Abstract
Background: Interstitial cells of Cajal (ICC) are required for normal intestinal motility. ICC are found throughout the human colon and are decreased in the sigmoid colon of patients with slow transit constipation.
Aims: The aims of this study were to determine the normal distribution of ICC within the human colon and to determine if ICC are decreased throughout the colon in slow transit constipation.
Patients: The caecum, ascending, transverse, and sigmoid colons from six patients with slow transit constipation and colonic tissue from patients with resected colon cancer were used for this study.
Methods: ICC cells were identified with a polyclonal antibody to c-Kit, serial 0.5 μm sections were obtained by confocal microscopy, and three dimensional software was employed to reconstruct the entire thickness of the colonic muscularis propria and submucosa.
Results: ICC were located within both the longitudinal and circular muscle layers. Two networks of ICC were identified, one in the myenteric plexus region and another, less defined network, in the submucosal border. Caecum, ascending colon, transverse colon, and sigmoid colon displayed similar ICC volumes. ICC volume was significantly lower in the slow transit constipation patients across all colonic regions.
Conclusions: The data suggest that ICC distribution is relatively uniform throughout the human colon and that decreased ICC volume is pan-colonic in idiopathic slow transit constipation.
Keywords: motility, smooth muscle, intestine, interstitial cells of Cajal, slow transit constipation
Interstitial cells of Cajal (ICC) are required for normal intestinal motility. Their role as intestinal pacemakers has been established in a number of model systems. Mice lacking ICC networks do not have an electrical slow wave and demonstrate absent or delayed intestinal motility.1,2 Dissociated ICC display the ability to produce an electrical slow wave and are the source of smooth muscle rhythmic electrical activity in the normal intestine.3–5 ICC have been shown to be diminished or lost in human disease processes with associated alterations in gastric and small intestinal motility. Among these conditions are diabetic gastroparesis,6 intestinal pseudo-obstruction,7,8 and congenital absence of the enteric nervous system.9 Less is understood about the role of ICC in the colon as colonic ICC and colonic motility appear to be preserved in the w/wv mutant,10 the ICC deficient mouse model most studied to date. Data on human colonic ICC published to date are conflicting (reviewed by Vanderwinden and Rumessen11).
We have focused our studies on idiopathic slow colonic transit constipation (STC), a disorder with measurably delayed movement of materials through the colon. Primary gastrointestinal causes of constipation are generally divided into normal transit constipation, STC, and pelvic floor dysfunction. Balloon expulsion measurements and anorectal angle studies can assess pelvic floor dysfunction. Impaired colon motility can be documented with marker tests or scintigraphic tests that determine colon transit times. Both forms of constipation can produce significant morbidity. STC is generally managed with aggressive medical regimens of laxatives, including bulking and osmotic agents, although the efficacy of bulking agents in STC is doubtful. When combinations of these medications fail to provide symptomatic relief, surgical treatment is warranted. For individuals with delayed colon transit and normal pelvic floor function, a subtotal colectomy with ileorectal anastomosis is generally recommended12–14 but some reports demonstrate variable results.15 Some authors continue to argue for segmental resections.16
The aetiology of STC remains unclear. Abnormalities in the neuronal networks of the colon have been demonstrated in patients with constipation but the specific neuronal defect remains unclear. In particular, alterations in nitric oxide synthase (NOS) and vasoactive intestinal peptide (VIP) containing neurones have been demonstrated.17–20 Our group previously reported an alteration of ICC within the sigmoid colon of constipated patients. Specifically, the total ICC volume is decreased in the sigmoid colon of STC patients compared with controls and the defect appears throughout the thickness of the sigmoid colon.21
In the present study, we used immunohistochemistry and three dimensional computer reconstruction to determine the distribution of ICC in all regions of the normal human colon and to determine if, in STC, ICC volume and distribution are altered in all regions of the human colon.
MATERIALS AND METHODS
Procurement of human tissue was approved by the Institutional Review Board of the Mayo Clinic. All tissue was immediately snap frozen after procurement and stored at −70° C until use.
