Abstract
Background and aims: Patients with preascitic liver cirrhosis display significant renal sodium retention in the upright posture and an exaggerated natriuresis during recumbency. To date, intrarenal sodium handling in these patients has not been studied using lithium clearance and fractional excretion techniques during recumbency and orthostatism.
Methods: Ten patients with preascitic (Child-Pugh A) liver cirrhosis and 10 healthy subjects underwent the following measurements during recumbency and then after four hours of standing: (a) active renin and aldosterone plasma levels; and (b) renal clearance of creatinine, sodium, potassium, and lithium (an index of fluid delivery to the loop of Henle).
Results: Unlike the control group, in the upright posture patients had significantly lower values of lithium clearance and fractional excretion compared with recumbency (21.6 (8.6) v 30.5 (10.2) ml/min (p<0.03) and 12.8 (4.4)% v 20.8 (4.9)% (p<0.01), respectively). Our patients showed maintenance of the glomerular-tubular balance—that is, the correlation between creatinine clearance and proximal tubular reabsorption of fluid—during both recumbency and in the upright posture (r=0.96, p<0.001; r=0.97, p<0.001, respectively). In contrast, patients displayed tubuloglomerular feedback only in the supine position. This was demonstrated by the observation of a negative correlation between lithium fractional excretion (a measure of the fractional delivery of sodium to the distal nephron) and filtered sodium load only in recumbency (r=−0.73; p< 0.03) and not during standing (r=0.22; p> 0.05).
Conclusions: This study suggests that both the reduction in fluid and sodium delivery to the distal nephron and loss of tubuloglomerular feedback (the mechanism increasing glomerular filtration rate when the distal tubule is reached by a reduced sodium load) contribute towards the tendency to sodium retention in compensated cirrhosis during prolonged upright posture.
Keywords: cirrhosis, sodium retention, lithium clearance, upright posture, ascites
In patients with preascitic liver cirrhosis there is expansion of extracellular fluid volume, as demonstrated by several experimental observations. These range from evidence of augmented distribution volume of 131I labelled albumin to ascertainment of increased end diastolic left ventricular diameter at this disease stage.1–8
Experimental studies in different animal species with cirrhosis have demonstrated that renal sodium retention precedes ascites formation, leading to plasma volume expansion.9–12
In patients with compensated cirrhosis, sodium retention develops mainly during standing. This phenomenon is thought to be aldosterone dependent, as suggested by the observation of an inverse correlation between slightly increased aldosterone plasma levels and renal excretion of sodium in the upright posture.13 In contrast, hypernatriuresis characterises patients with compensated liver cirrhosis undergoing bed rest and prevents a progressive expansion of extracellular fluid volume and ascites accumulation.6,14 Bed rest induced hypernatriuresis is thought to be caused by decreased tubular sodium reabsorption, being independent of any variation in glomerular filtration rate and filtered sodium load.6,14
Controversies on this simple view of the mechanisms of sodium and water retention in compensated cirrhosis patients arise when several experimental observations are considered. The renin-angiotensin-aldosterone axis is frequently suppressed, at least in supine patients, even during the early phases of overt ascites development,4–6 with recent evidence of an actual lack of correlation between aldosterone plasma concentration and sodium excretion.15 The central role of aldosterone in sodium retention which occurs in standing compensated cirrhotics is based on a single observation of a significant correlation between high-normal plasma levels of the hormone and sodium excretion.13 A previous authoritative investigation found proximal rather than distal tubular retention of sodium in compensated cirrhosis.16
To date, no study has investigated directly the behaviour of the different tubular segments of the nephron that follows passage from the reclining to the upright position in patients with cirrhosis. Hence the first aim of the study was to evaluate these tubular events using lithium clearance and fractional excretion techniques performed during recumbency and after standing in patients with compensated liver cirrhosis. A second aim was to assess in compensated cirrhosis (both in the supine and erect postures) maintenance of two relevant mechanisms of regulation of tubular sodium handling: the glomerular-tubular balance—that is, dependence of proximal tubular reabsorption of fluid and sodium on values of glomerular filtration rate (GFR)17–19—and tubuloglomerular feedback—that is, the mechanism increasing GFR when the distal tubule is reached by a reduced sodium load and vice versa.20–22
MATERIALS AND METHODS
Subjects
Ten healthy volunteers (five men, five women; age range 28–62 years) and 10 patients (seven men, three women; age range 41–67 years) with biopsy proven liver cirrhosis of functional stage A, according to the Child-Pugh classification,23 were studied. Clinical and laboratory findings in the controls and cirrhotic patients are shown in table 1 ▶. All patients were oedema and ascites free, as assessed by abdominal ultrasonography. Patients with past variceal bleeding, previous evidence of ascites, or diuretic consumption were excluded. No patient had heart failure, intrinsic renal disease, arterial hypertension, diabetes mellitus requiring drug therapy, or endocrine disease. No steroids, prostaglandin synthesis inhibitors, amines, or antihypertensive drugs were administered for at least one month before the study. Alcohol consumption was stopped in all patients at least three months previously and in controls not less than two weeks before the study. This study was performed in compliance with the 1975 Declaration of Helsinki ethical guidelines and all patients gave informed consent before participation.
