Abstract
Background: Although the prognosis in malignant resectable intraductal papillary mucinous tumours of the pancreas (IPMT) is often considered more favourable than for ordinary pancreatic ductal adenocarcinoma, the long term outcome remains ill defined.
Aims: To assess prognostic factors in patients with malignant IPMT after surgical resection, and to compare long term survival rates with those of patients surgically treated for ductal adenocarcinoma.
Methods: Seventy three patients underwent surgery for malignant IPMT in four French centres. Clinical, biochemical, and pathological features and follow up after resection were recorded. Patients with invasive malignant IPMT were matched with patients with pancreatic ductal adenocarcinoma, according to age and TNM stages; survival rates after resection were compared.
Results: Surgical treatment for IPMT were pancreaticoduodenectomy (n=46), distal (n=14), total (n=11), or segmentary (n=2) pancreatectomy. The operative mortality rate was 4%. IPMT corresponded to in situ (n=22) or invasive carcinoma (n=51). In the latter group, 17 had lymph node metastases. Overall median survival was 47 months. Five year survival rates in patients with in situ and invasive carcinoma were 88% and 36%, respectively. On univariate analysis, abdominal pain, preoperative high serum carbohydrate antigen 19.9 concentrations, caudal localisation, invasive carcinoma, lymph node metastases, peripancreatic extension, and malignant relapse were associated with a fatal outcome. Using multivariate analysis, lymph node metastases were the only prognostic factor (OR 7.5; 95% CI: 3.4 to 16.4). Overall five year survival rate was higher in patients with malignant invasive IPMT compared with those with pancreatic ductal carcinoma (36 v 21%, p=0.03), but was similar in the subset of stage II/III tumours.
Conclusions: The prognosis of patients with resected in situ/invasive stage I malignant IPMT is excellent. In contrast, prognosis of locally advanced forms is as poor as in patients with pancreatic ductal adenocarcinoma.
Keywords: pancreas, intraductal papillary mucinous tumours
Intraductal papillary mucinous tumours of the pancreas (IPMT) are rare pancreatic tumours, characterised by an adenomatous proliferation of pancreatic duct epithelium that may involve the main pancreatic duct or branch ducts alone or in combination.1,2 Different stages from hyperplasia to invasive carcinoma can be observed.3 Diagnostic and therapeutic problems stem from the difficulties in the preoperative determination of malignant transformation and extent of duct involvement.4–8 The prognosis in patients with IPMT depends on malignant transformation that occurs in 30% to 60% of cases, of which 17% to 43% are invasive cancers.6,9–11 Because of the established adenoma-carcinoma sequence, surgical resection of the tumour is the treatment currently recommended.4 Surgical resection of benign IPMT is associated with an overall five year survival exceeding 90%.12,13 In contrast, outcome and prognostic factors of patients operated on for malignant IPMT are less well defined and a wide spectrum of survival rates can be found in the literature.6,9,10,14 It has been suggested that the prognosis of malignant invasive IPMT is much better than that of ordinary pancreatic ductal adenocarcinoma, but direct comparisons between these two forms of pancreatic cancer are lacking and therefore definitive conclusions cannot be drawn.6,9,15
The aims of this study were: (1) to define the clinical, biochemical and pathological features and long term outcome after surgical resection in a large multicentric series of patients including only malignant IPMT, (2) to determine the prognostic factors for survival, and (3) to compare long term survival after surgical resection between patients with malignant invasive IPMT and a matched group of patients with pancreatic ductal adenocarcinoma of similar age and TNM stages.
METHODS
Selection of patients
Selection of patients required three stages: (1) all patients who underwent tumour resection for IPMT between 1987 and 1999 in four French centres were reviewed; (2) in each centre, only patients who underwent surgical resection for malignant IMPT were selected by a single local pathologist. Other malignant cystic lesions were excluded; (3) all surgical specimens were reviewed by one central pathologist (BT) who confirmed the diagnosis and its stage. Seven patients were rejected by this pathologist because of an incorrect diagnosis. Patients were also excluded if their clinical data (symptoms and diagnostic delay) were incomplete.