Immunocytochemistry
Colon samples were fixed either with 4% paraformaldehyde in 0.1 M sodium phosphate buffer (PBS), pH 7.4, or Zamboni's fixative (Newcomer Supply, Middleton, Wisconsin, USA) for four hours or held overnight at 4°C. Next, samples were washed three times with 0.1 M PBS, transferred to 30% sucrose in 0.1 M PBS, and refrigerated for up to 24 hours until sectioned. Serial longitudinal, transverse, and horizontal sections (40–150 μm thick) were cut with a Cryostat (Miles Inc., Elkhart, Indiana, USA), placed in spot plates, and flooded with 0.1 M PBS with 0.3% Triton X-100 (Sigma, St Louis, Missouri, USA) and 5% normal donkey serum for two hours to permeabilise the tissue and reduce background staining. Immunohistochemical staining was performed with a c-Kit rabbit polyclonal antibody (1:400 dilution; MBL, Nagoya, Japan) and a c-Kit goat polyclonal antibody (1:200 dilution; Santa Cruz Biotech, California, USA). No significant difference was noted between the two c-Kit antibodies used. Immunoreactivity was demonstrated by incubating the tissues with primary antibodies (overnight, 4°C). Bound antibodies were visualised by incubating tissues for two hours with fluorescent labelled secondary antibodies to rabbit or mouse IgG (Jackson ImmunoResearch, West Grove, Pennsylvania, USA). After washing with PBS, sections were mounted on slides, air dried overnight at room temperature, and coverslipped. Non-specific labelling for c-Kit was assessed by omitting the primary antibody and by preabsorbing with the purified antigen in separate experiments. No staining was seen with either control. Additionally, non-immune serum produced no staining.
Volume determination of ICC and neural networks
Immunostained tissues were examined with a laser scanning confocal microscope (Zeiss 310, Oberkochen, Germany). Cy3 fluorescence was visualised using an Argon/Krypton ion laser at 568 nm for excitation and emission taken over 590 nm.
Each specimen examined was divided into four anatomical regions: longitudinal muscle (LM), myenteric plexus region (MPR), circular muscle (CM), and submucosal border (SMB). Images were collected using an oil immersion ×40 objective (NA 1.3) and an oil immersion ×100 objective (NA 1.3). The 100× objective was used to ensure that weakly stained ICC were included in the quantitative analyses. In every region, four areas in the X-Y plane were examined. Under the 40× oil objective, the maximum area scanned in the horizontal X-Y plane was 320 μm×320 μm; under the 100× oil objective, the area scanned was 128 μm×128 μm. Optical sections (512×512 pixels) were recorded at 0.5 μm depth (optical Z axis) increments through each of the four adjacent areas. The volume (μm3) of total tissue (including interstitial space) was calculated by: area of the X-Y plane (μm2)×(number of optical sections−1)×0.5 (distance between top of two adjacent sections). The volumes obtained from each of the four adjacent areas were combined and averaged.
The volume occupied by ICC was calculated as previously described.21 Files of the stored confocal images were changed to ANALYZE format software (Mayo Foundation, Rochester, Minnesota, USA) and converted into a three dimensional dataset by applying a three way interpolation algorithm. The gray scale dataset was reconstructed in three dimensions using volume rendering and appropriate thresholds to remove background noise. To avoid over thresholding of less intensely fluorescent structures such as the fine processes in ICC networks, the threshold value was slowly increased eliminating voxels containing noise. As fine processes emerged, they were stored in an object file. The threshold was again increased until all visible processes were extracted. A surface shading algorithm was used to emphasise ICC surface features. The volume of the ICC network was obtained directly from the volume data set after mathematically correcting for focal anomaly.22 Mast cells were identified by their typical round shape and size and excluded from the analysis.
The investigator performing the data analysis was blinded to the clinical diagnosis—that is, control versus STC. The sigmoid colon specimens used in this study (both control and STC specimens) were different than those previously reported.21
Statistics
Values in the text are mean (SEM). Statistical differences among different regions were tested using an analysis of variance (ANOVA); p values <0.05 were considered statistically significant.