Table 1.
Clinical and biochemical data in controls and in patients with liver cirrhosis
| Controls (n=10) | Cirrhotic patients (n=10) | |
| Age (years) | 49 (7) | 53 (8) |
| Aetiology | ||
| Alcoholic | 3 | |
| Hepatitis C virus | 6 | |
| Hepatitis B virus | 1 | |
| Serum bilirubin (mg/dl) | 0.8 (0.3) | 1.3 (0.6) |
| Prothrombin time (%) | 97 (5) | 72 (13) |
| Serum albumin (g/dl) | 4.3 (0.5) | 3.6 (0.3) |
| Alkaline phosphatase (IU/l)* | 187 (56) | 275 (182) |
| ALT (IU/l)† | 35 (14) | 129 (53) |
| Platelet count (cell/mm3) | 286 000 (102 000) | 72 400 (16 300) |
| Oesophageal varices (present/absent) | 8/2 | |
| Plasma creatinine (mg/dl) | 0.6 (0.1) | 0.9 (0.1) |
| Plasma sodium (mEq/l) | 141 (2) | 136 (4) |
| Plasma potassium (mEq/l) | 4.2 (0.5) | 4.2 (0.6) |
Data are mean(SD).
ALT, alanine aminotransferase.
*Normal value <280 U/l.
†Normal value <36 U/l.
Study protocol
Before the study, patients and controls underwent an equilibration period of five days during which they received an unrestricted diet providing 180–200 mEq of sodium and 60–80 mEq of potassium daily. In phase 1, at 2200 on the day before the study, lithium carbonate 600 mg was administered orally. After an overnight fast spent supine, the following morning a urine collection period was started at 0800. Urine was collected by spontaneous voiding. Blood samples were taken at the beginning and end of the urine collection period which lasted four hours (until 1200). Water intake was fixed at 1 litre over the course of the four hour period and subjects were kept supine throughout the study. Blood samples at the beginning and at 1200 were analysed for plasma concentrations of sodium (P-Na), potassium (P-K), lithium (P-Li), and creatinine (P-Cr). Blood samples at the beginning of the clearance period were also analysed for plasma active renin (AR) and aldosterone concentrations. Urine samples were analysed for lithium (U-Li), sodium (U-Na), potassium (U-K), and creatinine (U-Cr) concentrations during the whole of the four hour clearance period. At 2200 on the same day there was a second oral administration of 600 mg of lithium carbonate. In phase 2, on the following day, a further four hour clearance period was started at 0800 and the same blood and urine samples were obtained as described in phase 1, except for determinations of plasma levels of AR and aldosterone which were assessed at 1200. In this phase both patients and controls were asked to have a water intake of 1 litre and to remain in the standing position for the four hour period (slow walking and brief periods in the sitting position were allowed).