Clinical and biochemical data
The following clinical data were recorded: age, sex, clinical signs leading to the diagnosis, and the delay between diagnosis and surgery. Serum tumour markers including carbohydrate antigen 19–9 (CA 19–9) and carcinoembryonic antigen (CEA) were recorded when available. Analysis of imaging data was not the aim of our study. Clinical data of 41 patients have been previously published in part.5,6,11,12,17
Pathological data
Pathological examinations, including macroscopic examination and histological examination for all patients from different centres, were reviewed by one pathologist (BT) to achieve homogeneous interpretation for the following variables: (a) diagnosis of IPMT defined by proliferation of pancreatic duct epithelium with mucus production, (b) signs of malignant transformation with a distinction between invasive adenocarcinoma and in situ carcinoma (the latter defined as the presence of marked cellular atypia with cytoplasmic nuclear changes and loss of nuclear polarisation without disruption of the basement membrane),3 (c) characterisation of tumours with respect to: involvement of main pancreatic duct, branch ducts, or both, continuity of tumour extension, size (in the presence of measurable solid lesions or the perpendicular diameter of the largest cystic dilatation, the length of pancreatic duct involvement was used in cases of high grade dysplasia), (d) determination of lymph node involvement and extra-pancreatic extension. The presence of marked mucus production that characterises colloid forms of IMPT was also recorded. Results from frozen section examination of the pancreatic cut-surface margin were considered when available.
Survival analysis
Postoperative survival was determined from the date of surgery to last follow up or patient death. Disease free survival was determined in patients free of recurrent disease at the last contact with the reference physicians in each centre. Time to disease relapse and treatment options for recurrent disease were recorded. Prognostic factors were analysed. Long term survival in patients after surgery for invasive malignant IPMT was compared with that of patients who underwent surgery with curative intent in the same four institutions over the same time period for pancreatic ductal adenocarcinoma, with exclusion of patients who died within 30 days after surgery. Only patients with invasive IPMT could be matched because of the absence of patients surgically treated for ductal adenocarcinoma with in situ carcinoma stages. While the TNM classification16 has been defined for pancreatic ductal adenocarcinoma, we extrapolated the 4th edition of the UICC TNM classification for malignant invasive IPMT, to compare two homogenous groups: stage I (limited to pancreas, duodenum, bile duct, or peripancreatic tissues), stage II (stomach, spleen, colon, or adjacent large vessels involvement), stage III (lymph node involvement), stage IV (distant metastasis). Patients were matched for age (± 5 years), TNM stages, and centre by centre when possible.
Statistical analysis
Comparisons of clinical and pathological parameters were performed using the χ2 test or Fisher’s exact test (if small number) for qualitative variables. Student’s t test was used for quantitative variables and a non-parametric test (Mann-Whitney test) if their distribution were abnormal. Survival curves were calculated using the Kaplan-Meier method. Univariate analysis was performed for factors influencing survival using the log rank test and results were expressed as the relative risk using a 95% confidence interval. Multivariate analysis was performed using the Cox model. The level of statistical significance was determined at 5% in all cases.
RESULTS
Clinical and biochemical data
Seventy three patients (49 men and 24 women) with a median age of 66 years (range 25–89) and a histological diagnosis of malignant IPMT were included. The clinical manifestations leading to the diagnosis were as follows: acute pancreatitis (n=20), abdominal pain (n=13), jaundice or ascending cholangitis (n=12), diarrhoea (n=9), weight loss (n=8), diabetes (n=4), palpable abdominal mass (n=2). The diagnosis was incidental in five patients undergoing abdominal ultrasound for an unrelated reason. The median delay between the onset of the first symptoms and surgery was nine months (range 0–196). Preoperative serum tumour marker (CEA and CA 19.9) concentrations were available in 38 and 46 patients, respectively. The CA 19.9 concentration was above twice the upper normal range in 37% and CEA in 16% of patients.
Surgical procedures, macroscopic examination, and histological examination
The following surgical procedures were performed: pancreaticoduodenectomy (n=46), medial (n=2), distal (n=14), and complete pancreatectomy (n=11). Because of tumour extension, a segmental colectomy and left adrenal gland resection were required in two and one patients, respectively. Surgical procedures according to IPMT localisation were similar in the five centres.
Pancreatic tumour localisation was as follows: head/body (n=49), neck (n=3), tail (n=14), diffuse (n=6), and bifocal (n=1). The topography of pancreatic duct involvement included main pancreatic duct only (n=9), branch ducts only (n=8), and combined (n=56). The median tumour size was 30 mm (range 9–130). In 13 patients without measurable solid lesions, the median diameter of cystic dilatation was 20 mm (range 6–70).
