Abstract
Background: 5-Aminosalicylates are extensively prescribed for the treatment of ulcerative colitis but have a wide range of described adverse effects.
Aims: To determine whether serious adverse effect profiles differ for sulphasalazine and mesalazine.
Methods: Analysis of suspected serious adverse reactions reported to the Committee on Safety of Medicines of the UK in 1991–1998. Adverse effect profiles were categorised for interstitial nephritis, pancreatitis, serious skin reactions, hepatitis and hepatic failure, and blood dyscrasias. Report rates were calculated using prescribing data from the Department of Health and compared for mesalazine and sulphasalazine. Further analysis was undertaken for sulphasalazine according to disease indication of inflammatory bowel disease or rheumatoid arthritis.
Results: A total of 4.7 million prescriptions were dispensed for sulphasalazine compared with 2.8 million for mesalazine. Interstitial nephritis was only described for mesalazine, with 11.1 reports per million prescriptions. Pancreatitis was reported seven times as frequently for mesalazine (7.5 per million prescriptions) compared with sulphasalazine (1.1 per million prescriptions) (odds ratio (OR) 7.0; 95% confidence interval (CI) 2.6–18.6; p<0.001). There were no reports of serious skin disorders in patients prescribed sulphasalazine for inflammatory bowel disease. Blood dyscrasias were reported significantly more often in patients receiving sulphasalazine for rheumatoid arthritis than for inflammatory bowel disease (OR 5.31; 95% CI 2.6–11.0; p<0.001), and there was a similar trend for hepatic disorders.
Conclusions: Spontaneous reports suggest that within the five sets of disorders considered, there is no evidence to indicate a safety advantage of mesalazine over sulphasalazine in the treatment of inflammatory bowel disease. Pancreatitis and interstitial nephritis appear significantly more common with mesalazine, and advice on renal monitoring in patients who receive mesalazine may need reinforcing.
Keywords: mesalazine, sulphasalazine, interstitial nephritis, pancreatitis, inflammatory bowel disease, rheumatoid arthritis
5-Aminosalicylic acid (5-ASA) is the active constituent of sulphasalazine in therapy for inflammatory bowel disease (IBD)1 with its sulphapyridine constituent perceived as a dispensable, but toxic, transport molecule.2 Pharmaceutical development has concentrated on delivering 5-ASA to small intestinal and colonic mucosa using inert carrier mechanisms. The success of this approach in IBD has largely been assessed in clinical trials designed to compare the efficacy of therapy rather than adverse events,3 although occasional renal effects of high dose mesalazine have been noted.4 However, the relatively small number of patients included in such studies allows comparison of only the commonest reactions, which are often minor and not life threatening. Medically important but serious rare reactions may not become apparent until large numbers of patients have been exposed to a particular compound. Analysis of the adverse effects of sulphasalazine is complicated because the compound is now widely prescribed in rheumatoid arthritis (RA).5 It cannot be assumed that adverse effects attributable to sulphasalazine, or any other compound, will occur with the same frequency, irrespective of disease type. Furthermore, the increased cost of mesalazine (£32.69/month) compared with sulphasalazine (£7.74/month)6 in IBD treatment needs to be justified.
We sought to examine differences in the serious adverse reaction profiles associated with sulphasalazine and mesalazine treatment for IBD, and to compare the frequency of serious adverse reactions with sulphasalazine in patients with RA and IBD. We therefore examined spontaneous reports of suspected adverse reactions to the Committee on Safety of Medicines (CSM) for sulphasalazine and mesalazine and related these reports to total numbers of prescriptions issued over a nine year period.
METHODS
Suspected adverse drug reactions reported to the CSM for sulphasalazine and mesalazine from January 1991 to December 1998 were analysed. Serious life threatening reactions studied in detail were interstitial nephritis, pancreatitis, serious skin reactions, hepatitis or hepatic failure, and blood dyscrasias. Only interstitial nephritis was studied as a specific renal adverse reaction although the overall number of renal reactions was evaluated. Skin reactions included were Stevens-Johnson reactions, erythema multiforme, bullous dermatitis, toxic pustuloderma and epidermal necrolysis, and exfoliative dermatitis. Blood dyscrasias included were bone marrow depression, aplastic anaemia, agranulocytosis and neutropenia, and thrombocytopenia. Numbers of reports were examined according to whether prescriptions were for IBD, RA, or unclear (categorised as “other”).