Determinations
P-Na, U-Na, P-Li, U-Li, P-K, and U-K were measured by a flame photometer (Instrumentation Laboratory model 143, Paderno Dugnano, Italy). P-Cr and U-Cr were determined colorimetrically. Determination of human AR was performed on EDTA plasma. AR was determined by a two site immunoradiometric assay for measurement of active renin protein (Nichols Institute Diagnostics, San Juan, California, USA). Plasma AR values are given in μU/ml. Plasma aldosterone concentrations were evaluated by radioimmunoassay using a kit from Sorin Biomedica (Saluggia, Italy) supplying solid phase antibody coated tubes. Plasma aldosterone values are given in pg/ml.
Calculations
P-Li was calculated by the formula15,24:
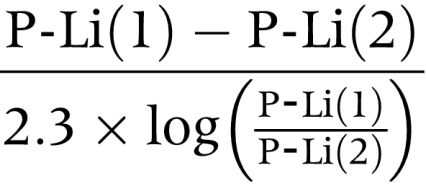 |
where P-Li (1) and P-Li (2) are lithium plasma concentrations at the beginning of the urine collection period (0800) and at 1200, respectively. This formula accounts for the decrease in lithium plasma concentration during the four hour study. P-Na, P-K, and P-Cr were calculated as the means of the corresponding values at the beginning and end of the respective urine collection periods. Lithium clearance (CLi), sodium clearance (CNa), potassium clearance (CK), and creatinine clearance (CCr) were calculated by the conventional formula:
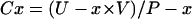 |
where U-x is urinary and P-x is plasma concentrations of x, and V is urinary output (ml/min). CCr was considered as a measure of GFR. Fractional sodium excretion (FENa), fractional potassium excretion (FEK), and fractional lithium excretion (FELi) were calculated by the ratio of CNa, CK, and CLi to CCr, respectively.
Assuming that lithium is reabsorbed in the proximal tubule in parallel with sodium and water, and that it is neither secreted nor reabsorbed beyond the proximal tubule, from urinary sodium excretion (UNaV), CLi, CNa, and CCr, the following parameters were derived according to Boer and colleagues25:
Filtered sodium load (FlNa)= P-Na×CCr (mEq/min)
Absolute distal fluid delivery (DD)=CLi (ml/min)
Proximal tubular reabsorption of fluid (PFR)=CCr−CLi (ml/min)
Absolute distal sodium delivery (DDNa)=CLi×P-Na (mEq/min)
Proximal tubular reabsorption of sodium (PTRNa)= FlNa−DDNa (mEq/min)
Absolute distal sodium reabsorption (DRNa)=DDNa−UNaV (mEq/min)
Distal fractional sodium reabsorption (DFRNa)=DRNa/DDNa×100 (%)
Finally, it should be noted that throughout the paper, the expression “distal tubule” indicates all segments of the renal tubule beyond the proximal segment.
Statistical analysis
Results are expressed as mean (SD). Comparisons between measurements obtained in the supine position and during standing were carried out using the Wicoxon signed rank test. Comparisons between patients and controls were carried out by the Wilcoxon rank sum test.26 Correlation coefficients were derived using Spearman’s rank correlation.27 Significance was accepted at the 5% probability level.
RESULTS
Supine period
Hormones (table 2 ▶)
Table 2.