At histological examination, 22 patients (30%) had in situ carcinoma and 51 (70%) invasive carcinoma. Table 1 ▶ shows the comparison of their clinical and pathological characteristics and outcome. Metastatic lymph nodes were found in 17 patients (33%) with invasive IPMT. Distribution of tumour stages according the TNM classification16 was as follows: stage I (n=27), II (n=7), and III (n=17). A colloid form of IPMT was observed in 33 patients. Tumour involvement was found to be continuous along pancreatic ducts in all patients except one. In this patient, foci of in situ carcinoma in both main and branch ducts were found in the pancreatic head and tail separated by a length of normal main pancreatic duct. Histological examination of the resected pancreatic specimen margins in the 73 patients demonstrated normal tissue (n=50), simple hyperplasia (n=9), atypical hyperplasia (n=7), in situ carcinoma (n=4), or invasive adenocarcinoma (n=3). In a subset of 41 patients, frozen section examination of the pancreatic cut surface was performed intraoperatively and resulted in extension of resection in 13 because of the diagnosis in remaining ducts of simple or atypical hyperplasia. Two patients treated for in situ carcinoma were found to have additional in situ carcinoma in pancreatic resection margins and subsequently underwent total pancreatectomy.
Table 1.
Clinical, biochemical, pathological features and follow up after resection in 73 patients with malignant IPMT according to their pathological status (in situ or invasive carcinoma)
| In situ (n=22) | Invasive (n=51) | p Value | |
| Sex (male/female) | 14/8 | 35/16 | NS |
| Median age (y) | 62.6 (25–89) | 63.8 (38–81) | NS |
| First symptom | |||
| Acute pancreatitis | 8 | 12 | NS |
| Abdominal pain | 4 | 9 | NS |
| Jaundice | 2 | 10 | NS |
| Diarrhoea | 3 | 6 | NS |
| Weight loss | 2 | 6 | NS |
| Diabetes | 2 | 2 | NS |
| Mass | 0 | 2 | NS |
| Asymptomatic | 1 | 4 | NS |
| Median diagnosis delay (months) | 24 (0–196) | 3 (0–180) | 0.01 |
| CEA (n=38)* ≥2N | 0/12 | 6/26 | NS |
| CA 19.9 (n=46)* ≥2N | 1/12 | 16/34 | 0.03 |
| Tumour location | |||
| Head/corpus | 14 | 35 | NS |
| Tail | 2 | 12 | NS |
| Diffuse | 4 | 2 | NS |
| Duct involvement | |||
| Main duct or combined | 18 | 47 | NS |
| Branch ducts only | 4 | 4 | NS |
| Median tumour size (mm) (n=60)* | 23 (9–40) | 36 (10–130) | 0.01 |
| Pancreatic cut surface | |||
| Normal | 17 | 33 | NS |
| Simple hyperplasia | 2 | 7 | NS |
| Atypical hyperplasia | 1 | 6 | NS |
| In situ carcinoma | 2 | 2 | NS |
| Invasive carcinoma | 0 | 3 | NS |
| Metastatic lymph nodes | 0 | 17 | 0.002 |
| Cancer relapse | 0 | 28 | 0.001 |
| Median follow up (months) | 28 (0–145) | 23 (0–115) | NS |
| Postoperative death | 1 | 2 | NS |
| Five year survival (SE) | 88% ( 8) | 36% ( 9) | 0.001 |
IPMT, intraductal papillary mucinous tumours of the pancreas; CEA, carcinoembryonic antigen; CA 19.9, carbohydrate antigen 19-9; NS, p>0.05. N, upper limit of the normal laboratory value. *Available data.
Postoperative course
Overall mean and median follow up after surgery were 32.7 and 25 months (range 0–145), respectively. Postoperative inhospital deaths occurred in three (4%) patients (one with in situ carcinoma and two with invasive carcinoma) related to respiratory distress (n=1), intravascular coagulopathy (n=1), and unknown cause (n=1). The first 30 day morbidity included infection (n=9), pancreatic fistula (n=4), haemorrhage (n=3), abdominal occlusion (n=2). Adjuvant radiotherapy and/or chemotherapy were performed in seven patients, who had either in situ/invasive carcinomas at the pancreatic resection margins or lymph node involvement.