RESULTS
Demographics
All STC patients were female and between the ages of 18 and 55 years. Constipation was present in all patients for at least 10 years. Evaluations included barium enema or colonoscopy to rule out structural lesions. Colon motility, as assessed by marker studies, was significantly prolonged (>100 hours) in all patients. Significant pelvic floor dysfunction was excluded with anorectal manometry and balloon expulsion. One patient demonstrated a mildly abnormal balloon expulsion test and a markedly prolonged colonic transit test. Colonic resection was performed after the patient underwent pelvic floor retraining with no improvement in symptoms. All STC patients failed a medical regimen of stimulant and osmotic laxatives as well as bulking agents prior to colectomy.
All control patients underwent colectomy for colon cancer. Because patients underwent segmental colectomy, samples were obtained from more than six patients so that a total of six samples were analysed for each colon region. Control ages ranged from 23 to 82 years. Four patients were male and eight were female.
ICC distribution in normal human colon
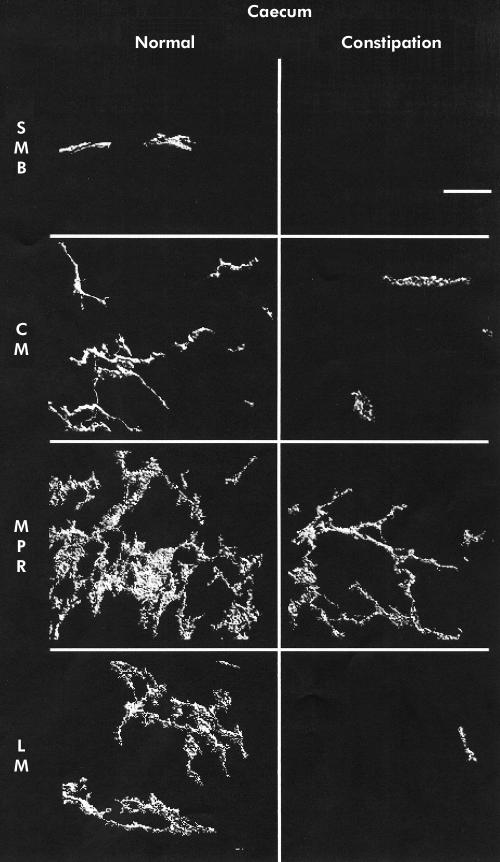
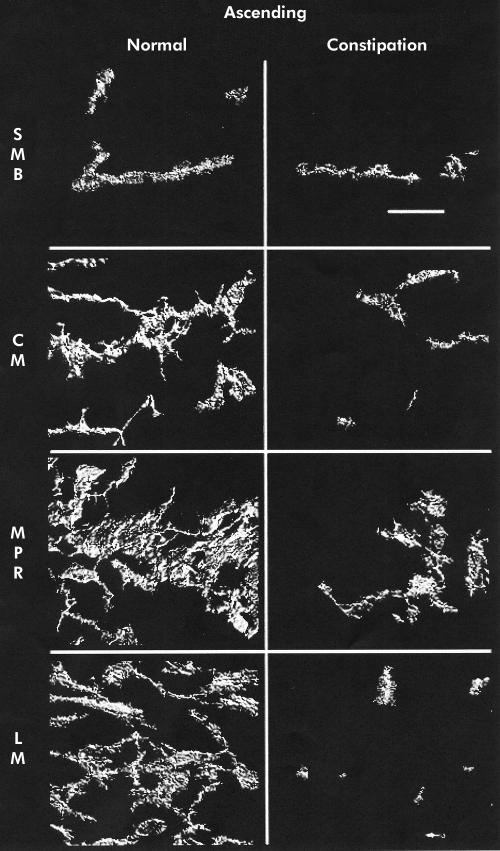
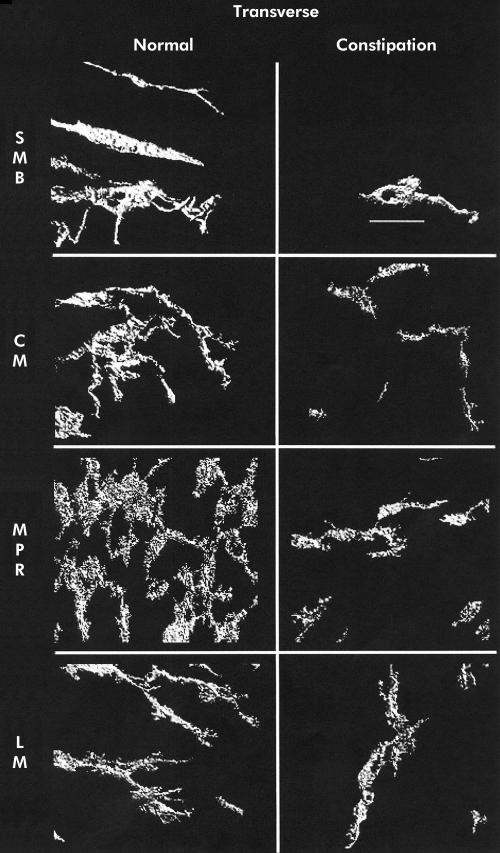
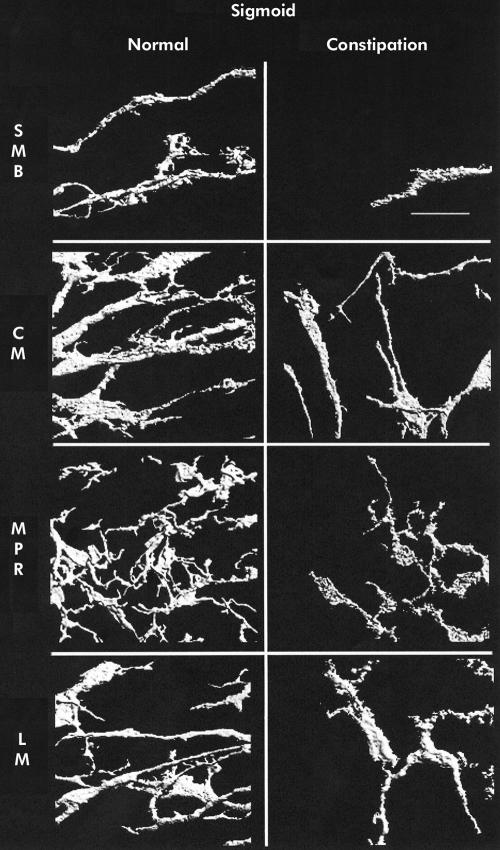