Renal function evaluated during four hours of standing and during maintenance of the supine position
| Supine | Standing | |
| Plasma AR concentration (μU/ml) | ||
| Controls | 22.3 (11.5) | 41.5 (36.2)** |
| Cirrhosis | 18.1 (5.9) | 36.7 (37.2)** |
| Plasma A concentration (pg/ml) | ||
| Controls | 105.0 (55.0) | 167.8 (40.8)** |
| Cirrhosis | 77.9 (63.9) | 200.8 (81.2)** |
| GFR (ml/min) | ||
| Controls | 112.5 (15.9) | 119.2 (37) |
| Cirrhosis | 147 (22.8)†† | 145.6 (36.5)†† |
| UNaV (μEq/min) | ||
| Controls | 159 (105) | 148 (128) |
| Cirrhosis | 146 (81) | 135 (78) |
| FlNa (mEq/min) | ||
| Controls | 15.8 (2.1) | 16.8 (5.1) |
| Cirrhosis | 19.9 (6.0)†† | 19.8 (12.4)†† |
| FENa (%) | ||
| Controls | 1.09 (0.87) | 0.88 (0.20) |
| Cirrhosis | 0.73 (0.48) | 0.68 (0.10)† |
| CLi (ml/min) | ||
| Controls | 26.9 (21.0) | 20.4 (14.2) |
| Cirrhosis | 30.5 (10.2) | 21.6 (8.6)* |
| PFR (ml/min) | ||
| Controls | 85.1 (50.3) | 101.3 (59.6) |
| Cirrhosis | 116.49 (42.2) | 123.9 (39.5) |
| PTRNa (mEq/min) | ||
| Controls | 11.90 (1.9) | 13.97 (3.0) |
| Cirrhosis | 15.70 (4.1)†† | 16.85 (6.5)†† |
| FELi (%) | ||
| Controls | 24.4 (8.2) | 17.6 (9.0) |
| Cirrhosis | 20.8 (4.9) | 12.8 (4.4)**†† |
| DDNa (mEq/min) | ||
| Controls | 3.88 (2.50) | 2.87 (2.10) |
| Cirrhosis | 4.22 (1.48) | 2.94 (0.90) |
| DRNa (mEq/min) | ||
| Controls | 3.42 (1.5) | 2.72 (0.3) |
| Cirrhosis | 4.10 (1.3) | 2.81 (2.9) |
| DFRNa (%) | ||
| Controls | 95.6 (4.0) | 94.6 (1.1) |
| Cirrhosis | 97.1 (1.2) | 95.5 (1.0) |
Data are mean (SD)
*p<0.03, **p<0.01 compared with values measured while supine (Wilcoxon signed rank test); †p<0.03, ††p<0.05 compared with controls (Wilcoxon rank sum test).
A, aldosterone; AR, active renin; CLi, lithium clearance; DDNa, distal sodium delivery; DRNa, distal sodium reabsorption; DFRNa, distal fractional sodium reabsorption; FELi, fractional lithium excretion; FENa, fractional sodium excretion; FlNa, filtered sodium load; GFR, glomerular filtration rate; PFR, proximal tubular reabsorption of fluid; PTRNa, proximal tubular reabsorption of sodium; UNaV, urinary sodium excretion.
While reclining, AR and aldosterone plasma levels did not differ significantly between healthy controls and patients, with slightly depressed values of both hormones in the cirrhotic group.
Renal function (table 2 ▶)
UNaV and FENa were similar in controls and cirrhotics. Conversely, GFR, FlNa, and PTRNa were significantly higher in patients than controls. All parameters of lithium and potassium excretion did not differ significantly between healthy controls and patients.
Correlations
There were close positive correlations between GFR and the absolute amount of fluid reabsorbed by the proximal renal tubule both in controls and patients (r=0.93, p<0.001 and r=0.96, p<0.001, respectively). In patients, we observed a negative correlation between FELi and FlNa (r=−0.73, p<0.03) (fig 1 ▶); the same correlation was observed in the control group (r=−0.75, p<0.03). In patients, aldosterone was slightly correlated with values of DRNa but this was not significant (r=0.49, p>0.05). We found no significant correlations between plasma aldosterone and urinary sodium excretion values in patients or controls (r=−0.07, p>0.05 and r=−0.44, p>0.05, respectively).
Figure 1.
Cirrhotic patients. Inverse correlation between filtered sodium load and fractional lithium excretion while supine, demonstrating the preservation of tubuloglomerular feedback (see text). Correlation coefficient derived using Spearman’s rank correlation.
Standing period
Hormones (table 2 ▶)
After the four hour standing period, plasma AR and aldosterone levels did not differ significantly between controls and patients, although aldosterone values were slightly increased in the cirrhotic group. In the standing position, aldosterone and AR plasma levels increased significantly in both controls and patients, with the incremental value of plasma aldosterone (Δ increase) significantly higher in patients compared with controls (122.5 (15.01) v 62.3 (28.01) pg/ml; p<0.05).