Total numbers of prescriptions dispensed in the community were obtained from the Department of Health for sulphasalazine and mesalazine for the same period. Mesalazine prescriptions were aggregated for “Asacol”, “Pentasa”, and “Salofalk”. There were insufficient reports and prescription numbers for the linked 5-aminosalicylate molecule “Olsalazine” and the para-aminobenzoate linked molecule “balsalazide” to allow similar study. Suspected adverse reaction rates were then calculated per million prescriptions. Use of sulphasalazine in treating RA or IBD was estimated by reference to published data from the General Practitioner Research Database for the period 1985–937 and rates were calculated separately for the two diseases.
Statistical methods
We used χ2 analysis to assess levels of probability, with odds ratios (OR) and 95% confidence intervals (CI), to compare suspected adverse report rates between mesalazine and sulphasalazine for all suspected serious adverse reports and between RA and IBD related events for sulphasalazine.
RESULTS
A total of 2400 suspected adverse drug reactions were reported for sulphasalazine to the CSM between 1 January 1991 and 31 December 1998 compared with 1100 for mesalazine. The number of sulphasalazine prescriptions issued over this time period was 4.67×106 compared with 2.8×106 prescriptions for mesalazine. When the total numbers of reports were related to the number of prescriptions issued over this time period, slightly greater overall numbers were recorded for sulphasalazine (514 reactions/million prescriptions) compared with mesalazine (393 reactions/million prescriptions) (OR 1.31; 95% CI 1.22–1.40).
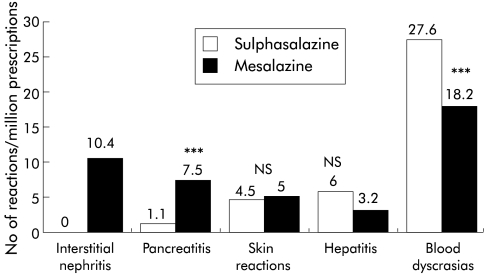
The adverse reaction profiles for each drug (table 1 ▶) showed that blood dyscrasias formed nearly 75% of the 183 serious reports studied for sulphasalazine but represented less than half of the 124 for mesalazine (p<0.001). Numbers of reported fatal blood dyscrasias were small: seven for sulphasalazine and five for mesalazine. Interstitial nephritis and pancreatitis reports formed 40% of reports for mesalazine but only 3% of those for sulphasalazine (p<0.001). Reports of suspected serious skin reactions for sulphasalazine were limited to patients with RA or other diagnoses and no cases were reported in patients with IBD. Interstitial nephritis accounted for 31% (29/93) of the total suspected adverse renal events for mesalazine but there were no such reports for sulphasalazine within the overall total of 27 adverse renal events reported for sulphasalazine. When prescription numbers were included and suspected adverse reactions rates were calculated (fig 1 ▶), a clear excess of reports was evident for interstitial nephritis (p<0.001) and pancreatitis (p<0.001, OR 7.0, 95% CI 2.6–18.6) for mesalazine compared with sulphasalazine and a moderate excess of reports of blood dyscrasias for sulphasalazine (OR 1.5, 95% CI 1.1–2.1). There were no significant differences in the frequency of serious skin reactions or hepatic disorders (OR 1.8 95% CI 0.88–3.95; p>0.05).
Table 1.
Adverse reaction profiles for sulphasalazine and mesalazine according to disease indication for prescribing for the period 1991–1998 (absolute numbers)
| Sulphasalazine | Mesalazine | ||||||
| RA | IBD | Other* | Total | IBD | Other* | Total | |
| Interstitial nephritis (23%) | 0 | 0 | 0 | 0 (0%) | 29 | 0 | 29 |
| Pancreatitis (17%) | 2 | 2 | 1 | 5 (3%) | 18 | 3 | 21 |
| Skin reactions (11%) | 15 | 0 | 6 | 21(11%) | 12 | 2 | 14 |
| Hepatitis (7%) | 15 | 9 | 4 | 28 (15%) | 8 | 1 | 9 |
| Blood dyscrasias (42%) | 80 | 25 | 24 | 129 (71%) | 48 | 3 | 51 |
| Total | 112 | 36 | 35 | 183 | 115 | 9 | 124 |
IBD, inflammatory bowel disease; RA, rheumatoid arthritis.
