitored with an antimony micro pH electrode od 0.3 mm (Microelectronics Department, University of South Australia, South Australia) installed within one of the assembly lumina and emerging 3 cm proximal to the centre of the LOS sleeve. Data acquisition and analysis were performed on a Macintosh Quadra 700 with software based on National Instruments’ Labview (MAD software, Royal Adelaide Hospital, C Malbert).
Manometric protocol
The assembly was positioned with the mid point of the sleeve straddling the LOS. After positioning and with the infant in the right lateral posture, the feed was gavaged over 15–30 minutes. Oesophageal pH and spontaneous oesophageal body and LOS motor patterns were then recorded during the feed and for four hours.
Analysis of manometric and pH tracings
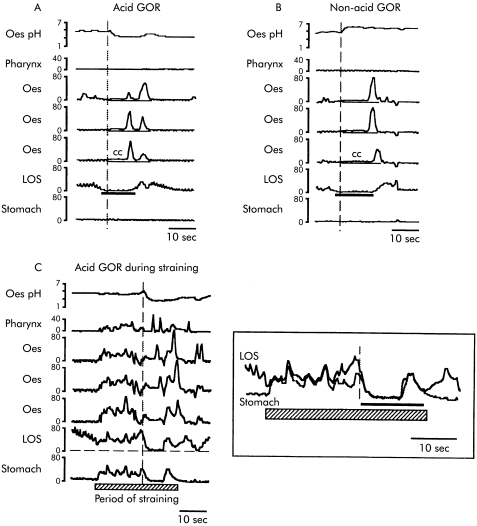
Analyses were performed by investigators (TO and MB) who were blinded to the clinical status of the infants. Swallow related LOS relaxations and TLOSRs were defined using previously described criteria.4 A common cavity reflux episode (fig 1 ▶) was defined as an abrupt sustained increase (≥2 mm Hg) in intraoesophageal pressure to equal intragastric pressure. Abdominothoracic straining was identified by sustained increases (≥5 mm Hg) of intragastric pressure for at least 10 seconds, associated with a corresponding rise in oesophageal body pressure. In practice, strain events shorter than 10 seconds occurred infrequently and were excluded. The computerised data analysis system made it possible to recognise the occurrence of LOS relaxation and common cavities during straining by comparison of the pressure differential between the gastric, sleeve, and oesophageal sensors (fig 1C ▶). These sustained strain patterns are different to the short duration (<5 seconds) strain patterns previously described in association with regurgitation in term infants.12
Figure 1.
Example tracings of transient lower oesophageal sphincter relaxations (TLOSRs) triggering (A) acid gastro-oesophageal reflux (GOR), (B) non-acid GOR, and (C) acid GOR during abdominothoracic straining. In (A) and (B), TLOSR (indicated by the horizontal black line) triggers a common cavity (cc) episode the onset of which is closely associated with the onset of the pH change (vertical dotted line). In (C), abdominothoracic straining (hatched bar) causes a sustained (>10 seconds) increase in intraluminal pressure across all channels. If LOS and gastric pressure recordings are superimposed (see insert), the occurrence of a TLOSR at the end of the straining episode can easily be identified by a prolonged equalisation LOS and gastric pressures (horizontal black line) which is associated with the onset of the GOR episode (vertical dotted line).
Recognition of GOR episodes
The occurrence of both acid and non-acid GOR was identified using manometry in conjunction with oesophageal pH measurement. Acid GOR episodes were defined as drops in oesophageal pH of 0.5 pH units or more over five seconds (fig 1A ▶). In the case of small pH drops (those between 0.5–1.0 pH unit in magnitude), recognition of a common cavity episode with an onset within ±2 seconds of the onset of the pH drop was an essential additional requirement. Non-acid GOR episodes were identified by the presence of a common cavity episode with either no pH drop or even a pH increase which occurred within ±2 seconds of the onset of the common cavity and were not associated (±5 seconds) with the passage of oesophageal peristalsis and therefore unlikely to be due to the buffering effect of saliva (fig 1B ▶).