Renal function (table 2 ▶)
During the standing period, compared with controls cirrhotic patients showed significantly higher values for GFR, FlNa, and PTRNa (all p<0.05) and lower values for FENa (p<0.03) and FELi (p<0.05). All of the remaining parameters of electrolyte excretion or tubular function did not differ between patients and controls. In the standing position, cirrhotic patients showed a significant reduction in CLi (p<0.03) and in FELi (p<0.01), a measure of the fraction of filtered sodium load that escapes proximal sodium reabsorption.24,25 DFRNa did not change significantly with the change from recumbency to the upright posture. Other parameters of renal electrolyte handling remained unchanged in patients during the standing period. None of the studied parameters changed in the control group as a consequence of changing from recumbency to the upright posture. Lastly, the ratio FlNa/FELi was significantly higher in the upright posture in patients compared with the supine position (1.87 (1.32) v 0.88 (0.63); p<0.02), indicating that for a given value of filtered sodium load they had a lower fraction of this amount of electrolyte reaching Henle’s loop.
Correlations
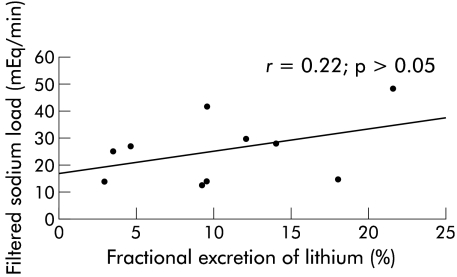
There were close positive correlations between glomerular filtration and absolute amount of fluid reabsorbed by the proximal renal tubule both in controls and patients (r=0.95, p<0.001 and r=0.97, p<0.001, respectively). During standing, the correlation (observed while reclining) between FELi and FlNa in patients was lost (r=0.22; p>0.05) (fig 2 ▶); in contrast, this correlation was maintained in healthy controls (r=−0.65, p<0.05). Plasma aldosterone did not correlate with values for UNaV, DRNa, or DFRNa in patients or controls.
Figure 2.
Cirrhotic patients. Lack of a significant correlation between filtered sodium load and fractional lithium excretion while standing, as an expression of tubuloglomerular feedback loss. Correlation coefficient derived using Spearman’s rank correlation.
DISCUSSION
We have shown that patients with compensated cirrhosis exhibit an increase in fluid and sodium reabsorption at the level of the proximal convoluted tubule when in the standing position, leading to a reduction in both fluid delivery and the fraction of the filtered sodium load that reaches Henle’s loop with respect to values measured while in the supine position (table 2 ▶). As our preascitic cirrhotics showed values of GFR significantly higher than normal both in recumbency and in the upright posture, according to previous reports,16,28,29 CLi in standing patients, although reduced with respect to values measured while supine, was still in the low-normal range. However, the excretion fraction of lithium was found to be significantly reduced in standing patients with respect to upright controls (table 2 ▶).
The higher values for the FlNa/FELi ratio in patients in the upright posture underscore retention of sodium by the proximal nephron in this body position. In fact, this observation implies that for a given value of filtered sodium load, a lower fraction of this amount of electrolyte reaches Henle’s loop. These findings may account for the previous observation of the blunted natriuretic effects of bumetanide (a diuretic acting on the Na+-K+-2Cl− pump of the ascending limb of Henle’s loop) in standing cirrhotic patients, possibly caused by a posture dependent increase in sodium retention in the proximal nephron.30
During the course of our short (four hours) period of observation, we found only a non-significant tendency towards reduction of urinary sodium excretion in both groups of subjects during standing because in the upright posture the distal nephron of patients and controls did not show any increase in sodium reabsorption, in spite of the observed (and expected) rise in aldosterone plasma levels. Indeed, both controls and patients showed slightly lower values for distal fractional sodium reabsorption (DFRNa) during standing with respect to recumbency (table 2 ▶).