*Other includes unknown diagnosis and diagnoses other than RA, such as psoriatic athropathy and non-specific arthritis.
4.7×106 sulphasalazine and 2.8×106 mesalazine prescriptions were issued.
For sulphasalazine, disease indications for prescribing: RA=1.75×106 prescriptions, IBD=2.92×106 prescriptions.
Figure 1.
Total suspected serious adverse reactions for sulphasalazine and mesalazine per million prescriptions for the period 1991–1998 (χ2 test: ***p<0.001; NS, not significant).
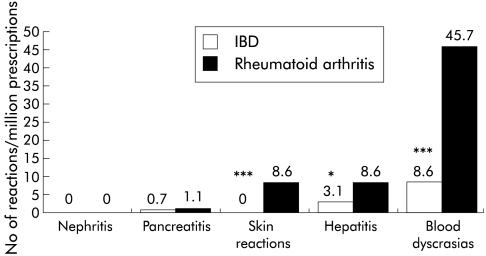
For sulphasalazine, the adverse event profiles were analysed according to disease indication of IBD or RA (fig 2 ▶). In the period considered, 37.5% of prescriptions issued for sulphasalazine in participating practices in the General Practitioner Research Database were for RA and 62.5% for IBD. Blood dyscrasias were reported over five times as often as expected in patients with RA as in those with IBD (OR 5.3; 95% CI 2.6–11.0; p<0.001). Hepatitis and hepatic failure were also reported more frequently with RA (p<0.01) while serious skin reactions were only reported in patients with RA.
Figure 2.
Suspected adverse reactions for sulphasalazine according to disease indication per million prescriptions for the period 1991–1998 (χ2 test: *p<0.01, ***p<0.001). Only cases with a specific diagnosis of inflammatory bowel disease (IBD) or rheumatoid arthritis were included.
DISCUSSION
These findings suggest that the serious adverse reaction profiles of sulphasalazine and mesalazine differ markedly. Interstitial nephritis was reported only for mesalazine while pancreatitis was over seven times as commonly reported for mesalazine compared with sulphasalazine. Blood dyscrasias, hepatitis and hepatic failure, and serious skin reactions were also reported more commonly in patients receiving sulphasalazine for RA rather than IBD, and calculated rates per million prescriptions were also markedly higher in patients with RA than in those with IBD.
Spontaneous reporting of suspected adverse reactions cannot be used to determine true rates of reaction. Reporting rates are generally much lower than actual rates, as shown for example by comparison of reporting rates of suspected serious adverse reactions to non-steroidal anti-inflammatory drugs with observed frequencies of events in clinical practice.8,9 Whether doctors do or do not report adverse reactions reflects unknown factors. Reporting may have been more common in the period studied for mesalazine because it was relatively newly released, because a specific warning of possible renal toxicity had been issued,10 and because sulphasalazine takers were likely to be established rather than new takers. Estimation of event rates per million prescriptions of sulphasalazine for RA and IBD also lack precision because of the differing but overlapping time periods of adverse reaction surveillance and prescription collection. However, computerised general practice data have shown that the risk of blood disorders attributable to sulphasalazine was 10 times higher for RA patients than for patients with IBD,7 a value of the same order of magnitude as the fivefold difference found by us for spontaneous reports. Even taking into account all of the potential biases in spontaneous reports, the differences seen here in rates of pancreatitis, interstitial nephritis, and blood dyscrasias are unlikely to reflect chance.
Interstitial nephritis is recognised as a complication of mesalazine treatment,11 and an idiosyncratic hypersensitivity reaction with the use of sulphasalazine,12 while tubular proteinuria,13 renal calculi, AA amyloidosis, and glomerulonephritis14 occur in untreated IBD. 5-ASA resembles phenacetin structurally, a well known cause of interstitial nephritis and papillary necrosis,15 and systemic absorption and urinary concentrations of 5-ASA have been found to be higher with mesalazine than with azo-bond 5-ASA preparations.16
The incidence of mesalazine associated interstitial nephritis has varied from one recorded case in 15017 and none in 50018 to renal impairment with elevated serum creatinine in 0.2% of patients, as found in the largest study so far reported involving 2940 patients.19 Renal impairment has been associated with higher 5-ASA doses,4 and tubular proteinuria has been found in up to one third of patients treated with doses over 3 g daily.20
It has been recommended that serum urea and creatinine levels should be monitored every four weeks for the first three months, every three months for a year, and annually thereafter in patients receiving any mesalazine containing preparation.21 Drug withdrawal restores renal function if done early, with 85% of cases of renal impairment resolved where treatment was stopped within 10 months.18 The British National Formulary warns of the risk of interstitial nephritis but does not recommend renal monitoring.6
Pancreatitis was commonly found by us, and in a French study of mesalazine as Pentasa,22 and mesalazine associated pancreatitis is well described on the World Health Organisation adverse reaction database.23 Sulphasalazine associated pancreatitis by comparison is rarely reported.