Measurement of GE rate
Gastric half emptying time was measured with the 13C-octanoic acid breath test. 13C-labelled octanoic acid (50 μl) was added to the infant’s regular feed and breath samples were taken as previously described.9,13 Breath samples were analysed for 13CO2 content using an isotope ratio mass spectrometer. 13CO2 excretion rate was used to calculate half GE time using the established non-linear regression model.9,13
Statistical analysis
Normally distributed group data were compared with an ANOVA technique (F test and/or Scheffe’s test). Non-parametric grouped data were compared using the Mann-Whitney test. Interrelationships between variables were determined by Spearman-Rank correlation. Proportionate data were compared by χ2 test. A p value <0.05 was considered statistically significant.
Associations between random variables (for example, TLOSRs and abdominal straining) were determined by calculating the 95% confidence intervals (CI) of the odds ratio. If CI was greater than 1 or less than −1, a significant association between variables was indicated.
RESULTS
Evaluation of patient symptoms, pH monitoring, and follow up
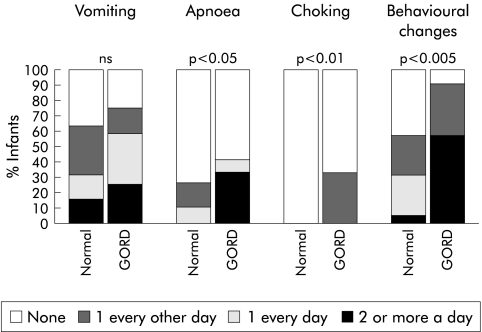
Infants assessed clinically to have GORD were older postnatally than normals (45 (8) v 25 (5) days old, respectively; p<0.05) and were more likely to be receiving xanthine therapy (71% v 38% on xanthines; p<0.01). No other significant age, size, or feeding differences (type, volume) were observed between the groups. Numbers of infants receiving two, three, and four hourly feeds were 4 (29%), 3 (21%), and 7 (50%), respectively, for the GORD group and 3 (14%), 7 (32%), and 12 (54%), respectively, for normals (NS). Adequate symptom assessment charting was completed in 19 normals and 12 patients with GORD. Analysis of the reflux symptom assessment charts confirmed more frequent symptoms overall in the GORD group; apnoea, chocking, and behavioural changes in particular (fig 2 ▶). Infants in the GORD group had a median reflux index of 15.1% (interquartile range 9.8–30.0%), 10 infants (72%) had a reflux index of >10%, 3 (21%) had a reflux index of 5–10%, and one had a reflux index <5%. Median reflux indexes for two, three, and four hourly fed infants with GORD were 8.7%, 17.4%, and 41.4% respectively (p<0.01).
Figure 2.
Incidence of symptoms in normal infants and patients with gastro-oesophageal reflux disease (GORD). p values for χ2 analysis are shown.
The follow up reflux questionnaire performed at three months after discharge was completed for 21 normals and 10 infants in the GORD group. Of the normals, 17 (81%) continued to be reflux free while eight (80%) of the infants in the GORD group continued to be symptomatic.
TLOSRs and GOR
TLOSR was the predominant mechanism of GOR triggering, accounting for 50–100% (median 91.5%) of all GOR episodes in the 36 patients. In all, 726 TLOSRs were recorded; of these, 105 (14%) were associated with acid GOR. Two hundred and sixty five (37%) TLOSRs were associated with non-acid GOR and 356 (49%) did not result in GOR, as defined by our criteria. Abdominal straining occurred commonly (average 41 (2) strains/study, range 13–64). Of all of the TLOSRs observed, 315 (43%) occurred during abdominothoracic straining with 71 associated with acid GOR. When a TLOSR occurred during abdominothoracic straining, acid GOR was more likely to occur than when a TLOSR was not associated with straining (odds ratio 4.32 (2.71, 6.88); p<0.001).