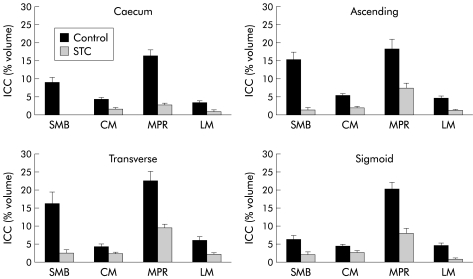
Mean (SEM) ICC volume in the SMB, CM, MPR, and LM in the caecum was 9.9 (1.4), 4.3 (0.5), 16.5 (1.5), and 3.6 (0.3)%, respectively. A representative set of images is shown in fig 1 ▶. Ascending colon ICC volumes were 15.3 (2.0), 5.3 (0.5), 18.4 (2.6), and 4.6 (0.6)% (representative images in fig 2 ▶). Transverse colon ICC volumes were 16.2 (3.1), 4.3 (0.6), 22.6 (2.6), and 6.1 (0.9)% (representative images in fig 3 ▶). Sigmoid colon ICC volumes were 6.3 (1.0), 4.4 (0.4), 20.3 (1.8), and 4.7 (0.4)% (representative images in fig 4 ▶). These data are shown graphically in fig 5 ▶. The distribution of ICC volume in each region did not differ significantly from one anatomical segment to another. In contrast, ICC volume was significantly higher in the MPR and SMB regions than in the CM and LM regions in all anatomical segments (p<0.05).
Figure 1.
Three dimensional reconstruction of c-kit immunoreactivity in the caecum for control tissue (left panel) and slow transit constipation (right panel) specimens. Each panel is of a representative sample. Panels represent separate reconstructions in the submucosal border (SMB), circular smooth muscle layer (CM), myenteric plexus region (MPR), and longitudinal muscle layer (LM). Scale bar is 50 μm.