Malignant tumour relapse occurred in 28 patients (38%) (including five patients with malignancy at resection margins) during the study period, with a median delay of 12 months (range 0–56), in 44 sites: remaining pancreas (n=16), lymph nodes (n=7) and metastases (n=21). No patients with in situ carcinoma had malignant tumour relapse (p=0.001 v patients with invasive cancer). Malignant relapse was treated as follows: extensive pancreatic surgical resection (n=5), systemic chemotherapy (n=5), combined radiotherapy-chemotherapy (n=1), radiotherapy alone (n=1), palliative medical support (n=16).
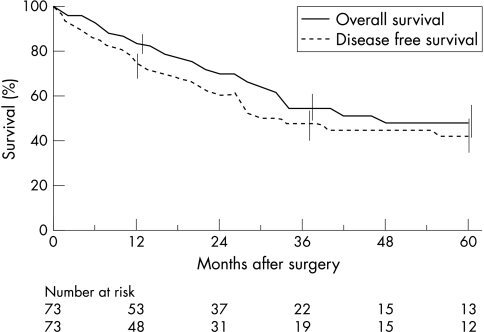
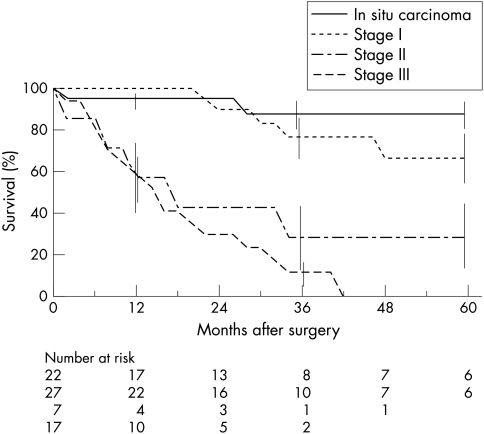
The mortality rate at the end of the study period was 38%. Twenty three patients died of IPMT and two without recurrence of IPMT: severe diabetes mellitus after total pancreatectomy (n=1) and accidental (n=1). The overall actuarial and disease free survival rates were 55% and 47% at three years and 48% and 42% at five years, respectively (fig 1 ▶). The five year survival rates in patients with in situ carcinoma and invasive carcinomas were 88% and 36%, respectively (p=0.001) (fig 2 ▶).
Figure 1.
Overall and disease free actuarial survival after resection of malignant IPMT in 73 patients, as assessed using Kaplan-Meier method. Vertical bars represent standard errors.
Figure 2.
Overall actuarial survival after resection of malignant IPMT in 73 patients according to TNM stage: in situ carcinoma (n=22), stage I invasive carcinoma (n=27), stage II (n=7), stage III (n=17). Vertical bars represent standard errors.
Predictive factors for survival
On univariate analysis (table 2 ▶), poor prognostic factors were initial abdominal pain, increased serum CA 19.9 concentrations (≥2N), distal pancreatic localisation, invasion of basal membrane, metastatic lymph nodes, and malignant relapse. A trend towards better survival was observed in patients with colloid forms of IPMT, but the difference was not significant (p=0.07). Administration of adjuvant treatments was not found to influence survival rates.
Table 2.
Prognostic factors on univariate analysis in 73 patients who underwent resection for malignant IPMT
| Variable | Patients (n) | p Value* | Odds ratio (95% CI) |
| Sex: male | 49 | NS | |
| Age >65 y | 41 | NS | |
| First symptom | |||
| Acute pancreatitis | 20 | NS | |
| Abdominal pain | 13 | 0.004 | 3.3 (1.4 to 7.7) |
| Jaundice | 12 | NS | |
| CEA (n=38)† ≥2N | 6 | NS | |
| CA 19.9 (n=46)† ≥2N | 17 | 0.02 | 2.7 (1.1 to 6.6) |
| Tumour location | |||
| Head/corpus | 49 | 0.01 | 0.3 (0.1 to 0.8) |
| Tail | 14 | 0.04 | 2.3 (1.0 to 5.2) |
| Diffuse | 6 | NS | |
| Duct involvement | |||
| Main duct or combined | 65 | NS | |
| Secondary ducts only | 8 | NS | |
| Tumour size (n=60)† >30 mm | 21 | NS | |
| Pathology | |||
| In situ carcinoma | 22 | 0.01 | 6.3 (1.5 to 27.0) |
| Invasive carcinoma | 51 | ||
| Pancreatic cut surface | |||
| Normal | 50 | NS | |
| Hyperplasia | 9 | NS | |
| Atypical hyperplasia | 7 | NS | |
| In situ carcinoma | 4 | NS | |
| Invasive carcinoma | 3 | NS | |
| Colloid form (n=51)† | 33 | NS | |
| Lymph node metastases | 17 | 0.0001‡ | 7.5 (3.4 to 16.4) |
| Peripancreatic extension: T3 | 12 | 0.01 | 2.8 (1.2 to 6.8) |
| Malignant relapse | 28 | 0.0001 | 9.0 (3.4 to 24.0) |
| Adjuvant treatment: radio/chemotherapy | 7 | NS | |
| Centre | |||
| Clichy | 24 | NS | |
| Lyon | 23 | NS | |
| Paris | 20 | NS | |
| Marseille | 6 | NS |
*Log rank test, number of patients for each variable compared with remaining patients. †Data available. IPMT, intraductal papillary mucinous tumours of the pancreas; CEA, carcinoembryonic antigen; CA 19.9, carbohydrate antigen 19-9; NS, p>0.05. N, upper limit of the normal laboratory value. ‡Also significant in multivariate analysis.