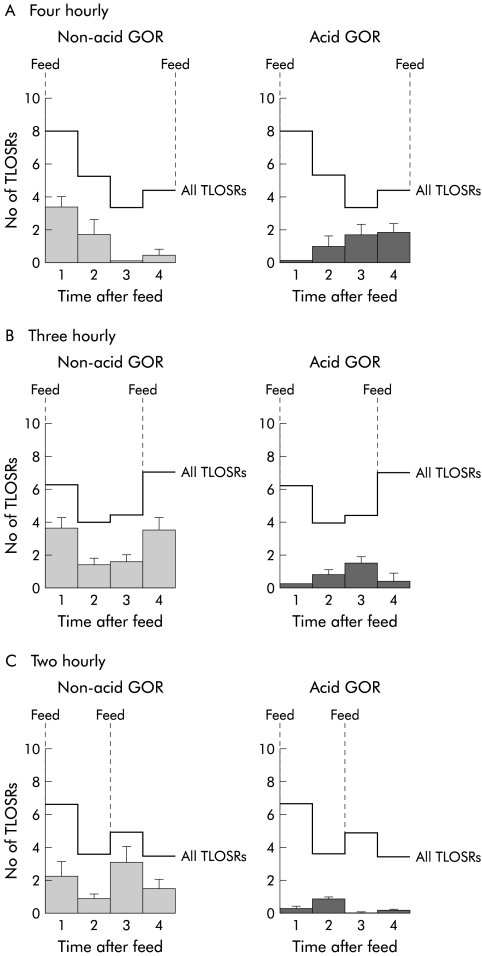
The occurrence of TLOSRs and the relationship between TLOSR and acid or non-acid GOR was significantly influenced by the time interval between feeds (two, three, or four hourly) (fig 3A–C ▶). In infants fed four hourly (that is, fed only once immediately prior to the commencement of the four hour manometric study) the number of TLOSRs peaked in the first hour (fig 3A ▶). In contrast, infants fed two hourly or three hourly (that is, fed on two occasions immediately prior to the study and then two or three hours into the study), a second peak in the number of TLOSRs was observed which corresponded to the one hour period immediately following the second feed (fig 3B, C ▶). Feeding therefore changed the pattern of occurrence of TLOSRs and acid/non-acid GOR during the period 2–4 hours postprandially. Because of this, further comparisons among patients were limited to an analysis of events occurring during the period from initial feed to two hours postprandially. During this period the overall number of TLOSRs and TLOSRs associated with GOR were not significantly altered by feed frequency (9.1 (2.1), 9.0 (0.8), and 12.5 (1.9) for two, three, and four hourly feeds, respectively).
Figure 3.
Occurrence of all transient lower oesophageal sphincter relaxations (TLOSRs) (lines) and TLOSRs associated with non-acid gastro-oesophageal reflux (GOR) (light shading) and acid GOR (dark shading) in infants receiving feeds at (A) four hourly, (B) three hourly, and (c) two hourly intervals. Data represent the mean number of events recorded during each one hour period of the four hour studies.
Feed type did not alter the rate of occurrence of TLOSRs (8.8 (0.8) v 11.6 (1.6) for EBM v formula, respectively; p=0.1) but EBM fed infants did have more acid GOR (13.3% v 7.0% for EBM v formula, respectively; p<0.05) and less non-acid GOR (32.2% v 43.5% for EBM v formula, respectively; p<0.05) in association with TLOSRs. Xanthine therapy did not significantly alter the number of TLOSRs (10.3 (1.2) v 9.6 (1.2) for xanthine therapy v no therapy, respectively) or their association with acid GOR (11.9% v 9.0% for xanthine therapy v no therapy, respectively) and non-acid GOR (41.0% v 33.5% for xanthine therapy v no therapy, respectively). The rate of occurrence of TLOSRs was not affected by postmenstrual age but there was a weak correlation between postnatal age and the rate of TLOSRs, with older infants having fewer TLOSRs (r=0.32, p<0.05).