To our knowledge, this is the first direct evidence of exclusive intratubular regulation of sodium handling, namely the capacity to moderate distal fractional sodium reabsoption when the distal nephron (namely the loop of Henle) is reached by a decreased load of electrolyte due to a change in body position. To some extent, this regulatory mechanism resembles (but in the opposite direction) the increase in distal sodium reabsorption that compensates for enhancement of distal fluid delivery induced by amino acid infusion in normal subjects.31 In contrast, this mechanism of compensation closely mimics depression of distal fractional sodium reabsorption following proximal tubular sodium retention induced by administration of antidopaminergic drugs in patients with compensated liver cirrhosis.32
Modulation of sodium handling beyond the proximal convoluted tubule must occur at an aldosterone independent distal tubular site, possibly the loop of Henle, or it could be dependent on selective stimulation of the macula densa as different degrees of perfusion of this tubular segment with sodium chloride containing solutions are known to modulate renin secretion.33,34
Although net sodium excretion was not significantly modified by changes in body position, we observed that compensated cirrhotics during standing had significantly reduced values of FENa with respect to healthy controls (table 2 ▶). This observation partially agrees with a previous study demonstrating subnormal values for absolute and fractional sodium excretion during brief periods of standing (two hours) in preascitic cirrhosis.13
The strict correlations between creatinine clearance and proximal tubular reabsorption of fluid (PFR) showed that compensated cirrhotic patients fully maintained the glomerular-tubular balance—that is, the mechanism increasing proximal tubular reabsorption of fluid and sodium when GFR augments and vice versa.17–19 This mechanism of regulation of sodium handling was observed both in the supine and standing positions, similar to that in the control group.
As FELi is a measure of the fractional delivery of sodium from the proximal tubule to the loop of Henle, the finding in the cirrhotic (fig 1 ▶) and control groups while reclining of an inverse correlation between this parameter and FlNa could be an expression of tubuloglomerular feedback (that is, the mechanism increasing GFR when the distal tubule is reached by a reduced sodium load and vice versa).20–22 A relevant observation is the following: at variance with controls, during standing, our patients lost the inverse correlation between FELi and FlNa (fig 2 ▶), possibly implying derangement of tubuloglomerular feedback as a further contribution to sodium retention during the upright posture in compensated cirrhosis. As we observed a prompt decrease in CLi and FELi in compensated patients in the standing position, even if not to subnormal values (table 2 ▶), the present evidence of derangement of the tubuloglomerular feedback (namely, the inability to upregulate GFR when the distal nephron is reached by a reduced fluid and salt load) may surely add to renal sodium retention during prolonged periods of standing.
In conclusion, our observations were of urinary indexes, which are indirect measurements of intratubular events and not actual measured values. However, the previous knowledge that in ascitic cirrhosis an avid proximal tubular sodium retention leads to unresponsiveness to diuretic administration30,35 and our finding of increased proximal sodium reabsorption with deranged tubuloglomerular feedback in standing compensated patients are sufficient reasons to recommend that cirrhotic patients spend a consistent amount of time in the supine position to avoid progressive sodium and water retention.
Acknowledgments
This work was supported by grants from the Ministero della Università e della Ricerca Scientifica (60%), 1997, and from the Italian Society of Gastroenterology (SIGE), 1997.
Abbreviations
AR, active renin
CCr, creatinine clearance
CK, potassium clearance
CLi, lithium clearance
CNa, sodium clearance
DD, distal fluid delivery
DDNa, distal sodium delivery
DRNa, distal sodium reabsorption
DFRNa, distal fractional sodium reabsorption
FEK, fractional potassium excretion, FELi, fractional lithium excretion
FENa, fractional sodium excretion
FlNa, filtered sodium load
GFR, glomerular filtration rate
P-Cr, plasma creatinine concentration
P-K, plasma potassium concentration
P-Li, plasma lithium concentration
P-Na, plasma sodium concentration
PFR, proximal tubular reabsorption of fluid
PTRNa, proximal tubular reabsorption of sodium
U-Cr, urinary creatinine concentration
U-K, urinary potassium concentration
U-Li, urinary lithium concentration
U-Na, urinary sodium concentration
UNaV, urinary sodium excretion
This study was presented in part at the 1999 Annual Meeting of the American Association for the Study of Liver Disease (AASLD), Dallas, Texas, USA, November 1999; at the 1999 Annual United European Gastroenterology Week (UEGW), Rome, Italy, November 1999; at the 2000 Annual Meeting of the Italian Association for the Study of the Liver (AISF), Rome, Italy, February 2000; and at the 35th Annual Meeting of the European Association for the Study of the Liver (EASL), Rotterdam, the Netherlands, May 2000.