Figure 2.
Three dimensional reconstruction of c-kit immunoreactivity in the ascending colon for control tissue (left panel) and slow transit constipation (right panel) specimens. Each panel is of a representative sample. Panels represent separate reconstructions in the submucosal border (SMB), circular smooth muscle layer (CM), myenteric plexus region (MPR), and longitudinal muscle layer (LM). Scale bar is 50 μm.
Figure 3.
Three dimensional reconstruction of c-kit immunoreactivity in the transverse colon for control tissue (left panel) and slow transit constipation (right panel) specimens. Each panel is of a representative sample. Panels represent separate reconstructions in the submucosal border (SMB), circular smooth muscle layer (CM), myenteric plexus region (MPR), and longitudinal muscle layer (LM). Scale bar is 50 μm.
Figure 4.
Three dimensional reconstruction of c-kit immunoreactivity in the sigmoid colon for control tissue (left panel) and slow transit constipation (right panel) specimens. Each panel is of a representative sample. Panels represent separate reconstructions in the submucosal border (SMB), circular smooth muscle layer (CM), myenteric plexus region (MPR), and longitudinal muscle layer (LM). Scale bar is 50 μm.
Figure 5.
c-kit immunoreactivity of interstitial cells of Cajal (ICC) expressed as per cent volume for the submucosal border (SMB), circular smooth muscle layer (CM), myenteric plexus region (MPR), and longitudinal muscle layer (LM) in control colonic tissue and in slow transit constipation (STC). Control versus STC are all p<0.05; n=6 for all data points. Values are mean (SEM).
ICC in slow transit colon
In STC patients, the caecum ICC volumes were 0 (0) in the SMB, 1.6 (0.4) in the CM, 2.8 (0.5) in the MPR, and 1 (0.3)% in the LM (representative images shown in fig 1 ▶). Ascending colon ICC volumes were 1.4 (0.5), 1.9 (0.3), 7.4 (1.2), and 1.3 (0.2)% (representative images shown in fig 2 ▶). Transverse colon ICC volumes were 2.5 (0.9), 2.4 (0.3), 9.5 (1.0), and 2.2 (0.3)% (representative images shown in fig 3 ▶). Sigmoid colon ICC volumes were 2.0 (0.7), 2.6 (0.4), 8.0 (1.4), and 0.8 (0.3)% (representative images shown in fig 4 ▶). As with control colon layers, there was no significant difference when each region was compared across colon segments, and the SMB and MPR values were higher compared with the LM and CM regions in all anatomical segments (p<0.05) (fig 5 ▶). ANOVA revealed a significantly lower ICC volume in slow transit patients compared with controls (p<0.05) and subsequent pairwise analysis revealed a significant difference at each region analysed (p<0.05).
DISCUSSION
In the normal human colon, we observed c-kit staining in each of the regions studied—that is, in the SMB, MPR, CM, and LM layers, and in all colonic segments analysed. A higher per cent volume of ICC was present in the SMB and MPR compared with the CM and LM layers. These data are similar to those reported for other species. No significant variation in the distribution of ICC volume among the caecum, ascending, transverse, and sigmoid colon was found. These results contrast with previous reports wherein the density of ICC has been reported to be higher in the myenteric plexus of the transverse colon.23 Methodological differences may explain the different results. Our tissues were snap frozen immediately after procurement and stored at −70°C until processing. We did not employ paraffin embedding which decreases c-Kit staining (unpublished results). Our scoring of ICC utilised computer reconstruction which allows for quantification of ICC volume and may be sensitive not only to changes in cell number but also cell and process size. Mast cells are the only other cell type known to express c-kit in the intestine. Previous reports suggest these cells are less than 8% of c-kit positive cells in the intestine,7 and hence were specifically excluded from the analysis.