TLOSR was the predominant mechanism of GOR in both normals and infants with GORD, being associated with 94% (range 70–100%) and 92% (range 50–100%) of all GOR episodes, respectively. Infants with GORD had similar numbers of TLOSRs to normals (9.5 (1.2) v 10.4 (1.1), respectively from 0–2 hours, 6.9 (1.0) v 9.7 (1.1), respectively, from 2–4 hours postprandially) but had a significantly higher number and proportion of TLOSRs associated with acid GOR (1.6 (0.4) v 0.6 (0.1) (p<0.05) and 16.5% v 5.9% (p<0.0001), respectively, from 0–2 hours; 1.7 (0.6) v 1.9 (0.4) (NS) and 25.0% v 19.7% (NS), respectively, from 2–4 hours postprandially). In contrast, the number and proportion of TLOSRs associated with non-acid GOR were similar (4.0 (0.9) v 3.7 (0.6) (NS) and 42.1% v 35.8% (NS), respectively, from 0–2 hours; 2.2 (0.5) v 3.5 (0.7) (NS) and 32.3% v 36.6% (NS), respectively, from 2–4 hours postprandially). Straining in association with TLOSR augmented the likelihood of acid GOR in both normals (odds ratio 2.26 (1.54, 3.31); p<0.0001) and GORD (odds ratio 2.39 (1.36, 4.21); p<0.0001).
Gastric emptying
For all infants, mean half GE time was 33 (1) minutes, and EBM fed infants had faster GE rates (28 (3) v 41 (6) minutes for EBM v formula, respectively; p<0.05). Infants receiving feeds at two, three, or four hourly intervals had different GE times, with longer intervals between feeds being associated with slower GE (25 (5) minutes, 30 (4) minutes, and 46 (5) minutes for two, three, and four hourly feeds, respectively; p<0.05). This effect was most likely due to differences in feed volumes administered which varied from 20 to 80 ml and were lower in the more frequently fed infants (22 (1) ml, 43 (2) ml, and 69 (3) ml in two, three, and four hourly fed, respectively; p<0.001). Xanthine therapy did not alter GE rate (33 (4) v 33 (5) minutes for xanthine therapy v no therapy respectively). Infants with GORD had similar half GE times to normals (32 (7) v 33 (3) minutes, respectively).
DISCUSSION
This is the first study to characterise oesophagogastric motor function and mechanisms of GOR in preterm and term infants with GORD. While TLOSR was the predominant mechanism of GOR in infants with GORD, the number of TLOSRs was similar to normal infants but GORD infants had a higher proportion associated with acid reflux. These data demonstrate that TLOSR is likely to be a major contributing factor to the pathophysiology of GORD in these babies.
Gastric distension (by feeding) stimulated TLOSRs with the frequency of feeding altering the pattern of TLOSRs and the relationship between TLOSRs and acid or non-acid GOR. This affect was most evident during the period from two to four hours postprandially and consequently differences seen between infants with GORD and normals were less apparent during this period due to the variability introduced because some infants were fed during this period. Abdominal straining was also an important factor increasing the likelihood of TLOSRs to trigger acid GOR episodes.
Early studies in supine adult reflux patients indicated that GORD was characterised by a higher number of TLOSRs triggered in response to a meal,14,15 whereas infants with GORD had a greater proportion of TLOSRs associated with acid GOR, particularly during straining. Similar observations have now been reported in adults with GORD16 and indicate that the differences between normals and patients with GORD may be due to sensory or anatomical variations that increase the likelihood for liquid (rather than gas) reflux to occur during TLOSR.
Our data clearly show that GE was not delayed in GORD patients and challenge the logic of acceleration of GE for the treatment of acid reflux. The GORD patients studied did not have significantly more regurgitation than normals, and therefore we are unable to comment on gastric acceleration for the treatment of infants with volume reflux. GORD patients were more likely to be receiving xanthine therapy but xanthines on their own did not appear to alter TLOSRs, GOR triggering by TLOSRs, or GE rate.