REFERENCES
- 1.Lieberman FL, Reynolds TB. Plasma volume in cirrhosis of the liver: its relation to portal hypertension, ascites and renal failure. J Clin Invest 1967;46:1297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis FW, Adair O, Rector WG jr. Arterial vasodilatation is not the cause of increased cardiac output in cirrhosis. Gastroenterology 1992;102:1024–9. [DOI] [PubMed] [Google Scholar]
- 3.Wong F, Liu P, Tobe S, et al. Central blood volume in cirrhosis: measurement with radionuclide angiography. Hepatology 1994;19:312–21. [PubMed] [Google Scholar]
- 4.Wilkinson SP, Smith JK, Clarke M, et al. Intrarenal distribution of plasma flow in cirrhosis as measured by transit renography: relationship with plasma renin activity and sodium and water retention. Clin Sci Mol Med 1977;52:469–75. [DOI] [PubMed] [Google Scholar]
- 5.Wilkinson SP, Smith JK, Williams R. Changes in plasma renin activity in cirrhosis: a reappraisal based on studies in 67 patients and “low renin” cirrhosis. Hypertension 1979;1:125–9. [DOI] [PubMed] [Google Scholar]
- 6.Trevisani F, Bernardi A, Gasbarrini A, et al. Bed-rest-induced hypernatriuresis in cirrhotic patients without ascites: does it contribute to maintain “compensation”? J Hepatol 1992;16:190–6. [DOI] [PubMed] [Google Scholar]
- 7.Rector WG Jr, Adair O, Hossack KF, et al. Atrial volume in cirrhosis: relationship to blood volume and plasma concentration of atrial natriuretic factor. Gastroenterology 1990;99:766–70. [DOI] [PubMed] [Google Scholar]
- 8.Sansoé G, Ferrari A, Baraldi E, et al. Endogenous dopaminergic activity in Child-Pugh A cirrhosis: potential role in renal sodium handling and in the maintenance of the clinical compensation. Eur J Clin Invest 1998;28:131–7. [DOI] [PubMed] [Google Scholar]
- 9.Levy M. Sodium retention and ascites formation in dogs with experimental portal cirrhosis. Am J Physiol 1977;233:F572–85. [DOI] [PubMed] [Google Scholar]
- 10.Levy M, Allotey JBK. Temporal relationship between urinary salt excretion and altered systemic hemodynamics in dogs with experimental cirrhosis. J Lab Clin Med1978;92:245–50. [PubMed] [Google Scholar]
- 11.Lòpez-Novoa JM, Rengel MA, Hernando L. Dynamics of ascites formation in rats with experimental cirrhosis. Am J Physiol 1980;238:F353–7. [DOI] [PubMed] [Google Scholar]
- 12.Jiménez W, Martinez-Pardo A, Arroyo V, et al. Temporal relationship between hyperaldosteronism, sodium retention and ascites formation in rats with experimental cirrhosis. Hepatology 1985;5:245–50. [DOI] [PubMed] [Google Scholar]
- 13.Bernardi M, Di Marco C, Trevisani F, et al. Renal sodium retention during upright posture in preascitic cirrhosis. Gastroenterology 1993;105:188–93. [DOI] [PubMed] [Google Scholar]
- 14.Trevisani F, Bernardi M, De Palma R, et al. Circadian variation in renal sodium and potassium handling in cirrhosis. Gastroenterology 1989;96:1187–98. [DOI] [PubMed] [Google Scholar]
- 15.Sansoé G, Ferrari A, Baraldi E, et al. Renal distal tubular handling of sodium in central fluid volume homoeostasis in preascitic cirrhosis. Gut 1999;45:750–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong F, Massie D, Hsu P, et al. Renal response to a saline load in well-compensated alcoholic cirrhosis. Hepatology 1994;20:873–81. [DOI] [PubMed] [Google Scholar]