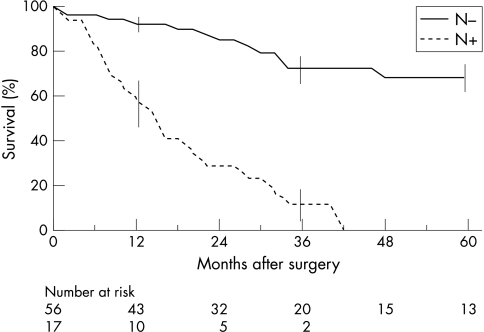
On multivariate analysis, only the presence of metastatic lymph nodes was predictive of death with a relative risk of 7.5 (95% CI 3.4 to 16.4) (fig 3 ▶).
Figure 3.
Overall actuarial survival after resection of malignant IPMT in 73 patients according to lymph node involvement. (N+: lymph node invasion, N−: absence of lymph node invasion). Vertical bars represent standard errors.
Survival in patients with invasive IPMT compared with ductal adenocarcinoma
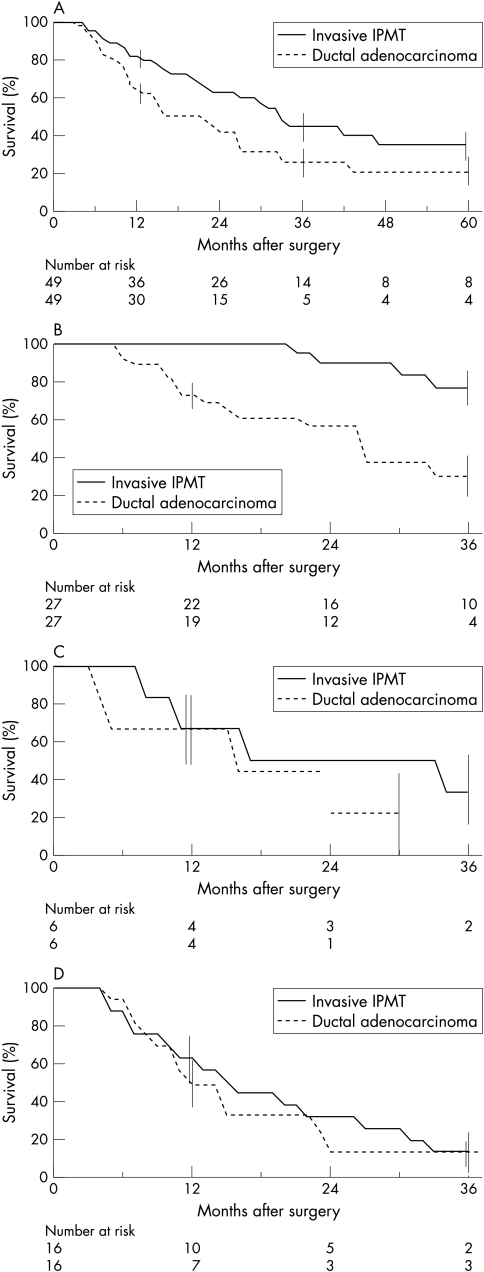
Patients with invasive IPMT (with exclusion of the two patients who died within 30 days after surgery) (n=49) were matched with patients surgically treated for pancreatic ductal adenocarcinoma (n=49) of similar age and TNM stages: stage I (n=27), stage II (n=6), stage III (n=16). Seven patients with IPMT and 11 with ductal adenocarcinoma received chemotherapy or radiotherapy, or both, after surgery (NS). The median survival durations were 33 months in patients with invasive IPMT compared with 22 months for those with ductal adenocarcinoma. Overall survival rates in patients with IPMT and ductal carcinoma were 36% and 21% at five years, respectively (fig 4A ▶). This difference was statistically significant (p=0.03). The overall survival rates in patients with IPMT compared with those with ductal adenocarcinoma were significantly different for stage I (67% v 23% at five years, respectively) (p<0.001) (fig 4B ▶), but not for stages II and III (figs 4C and 4D ▶ ▶).