In a study such as this, patient selection and grouping is made difficult by the absence of “gold standard” diagnostic criteria specific to this age group in which endoscopy is usually unavailable or inappropriate. “Normal” oesophageal pH monitoring scores have not been adequately defined in infants, particularly premature infants. Most pH monitoring studies16,17 have indicated that acid GOR is a common event; reflux index scores are much higher than levels considered clinically significant in adults and children. Furthermore, reflux parameters are altered by changes in arousal state, feed type, and posture.18–21 The disease status of the patients enrolled in this study was judged firstly on the basis of clinical assessment by a neonatologist and then further examined by reflux symptom charting and 24 hour oesophageal pH monitoring. Confirmation of the clinical diagnosis of GORD using pH monitoring has been used in other studies,22 one of these23 indicating that infants selected in this way have increased acid oropharyngeal aspirates which may in itself be useful diagnostically.
Our confirmatory studies showed that infants in the GORD group had more frequent symptoms than normals and most had pathological GOR on pH monitoring. These were the best criteria we could apply to a very challenging patient group in whom demonstration of unequivocal GORD is often difficult. More frequent feeding had a marked effect in reducing the reflux index in this group of infants characterised by very high oesophageal acid exposure times. The effect of feeding frequency on the reflux index is entirely predictable based on the known effect of feeding on intragastric pH24 and supports the clinical utility of the conservative option of decreased volume/more frequent feeding to treat mild reflux. Despite frequent feeding however all but one infant in the GORD group exhibited reflux indices that were greater than 5% and the three month follow up using an established reflux questionnaire indicated that 80% of these patients continued to exhibit significant symptoms three months after being enrolled in this study.
In conclusion, like older children and adults, TLOSR is an important factor in the pathophysiology of GORD in preterm and term infants while delayed GE does not appear to be. Further investigations are now needed to characterise intragastric pH in these babies.
Acknowledgments
This work was supported by grants from the National Health and Medical Research Council of Australia, the WCH Research Foundation, and the JH & JD Gunn Medical Research Foundation. Dr Benninga was supported by the Ter Meulen Fund, Royal Netherlands Academy of Arts and Sciences, the Netherlands Organisation for Scientific Research, and the Netherlands Digestive Diseases Foundation. We would like to thank Mr Malcolm Bakewell for invaluable technical support and Dr Charles Malbert for the computerised data acquisition and analysis software used.
Abbreviations
LOS, lower oesophageal sphincter
TLOSR, transient LOS relaxation
GOR, gastro-oesophageal reflux
GORD, GOR disease
GE, gastric emptying
EBM, expressed breast milk
REFERENCES
- 1.Novak D. Gastroesophageal reflux in the preterm infant. Clin Perinatol 1996;23:304–20. [PubMed] [Google Scholar]
- 2.Omari T, Miki K, Davidson G, et al. Characterisation of lower oesophageal sphincter relaxation in healthy preterm infants. Gut 1997;40:370–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Omari T, Benninga M, Barnett C, et al. Characterisation of esophageal body and lower esophageal sphincter motor function in the very premature neonate. J Pediatr 1999;135:517–21. [DOI] [PubMed] [Google Scholar]
- 4.Omari T, Barnett C, Snel A, et al. Mechanisms of gastroesophageal reflux in healthy premature infants. J Pediatr 1998;133:650–4. [DOI] [PubMed] [Google Scholar]