- 17.Peterson OW, Gushwa LC, Blantz RC. An analysis of glomerular-tubular balance in the rat proximal tubule. Pflugers Arch 1986;407:221–7. [DOI] [PubMed] [Google Scholar]
- 18.Haberle DA, von Baeyer H. Characteristics of glomerulotubular balance. Am J Physiol 1983;244:F355–66. [DOI] [PubMed] [Google Scholar]
- 19.Tack I, Wilcox CS, Tran-Van T, et al. Amino acid loading disrupts whole kidney proximal glomerular-tubular balance in humans. Clin Nephrol 1996;45:42–50. [PubMed] [Google Scholar]
- 20.Wright FS, Mandin H, Persson AE. Studies of the sensing mechanism in the tubuloglomerular feedback pathway. Kidney Int Suppl 1982;12:S90–6. [PubMed] [Google Scholar]
- 21.Blantz RC, Pelayo JC. A functional role for the tubuloglomerular feedback mechanism. Kidney Int 1984;25:739–46. [DOI] [PubMed] [Google Scholar]
- 22.Briggs JP, Schnermann J. The tubuloglomerular feedback mechanism: functional and biochemical aspects. Annu Rev Physiol 1987;49:251–73. [DOI] [PubMed] [Google Scholar]
- 23.Pugh RNH, Murray-Lion IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973;60:646–9. [DOI] [PubMed] [Google Scholar]
- 24.Thomsen K, Olsen OV. Renal lithium clearance as a measure of the delivery of water and sodium fron the proximal tubule in humans. Am J Med Sci 1984;288:158–61. [DOI] [PubMed] [Google Scholar]
- 25.Boer WH, Koomans HA, Dorhout Mees EJ. Lithium clearance during paradoxical natriuresis of hypotonic expansion in man. Kidney Int 1987;32:376–81. [DOI] [PubMed] [Google Scholar]
- 26.Kirkwood BR. Non-parametric methods. In: Kirkwood BR, ed. Essentials of medical statistics. Oxford: Blackwell Science Ltd, 1988:147–52.
- 27.Siegel S. Measures of correlation and their tests of significance. In: Siegel S, ed. Non-parametric statistics for the behavioral sciences. New York: McGraw-Hill, 1956:195–239.
- 28.Wong F, Massie D, Colman J, et al. Glomerular hyperfiltration in patients with well-compensated alcoholic cirrhosis. Gastroenterology 1993;104:884–9. [DOI] [PubMed] [Google Scholar]
- 29.Wood LJ, Massie D, McLean AJ, et al. Renal sodium retention in cirrhosis: tubular site and relation to hepatic disfunction. Hepatology 1988;8:831–6. [DOI] [PubMed] [Google Scholar]
- 30.Ring-Larsen H, Henriksen JH, Wilken C, et al. Diuretic treatment in decompensated cirrhosis and congestive heart failure: effect of posture. BMJ 1986;292:1351–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olsen NV, Hansen JM, Ladefoged SD, et al. Overall renal and tubular function during infusion of aminoacid in normal man. Clin Sci 1990;78:497–501. [DOI] [PubMed] [Google Scholar]
- 32.Sansoé G, Ferrari A, Baraldi E, et al. Dopaminergic control of renal tubular function in patients with compensated cirrhosis. Dig Dis Sci 2002;47:392–400. [DOI] [PubMed] [Google Scholar]
- 33.Lorenz JN, Wehprecht H, Schnermann J, et al. Characterization of the macula densa stimulus for renin secretion. Am J Physiol 1990;259:F186–93. [DOI] [PubMed] [Google Scholar]
- 34.Lorenz JN, Weihprecht H, Schnermann J, et al. Renin release from isolated juxtaglomerular apparatus depends on macula densa chloride transport. Am J Physiol 1991;260:F486–93. [DOI] [PubMed] [Google Scholar]
- 35.Gatta A, Angeli P, Caregaro L, et al. A pathophysiological interpretation of unresponsiveness to spironolactone in a stepped-care approach to the diuretic treatment of ascites in nonazotemic cirrhotic patients. Hepatology 1991;14:231–6. [PubMed] [Google Scholar]