Figure 4.
Comparison of overall actuarial survival after surgical resection in two groups of matched patients for age and TNM stage with invasive malignant IPMT or ductal adenocarcinoma. Vertical bars represent standard errors. (A) Overall populations (n=49 in each group); (B) stage I patients (n=27); (C) stage II patients (n=6); (D) stage III patients (n=16).
DISCUSSION
This study analysed outcome after surgery in a large series of patients with malignant IPMT and was the first to propose direct comparison of survival in patients undergoing surgery for either malignant IPMT or ductal pancreatic adenocarcinomas. Complete clinical and pathological data were recorded for 73 patients from four French centres. To reduce interobserver variability, all the resected specimens were reviewed by one pathologist.
Male/female ratio (2/1), age, clinical manifestations, and tumour localisation were in accordance with previous reports.1,4,9,17–19 In contrast, the proportion of main pancreatic duct involvement, either alone or combined with branch duct lesions (89%), was higher than that previously published.9,20 This may be explained by the selection of malignant forms of IPMT (that is, in situ or invasive), which as recently demonstrated involve the main pancreatic duct more frequently than branch ducts.11,20,21 The median delay between first symptoms and diagnosis of malignant IPMT (nine months) is shorter than previously reported (12 to 43 months)4,9; this result may reflect increased physician awareness, improvements in imaging methods and the selection in this study of malignant forms of IPMT.
The natural history of IPMT remains poorly defined. Most studies have reported an excellent prognosis in patients undergoing surgery for benign tumours.6,9,19,22 In contrast, a wide variability in five year survival rates has been reported (0% to 82%) in patients treated for malignant IPMT.1,4,8–10,14 In this study, the five year survival rate of 48% is in accordance with some previous surgical studies,10 except for two Japanese series that reported overall five year survival rates of 75% to 82%.8,9 Possible explanations for such discordance include: small numbers of patients studied, data selection from compiled series, lack of distinction between in situ and invasive carcinomas, exclusion of postoperative mortality, or unclear causes of deaths in some studies.8,9,19
In this study, the five year survival rate of patients undergoing surgery for in situ IPMT was excellent and mortality in such forms was only related to early or late surgical complications. As neither relapse nor cancer death was observed in this subgroup of patients, perioperative morbidity and mortality should be as low as possible. Resection in such patients should be adapted to tumour extension, however extensive resection has to be counterbalanced with long term complications such as brittle diabetes mellitus. Management in these patients requires careful decision making in reference centres with precise intraoperative evaluation including frozen section examination of pancreatic resection margins.