We have focused our studies on ICC because mounting evidence suggests a crucial role for this cell population in regulating intestinal function. Recent studies suggest that ICC mediate neurotransmission from enteric motor neurones.24,25 Moreover, ICC act as intestinal pacemaker cells to generate slow waves. Two complimentary lines of evidence support this notion. Firstly, mutations of the c-kit receptor disrupt ICC development and slow wave production in the intestine.2 Likewise, mutations of the c-kit ligand, steel factor, also disrupt intestinal ICC cell development and electrical rhythmicity.26 A second line of evidence demonstrates that ICC cells are sufficient to generate an electrical slow wave. ICC in primary culture systems exhibit a slow wave activity—that is, have the proper characteristics to be an intestinal pacemaker.3–5 Taken together, these studies suggest a central role for ICC in the regulation of intestinal motility.
Important anatomical differences exist in ICC slow wave generation. For example, the ICC of the MPR generate the slow wave within the small intestine.27,28 In the colon however the ICC of the SMB region appear to produce the slow wave.29 The present demonstration of a distinct SMB ICC network throughout the human colon is consistent with this functional observation. Previous reports have suggested that the transverse colon is the primary colonic pacemaker site30 and that the myenteric plexus ICC is denser in the transverse colon.23 In this study, we were unable to demonstrate an anatomical correlation with this functional observation as both the MPR and SMB ICC volume appeared to be relatively uniform throughout the colon, including the caecum.
In addition to describing ICC distribution in the normal colon, we found reduced ICC volume in idiopathic STC. This reduction was present across all colon regions, including the caecum, ascending, transverse, and sigmoid colon. Our present report replicates our previous findings of decreased ICC volume in the sigmoid colon of patients with STC21 and expands our findings to demonstrate that this decrease is pan-colonic. All of the patients in our study had been treated preoperatively with bulking agents as well as stimulant and osmotic laxatives, including anthraquinone preparations. Some concern exists over the possibility that laxatives may cause histological changes31 but controlled prospective trials in animals and humans demonstrate no neuronal changes with anthraquinone usage.32,33 Whether this lack of effect can be extrapolated to ICC is unknown. The average age of our patient sample was lower than that of the control sample. Effects of age on human ICC number or volume has not been published but a subanalysis of our data combining all control patients below the age of 40 years in this study with those of a previous study21 and comparing these with our controls over the age of 40 years revealed no age related differences in ICC volume (data not shown).
Multiple investigators have reported histological findings in STC. Earlier studies have shown no significant changes in myocytes or gross changes in the ICC populations of STC patients but these studies did not employ specific c-kit staining for ICC.20 Multiple studies have revealed changes in the VIP and NOS containing cell populations of the enteric nervous system of constipated patients17,18,20,34 but these results have not been consistent.
In addition to being affected in STC, ICC populations are diminished in other conditions of gastrointestinal hypomotility, both congenital and acquired. ICC cell numbers may be decreased in the aganglionic, hypomotile colon segments in Hirschsprung's disease,9 and in acquired intestinal hypoganglionosis.35 Electron microscopy has demonstrated disturbed ICC ultrastructure in ulcerative colitis wherein intestinal motility is often affected.36 Decreased ICC have been observed in the colons of individuals affected by Chagas' disease37 and in the stomachs of diabetics with gastroparesis.6 Alterations in ICC in these human diseases affecting intestinal motility support the notion that ICC alterations are important in the aetiology of STC. Whether STC is an acquired or congenital disorder, a mixture of both, and whether loss of ICC is primary or secondary to another lesion is not known but mounting evidence from experimental models and human disease increasingly point to a central role for ICC in the aetiology of human gastrointestinal dysmotility.
Acknowledgments
This work was supported by National Institutes of Health grants DK17238, DK52766, and DK57061. The authors thank Kristy Zodrow for secretarial assistance.