- 5.Holloway RH. The anti-reflux barrier and mechanisms of gastro-oesophageal reflux. Baillieres Clin Gastroenterol 2000;14:681–99. [DOI] [PubMed] [Google Scholar]
- 6.Hillemeier A, Grill B, McCallum R, et al. Esophageal and gastric motor abnormalities in gastroesophageal reflux during infancy. Gastroenterology 1983;84:741–6. [PubMed] [Google Scholar]
- 7.Ewer A, Durbin G, Morgan M, et al. Gastric emptying and gastro-oesphageal reflux in preterm infants. Arch Dis Child 1996;75:F117–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McClure R, Kristensen J, Grauaug A. Randomised controlled trial of cisapride in preterm infants. Arch Dis Child Fetal Neonatal Ed 1999;80:F174–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnett CP, Omari T, Haslam R, et al. Randomised double-blind crossover trial of the effect of cisapride on gastric emptying in preterm infants with feed intolerance. J Paediatr Child Health 2001;37:559–63. [DOI] [PubMed] [Google Scholar]
- 10.Omari T, Benninga M, Haslam R, et al. Lower esophageal sphincter position in premature infants cannot be correctly estimated with current formulas. J Pediatr 1999;135:522–5. [DOI] [PubMed] [Google Scholar]
- 11.Orenstein S, Cohn J, Shalaby T, et al. Reliability and validity of an infants gastroesophageal reflux questionnaire. Clin Pediatr 1993;32:472–84. [DOI] [PubMed] [Google Scholar]
- 12.Orenstein S, Dent J, Deneault L, et al. Regurgitant reflux vs non regurgitant reflux is preceded by rectus abdominis contraction in infants. Neurogastroenterol Motil 1994;6:271–7. [Google Scholar]
- 13.Van Den Driessche M, Peeters K, et al. Gastric emptying in formula-fed and breast fed infants measured with the 13C-octanoic acid breath test. J Pediatr Gastroenterol Nutr 1999;29:46–51. [DOI] [PubMed] [Google Scholar]
- 14.Holloway R, Dent J. Pathophysiology of gastroesophageal reflux. Lower esophageal sphincter dysfunction in gastroesophageal reflux disease. Gastroenterol Clin North Am 1990;19:517–35. [PubMed] [Google Scholar]
- 15.Dodds W, Dent J, Hogan W, et al. Mechanisms of gastroesophageal reflux in patients with reflux esophagitis. N Engl J Med 1982;307:1547–52. [DOI] [PubMed] [Google Scholar]
- 16.Sifrim D, Holloway J, Silney J, et al. Composition of the post prandial refluxate in patients with gastroesophageal reflux disease. Am J Gastroenterol 2001;96:647–55. [DOI] [PubMed] [Google Scholar]
- 17.Sutphen J, Dillard V. Effects of maturation and gastric acidity on gastroesophageal reflux in infants. Am J Dis Child 1986;140:1062–4. [DOI] [PubMed] [Google Scholar]
- 18.Newell S, Booth I, Morgan M, et al. Gastro-oesophageal reflux in preterm infants. Arch Dis Child 1989;64:780–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tobin J, McCloud P, Cameron D. Posture and gastro-oesophageal reflux: a case for left lateral positioning. Arch Dis Child 1997;76:254–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heacock H, Jeffery H, Baker J, et al. Influence of breast milk versus formula milk on physiological gastroesophageal reflux in healthy, newborn infants. J Pediatr Gastroenterol Nutr 1992;14:41–6. [DOI] [PubMed] [Google Scholar]
- 21.Jeffery H, Page M. Developmental maturation of gastro-oesophageal reflux in preterm infants. Acta Paediatr 1995;84:245–50. [DOI] [PubMed] [Google Scholar]
- 22.Ewer A, James M, Tobin J. Prone and left lateral positioning reduce gastro-oesophageal reflux in preterm infants. Arch Dis Child Fetal Neonatal Ed 1999;81:F201–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.James M, Ewer A. Acid oro-pharyngeal secretions can predict gastro-oesophageal reflux in preterm infants. Eur J Pediatr 1999;80:F174–7. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell DJ, McLure BG, Tubman TRJ. Simultaneous monitoring of gastric and oesophageal pH reveals limitations of conventional oesophageal pH monitoring in milk fed infants. Arch Dis Child 2001;84:273–6. [DOI] [PMC free article] [PubMed] [Google Scholar]