In contrast, patients with invasive IPMT had a high rate of cancer relapse and cancer deaths, in line with previous reports.6,10 Lymph node involvement remained the only predictive factor of death on multivariate analysis (OR 7.5). Some studies reported that tumour size (>6 cm), peripancreatic invasion, and lymph node involvement are of prognostic value.9,10,14 In this study, tumour size did not influence survival. The precise determination of size in malignant IPMT is often problematic because of the intrinsic cystic nature of these tumours, which are composed of numerous dilated ducts, while areas of solid (malignant) tumour components are difficult to individualise. While a high serum CA 19.9 concentration was found to predict poor prognosis in this study, as observed in pancreatic ductal adenocarcinoma, this factor was no longer significant on multivariate analysis.23 As lymph node involvement is of large prognostic importance, the question of extended surgical lymph node dissection, as proposed by Japanese authors,24 can be raised. This problem has never been formally resolved in patients with IPMT. It has been reported that extended lymph node dissection does not influence long term survival in patients with pancreatic ductal adenocarcinoma,25 but whether such strategies have an impact on survival in IPMT needs to be analysed in a prospective fashion. The prognosis in patients with IPMT is often considered more favourable than for ordinary pancreatic ductal adenocarcinoma,9,14,15 but a matched comparison between these two forms of pancreatic cancers has never been performed. To answer this question, we compared survival in patients who underwent surgical resection for invasive malignant IPMT or ductal adenocarcinoma according to age and TNM classification. In our series, the five year survival rate after resection for all stages of ductal adenocarcinoma was 21%, a rate similar to that previously reported.14,26–28 Overall survival rates were better in patients with IPMT compared with those treated for ductal adenocarcinomas of early stage (I), but this difference was no longer significant in the subgroups of patients with stage II or stage III tumours. The apparent better prognosis in malignant IPMT compared with ductal carcinomas may be partly attributable to the high prevalence of in situ or invasive stage I IPMT, which are earlier diagnosed because of symptoms resulting from ductal obstruction by mucus. However, when the tumour is locally advanced (peripancreatic extension or lymph node involvement), the prognosis is as poor as for ductal carcinomas. As there is no current evidence for efficacy of adjuvant therapy in pancreatic cancers, patients with locally advanced malignant IPMT should be included in the same protocols assessing adjuvant chemotherapy or radiotherapy regimens as patients with ductal adenocarcinoma.
In conclusion, lymph node involvement was the only prognostic survival factor in patients who underwent resection for malignant IPMT. Overall five year survival rate was 48% in patients with malignant IPMT after surgical resection, but a clear cut difference was observed according to the stage of disease. Prognosis is good in patients with in situ or stage I carcinoma, who should be treated with function preserving minimal pancreatectomy. In contrast, patients with locally advanced malignant IPMT (that is, stage II or III) had the same poor prognosis as patients with pancreatic ductal adenocarcinomas.
Acknowledgments
The authors thank Dr Marie-José Payan-Defais (Marseille), and Professor José Sahel (Marseille) for providing their patient data and assistance.
Abbreviations
CA 19
9, carbohydrate antigen 19
9; CEA, carcinoembryonic antigen; IPMT, intraductal papillary mucinous tumours of the pancreas
REFERENCES
- 1.Loftus EV Jr, Olivares-Pakzad BA, Batts KP, et al. Intraductal papillary-mucinous tumors of the pancreas: clinicopathologic features, outcome, and nomenclature. Gastroenterology 1996;110:1909–18. [DOI] [PubMed] [Google Scholar]
- 2.Itai Y, Ohhashi K, Nagai H, et al. “ Ductectatic “ mucinous cystadenoma and cystadenocarcinoma of the pancreas. Radiology 1986;161:697–700. [DOI] [PubMed] [Google Scholar]
- 3.Klöppel G, Solcia E, Longnecker DS, et al. Histological typing of tumours of the exocrine pancreas. 2nd edn. In: World Health Organisation. International histological classification of tumours. Berlin: Springer-Verlag, 1996.
- 4.Azar C, Van de Stadt J, Rickaert F, et al. Intraductal papillary mucinous tumors of the pancreas. Clinical and therapeutic issues in 32 patients. Gut 1996;39:457–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbe L, Ponsot P, Vilgrain V, et al. Tumeurs intra-canalaires papillaires mucineuses pancréatiques. Aspects cliniques et morphologiques chez 30 malades. Gastroenterol Clin Biol 1997;21:278–86. [PubMed] [Google Scholar]