Sulphasalazine intolerant patients have been shown to tolerate mesalazine, olsalazine, or balsalazide24 but serious skin conditions and hepatic disorders were reported here as frequently in patients prescribed sulphasalazine as mesalazine. Hepatotoxicity with severe hypersensitivity can occur when patients with known sulphasalazine allergy are prescribed mesalazine.25
Blood dyscrasias in sulphasalazine takers were also reported more frequently in patients with RA rather than IBD. Agranulocytosis and pancytopenia are well documented adverse reactions of sulphasalazine. In this study the commonest adverse reactions to both sulphasalazine and mesalazine were blood dyscrasias. This may be explained by the increased dose of sulphasalazine used in RA or a greater liability to the development of blood dyscrasias. The risk of agranulocytosis appears higher in the first three months than later.26,27
We conclude that there are important differences in adverse event patterns associated with mesalazine and sulphasalazine treatment. Interstitial nephritis and pancreatitis appear to be major risks with mesalazine, with blood dyscrasias being more prominent for sulphasalazine, but mainly in rheumatoid patients. Overall, the data suggest that the choice between sulphasalazine and mesalazine in IBD may be more evenly balanced than at first appeared.
Acknowledgments
Provision of suspected adverse drug reaction reports by the Medicines Control Agency of the UK, and prescription data by the Department of Health are gratefully acknowledged.
Abbreviations
5-ASA, 5-aminosalicylic acid
IBD, inflammatory bowel disease
RA, rheumatoid arthritis
CSM, Committee on Safety of Medicines
REFERENCES
- 1.Das KM, Eastwood MA, McManus JPA, et al. Adverse reactions during salicylazosulphapyridine therapy and the relation with drug metabolism and acetylator phenotype. N Engl J Med 1973;289:491–5. [DOI] [PubMed] [Google Scholar]
- 2.Azad Khan AK, Piris J, Truelove SC. An experiment to determine the active therapeutic moiety of sulphasalazine. Lancet 1977;2:892–5. [DOI] [PubMed] [Google Scholar]
- 3.Riley SA, Mani V, Goodman MJ, et al. Comparison of delayed-release 5-aminosalicylic acid (mesalazine) and sulfasalazine as maintenance treatment for patients with ulcerative colitis. Gastroenterology 1988;94:1383–9. [DOI] [PubMed] [Google Scholar]
- 4.Riley SA, Mani V, Goodman MJ, et al. Comparison of delayed release 5 aminosalicylic acid (mesalazine) and sulphasalazine in the treatment of mild to moderate ulcerative colitis relapse. Gut 1988;29: 669–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Capell HA, Maiden N, Madhok R, et al. Intention-to-treat analysis of 200 patients with rheumatoid arthritis 12 years after random allocation to either sulfasalazine or penicillamine. J Rheumatol 1998;25:1880–6. [PubMed] [Google Scholar]
- 6.British National Formulary, vol. 41. London: British Medical Association and Royal Pharmaceutical Society of Great Britain, 2001 :47–9.