Abbreviations
ICC, interstitial cells of Cajal
STC, slow transit constipation
SMB, submucosal border
CM, circular muscle
MPR, myenteric plexus region
LM, longitudinal muscle
NOS, nitric oxide synthase
VIP, vasoactive intestinal peptide
PBS, phosphate buffered saline
REFERENCES
- 1.Ward SM, Burns AJ, Torihashi S, et al. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol 1994;480(Pt 1):91–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huizinga JD, Thuneberg L, Kluppel M, et al . W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature 1995;373:347–9. [DOI] [PubMed] [Google Scholar]
- 3.Thomsen L, Robinson TL, Lee JC, et al. Interstitial cells of Cajal generate a rhythmic pacemaker current. Nat Med 1998;4:848–51. [DOI] [PubMed] [Google Scholar]
- 4.Lee JC, Thuneberg L, Berezin I, et al. Generation of slow waves in membrane potential is an intrinsic property of interstitial cells of Cajal. Am J Physiol 1999;277(2 Pt 1):G409–23. [DOI] [PubMed] [Google Scholar]
- 5.Koh SD, Kim TW, Jun JY, et al. Regulation of pacemaker currents in interstitial cells of Cajal from murine small intestine by cyclic nucleotides. J Physiol 2000;527(Pt 1):149–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He CL, Soffer EE, Ferris CD, et al. Loss of interstitial cells of Cajal and inhibitory innervation in insulin-dependent diabetes. Gastroenterology 2001;121:427–34. [DOI] [PubMed] [Google Scholar]
- 7.Isozaki K, Hirota S, Miyagawa J, et al. Deficiency of c-kit+ cells in patients with a myopathic form of chronic idiopathic intestinal pseudo-obstruction. Am J Gastroenterol 1997;92:332–4. [PubMed] [Google Scholar]
- 8.Kenny SE, Vanderwinden JM, Rintala RJ, et al. Delayed maturation of the interstitial cells of Cajal: a new diagnosis for transient neonatal pseudoobstruction. Report of two cases. J Pediatr Surg 1998;33:94–8. [DOI] [PubMed] [Google Scholar]
- 9.Vanderwinden JM, Rumessen JJ, Liu H, et al. Interstitial cells of Cajal in human colon and in Hirschsprung's disease. Gastroenterology 1996;111:901–10. [DOI] [PubMed] [Google Scholar]
- 10.Burns AJ, Torihashi S, Harney SC, et al. The effect of the c-kit mutation on development of the interstitial cell network in the murine stomach and colon. Neurogastroenterol Motil 1995;7:249. [Google Scholar]
- 11.Vanderwinden JM, Rumessen JJ. Interstitial cells of Cajal in human gut and gastrointestinal disease. Microsc Res Tech 1999;47:344–60. [DOI] [PubMed] [Google Scholar]
- 12.Lubowski DZ, Chen FC, Kennedy ML, et al. Results of colectomy for severe slow transit constipation. Dis Colon Rectum 1996;39:23–9. [DOI] [PubMed] [Google Scholar]
- 13.Nyam DC, Pemberton JH, Ilstrup DM, et al. Long-term results of surgery for chronic constipation. Dis Colon Rectum 1997;40:273–9. [DOI] [PubMed] [Google Scholar]
- 14.Pikarsky AJ, Singh JJ, Weiss EG, et al. Long-term follow-up of patients undergoing colectomy for colonic inertia. Dis Colon Rectum 2001;44:179–83. [DOI] [PubMed] [Google Scholar]
- 15.Knowles CH, Scott M, Lunniss PJ. Outcome of colectomy for slow transit constipation. Ann Surg 1999;230:627–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.You YT, Wang JY, Changchien CR, et al. Segmental colectomy in the management of colonic inertia. Am Surg 1998;64:775–7. [PubMed] [Google Scholar]
- 17.Koch TR, Carney JA, Go L, et al. Idiopathic chronic constipation is associated with decreased colonic vasoactive intestinal peptide. Gastroenterology 1988;94:300–10. [DOI] [PubMed] [Google Scholar]
- 18.Milner P, Crowe R, Kamm MA, et al. Vasoactive intestinal polypeptide levels in sigmoid colon in idiopathic constipation and diverticular disease. Gastroenterology 1990;99:666–75. [DOI] [PubMed] [Google Scholar]