- 6.Cellier C, Cuillerier E, Palazzo L, et al. Intraductal papillary and mucinous tumors of the pancreas : accuracy of preoperative computed tomography, endoscopic retrograde pancreatography and endoscopic ultrasonography, and long-term outcome in a large surgical series. Gastrointest Endosc 1998;47:42–9. [DOI] [PubMed] [Google Scholar]
- 7.Yamaguchi K, Ogawa Y, Chijiiwa K, et al. Mucin-hypersecreting tumors of the pancreas : assessing the grade of malignancy preoperatively. Am J Surg 1996;171:427–31. [DOI] [PubMed] [Google Scholar]
- 8.Sugiyama M, Atomi Y. Intraductal papillary mucinous tumors of the pancreas : imaging studies and treatment strategies. Ann Surg 1998;228:685–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kimura W, Sasahira N, Yoshikawa T, et al. Duct-ectatic type of mucin producing tumor of the pancreas. New concept of pancreatic neoplasia. Hepatogastroenterology 1996;43:692–709. [PubMed] [Google Scholar]
- 10.Yamao K, Ohashi K, Nakamura T, et al. The prognosis of intraductal papillary mucinous tumors of the pancreas. Hepatogastroenterology 2000;47:1129–34. [PubMed] [Google Scholar]
- 11.Terris B, Ponsot P, Paye F, et al. Intraductal papillary mucinous tumors of the pancreas confined to secondary ducts show less aggressive pathologic features as compared with those involving the main pancreatic duct. Am J Surg Pathol 2000;24:1372–7. [DOI] [PubMed] [Google Scholar]
- 12.Paye F, Sauvanet A, Terris B, et al. Intraductal papillary mucinous tumors of the pancreas: pancreatic resections guided by preoperative morphological assessment and intraoperative frozen section examination. Surgery 2000;127:536–44. [DOI] [PubMed] [Google Scholar]
- 13.Nakagohri T, Kenmochi T, Kainuma O, et al. Intraductal papillary mucinous tumors of the pancreas. Am J Surg 1999;178:344–7. [DOI] [PubMed] [Google Scholar]
- 14.Sohn TA, Yeo CJ, Cameron JL, et al. Intraductal papillary mucinous neoplasms of the pancreas: an increasingly recognized clinicopathologic entity. Ann Surg 2001;234:313–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shyr YM, Su CH, Tsay SH, et al. Mucin-producing neoplasms of the pancreas. Intraductal papillary and mucinous cystic neoplasms. Ann Surg 1996;223:141–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.UICC. TNM classification of malignant tumours. 4th edn. Hermanek P, Hutter RVP, Sobin LH, et al, eds. Berlin: Springer-Verlag, 1998.
- 17.Zamora C, Sahel J, Cantu DG, et al. Intraductal papillary or mucinous tumors (IPMT) of the pancreas: report of a case series and review of the literature. Am J Gastroenterol 2001;96:1441–7. [DOI] [PubMed] [Google Scholar]
- 18.Traverso LW, Peralta EA, Ryan JA Jr, et al. Intraductal neoplasms of the pancreas. Am J Surg 1998;175:426–32. [DOI] [PubMed] [Google Scholar]
- 19.Falconi M, Salvia R, Bassi C, et al. Clinicopathological features and treatment of intraductal papillary mucinous tumour of the pancreas. Br J Surg 2001;88:376–81. [DOI] [PubMed] [Google Scholar]
- 20.Kobari M, Egawa S, Shibuya K, et al. Intraductal papillary mucinous tumors of the pancreas comprise 2 clinical subtypes: differences in clinical characteristics and surgical management. Arch Surg 1999;134:1131–6. [DOI] [PubMed] [Google Scholar]
- 21.Sugiyama M, Atomi Y, Kuroda A. Two types of mucin-producing cystic tumors of the pancreas. Diagnosis and treatment. Surgery 1997;122:617–25. [DOI] [PubMed] [Google Scholar]
- 22.Siech M, Tripp K, Schmidt-Rohlfing B, et al. Intraductal papillary mucinous tumor of the pancreas. Am J Surg 1999;177:117–20. [DOI] [PubMed] [Google Scholar]
- 23.Safi F, Schlosser W, Falkenreck S, et al. Prognostic value of Ca 19–9 serum course in pancreatic cancer. Hepatogastroenterology 1998;45:253–9. [PubMed] [Google Scholar]
- 24.Nagakawa T, Nagamori M, Futakami F, et al. Results of extensive surgery for pancreatic carcinoma. Cancer 1996;77:640–5. [PubMed] [Google Scholar]
- 25.Pedrazzoli S, DiCarlo V, Dionigi R, et al. Standard versus extended lymphadenectomy associated with pancreatoduodenectomy in the surgical treatment of adenocarcinoma of the head of the pancreas : a multicenter, prospective, randomized study. Lymphadenectomy Study Group. Ann Surg 1998;228:508–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baumel H, Huguier M, Manderscheid JC, et al. Results of resection for cancer of the exocrine pancreas : a study from the French Association of Surgery. Br J Surg 1994;81:102–7. [DOI] [PubMed] [Google Scholar]
- 27.Conlon KC, Klimstra DS, Brennan MF. Long-term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5-year survivors. Ann Surg 1996;223:273–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nitecki SS, Sarr MG, Colby TV, et al. Long-term survival after resection for ductal adenocarcinoma of the pancreas. Ann Surg 1995;221:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]