- 7.Jick H, Myers MW, Dean AD. The risk of sulphasalazine and mesalazine associated blood disorders. Pharmacotherapy 1995;15:176–81. [PubMed] [Google Scholar]
- 8.CSM update. Non-steroidal anti-inflammatory drugs and serious gastrointestinal adverse reactions-2. BMJ 1986;292:1190–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langman MJS. Ulcer complications associated with anti-inflammatory drug use. What is the extent of the disease burden? Pharmacoepidemiol Drug Safety 2001;10:13–19. [DOI] [PubMed] [Google Scholar]
- 10.Anon. Nephrotoxicity associated with mesalazine. Current problems. Comm Safety Med 1990;30:2. [Google Scholar]
- 11.Thuluvath PJ, Ninkovic M, Calam J, et al. Mesalazine induced interstitial nephritis. Gut 1994;35:1493–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chester AC, Diamond LH, Schreiner GE. Hypersensitivity to salicylazosulfapryridine. Renal and hepatic toxic reactions. Arch Intern Med 1978;138:1138–9. [DOI] [PubMed] [Google Scholar]
- 13.Fraser JS, Muller AF, Smith DJ, et al. Renal tubular injury is present in acute inflammatory bowel disease prior to the introduction of drug therapy. Aliment Pharmacol Ther 2001;15:1131–7. [DOI] [PubMed] [Google Scholar]
- 14.Pardi DS, Temaine WJ, Sandborn WJ, et al. Renal and urologic complications of inflammatory bowel disease. Am J Gastroenterol 1998;93:504–14. [DOI] [PubMed] [Google Scholar]
- 15.Rocha GM, Michea LF, Peters EM, et al. Direct toxicity of nonsteroidal anti-inflammatory drugs for renal medullary cells. Proc Natl Acad Sci USA 2001;98:5317–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Staerk Laursen L, Stokholm M, Bukhave K, et al. Disposition of 5-aminosalicylic acid by olsalazine and three mesalazine preparations in patients with ulcerative colitis: comparison of intraluminal colonic concentrations, serum values and urinary excretion. Gut 1990;31:1271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fockens P, Mulder CJJ, Tytgat GNJ, et al. Comparison of the efficacy and safety of 1.5 compared with 3.0g oral slow-release mesalazine (Pentasa) in the maintenance treatment of ulcerative colitis. Eur J Gastroenterol Hepatol 1995;7:1025–30. [DOI] [PubMed] [Google Scholar]
- 18.World MJ, Stevens PE, Ashton MA, et al. Mesalazine associated interstitial nephritis. Nephrol Dial Transplant 1996;11:614–21. [DOI] [PubMed] [Google Scholar]
- 19.Hanauer SB, Verst-Brasch C, Regalli G. Renal safety of long-term mesalamine therapy in inflammatory bowel disease. Gastroenterology 1997;112(suppl):A991. [Google Scholar]
- 20.Schreiber S, Hamling J, Zehnter E, et al. Renal tubular dysfunction in patients with inflammatory bowel disease treated with aminosalicylate. Gut 1997;40:761–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corrigan G, Stevens PE. Interstitial nephritis associated with the use of mesalazine in inflammatory bowel disease. Aliment Pharmacol Ther 2000;14:1–6. [DOI] [PubMed] [Google Scholar]
- 22.Marteau P, Nelet F, Le Lu M, et al. Devaux C. Adverse events in patients treated with 5-aminosalicylic acid:1993–1994. Pharmacovigilance report for Pentasa in France. Aliment Pharmacol Ther 1996;10:949–56. [DOI] [PubMed] [Google Scholar]
- 23.Bergholm U, Langman M, Rawlins M, et al. Drug-induced acute pancreatitis. Pharmacoepidemiol Drug Safety 1995;4:329–34. [Google Scholar]
- 24.Giaffer MH, O’Brien CJ, Holdsworth CD. Clinical tolerance to three 5-aminosalicylic acid releasing preparations in patients with inflammatory bowel disease intolerant or allergic to sulphasalazine. Aliment Pharmacol Ther 1992;6:51–9. [DOI] [PubMed] [Google Scholar]
- 25.Hautekeete ML, Bourgeois N, Potvin P, et al. Hypersensitivity with hepatotoxicity to mesalazine after hypersensitivity to sulfasalazine. Gastroenterology 1992;106:1925–7. [DOI] [PubMed] [Google Scholar]
- 26.Huang JL, Hung IJ, Chen LC, et al. Successfully treated sulphasalazine-induced fulminant hepatic failure, thrombocytopenia and erythroid hypoplasia with intravenous immunoglobulin. Clin Rheum 1998;17:349–52. [DOI] [PubMed] [Google Scholar]
- 27.Keisu M, Ekman E. Sulfasalazine associated agranulocytosis in Sweden 1972–1989. Clinical features and estimation of its incidence. Eur J Pharmacol 1992;43:215–18. [DOI] [PubMed] [Google Scholar]