- 19.Dolk A, Broden G, Holmstrom B, et al. Slow transit chronic constipation (Arbuthnot Lane's disease). An immunohistochemical study of neuropeptide-containing nerves in resected specimens from the large bowel. Int J Colorectal Dis 1990;5:181–7. [DOI] [PubMed] [Google Scholar]
- 20.Faussone-Pellegrini MS, Infantino A, Matini P, et al. Neuronal anomalies and normal muscle morphology at the hypomotile ileocecocolonic region of patients affected by idiopathic chronic constipation. Histol Histopathol 1999;14:1119–34. [DOI] [PubMed] [Google Scholar]
- 21.He CL, Burgart L, Wang L, et al. Decreased interstitial cell of cajal volume in patients with slow-transit constipation. Gastroenterology 2000;118:14–21. [DOI] [PubMed] [Google Scholar]
- 22.Visser TD, Oud JL, Brakenhoff GJ. Refractive index and axial distance measurement in 3-D microscopy. Optic 1992;90:17–19. [Google Scholar]
- 23.Hagger R, Gharaie S, Finlayson C, et al. Regional and transmural density of interstitial cells of Cajal in human colon and rectum. Am J Physiol 1998;275(6 Pt 1):G1309–16. [DOI] [PubMed] [Google Scholar]
- 24.Ward SM, Beckett EA, Wang X, et al. Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J Neurosci 2000;20:1393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ward SM, Sanders KM. Interstitial cells of Cajal: primary targets of enteric motor innervation. Anat Rec 2001;262:125–35. [DOI] [PubMed] [Google Scholar]
- 26.Ward SM, Burns AJ, Torihashi S, et al. Impaired development of interstitial cells and intestinal electrical rhythmicity in steel mutants. Am J Physiol 1995;269(6 Pt 1):C1577–85. [DOI] [PubMed] [Google Scholar]
- 27.Hara Y, Kubota M, Szurszewski JH. Electrophysiology of smooth muscle of the small intestine of some mammals. J Physiol 1986;372:501–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szurszewski JH. Electrical basis for gastrointestinal motility. In: Johnson LR, ed. Physiology of the gastrointestinal tract. New York: Raven Press, 1987:1435–66.
- 29.Liu LW, Thuneberg L, Huizinga JD. Selective lesioning of interstitial cells of Cajal by methylene blue and light leads to loss of slow waves. Am J Physiol 1994;266(3 Pt 1):G485–96. [DOI] [PubMed] [Google Scholar]
- 30.Sarna SK, Bardakjian BL, Waterfall WE, et al. Human colonic electrical control activity (ECA). Gastroenterology 1980;78:1526–36. [PubMed] [Google Scholar]
- 31.Tzavella K, Schenkirsch G, Riepl RL, et al. Effects of long-term treatment with anthranoids and sodium picosulphate on the contents of vasoactive intestinal polypeptide, somatostatin and substance P in the rat colon. Eur J Gastroenterol Hepatol 1995;7:13–20. [PubMed] [Google Scholar]
- 32.Kiernan JA, Heinicke EA. Sennosides do not kill myenteric neurons in the colon of the rat or mouse. Neuroscience 1989;30:837–42. [DOI] [PubMed] [Google Scholar]
- 33.Riecken EO, Zeitz M, Emde C, et al. The effect of an anthraquinone laxative on colonic nerve tissue: a controlled trial in constipated women. Z Gastroenterol 1990;28:660–4. [PubMed] [Google Scholar]
- 34.Cortesini C, Cianchi F, Infantino A, et al. Nitric oxide synthase and VIP distribution in enteric nervous system in idiopathic chronic constipation. Dig Dis Sci 1995;40:2450–5. [DOI] [PubMed] [Google Scholar]
- 35.Faussone-Pellegrini MS, Fociani P, Buffa R, et al. Loss of interstitial cells and a fibromuscular layer on the luminal side of the colonic circular muscle presenting as megacolon in an adult patient. Gut 1999;45:775–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rumessen JJ. Ultrastructure of interstitial cells of Cajal at the colonic submuscular border in patients with ulcerative colitis. Gastroenterology 1996;111:1447–55. [DOI] [PubMed] [Google Scholar]
- 37.Hagger R, Finlayson C, Kahn F, et al. A deficiency of interstitial cells of Cajal in Chagasic megacolon. J Auton Nerv Syst 2000;80:108–11. [DOI] [PubMed] [Google Scholar]