Abstract
Background and aims: Osteopenia and osteoporosis are frequent in Crohn’s disease. However, there are few data on related vertebral fractures. Therefore, we evaluated prospectively the prevalence of osteoporotic vertebral fractures in these patients.
Methods: A total of 293 patients were screened with dual energy x ray absorptiometry of the lumbar spine (L1-L4) and proximal right femur. In 156 patients with lumbar osteopenia or osteoporosis (T score <−1), x ray examinations of the thoracic and lumbar spine were performed. Assessment of fractures included visual reading of x rays and quantitative morphometry of the vertebral bodies (T4-L4), analogous to the criteria of the European Vertebral Osteoporosis Study.
Results: In 34 (21.8%; 18 female) of 156 Crohn’s disease patients with reduced bone mineral density, 63 osteoporotic vertebral fractures (50 fx. (osteoporotic fracture with visible fracture line running into the vertebral body and/or change of outer shape) and 13 fxd. (osteoporotic fracture with change of outer shape but without visible fracture line)) were found, 50 fx. in 25 (16%, 15 female) patients and 13 fxd. in nine (5.8%, three female) patients. In four patients the fractures were clinically evident and associated with severe back pain. Approximately one third of patients with fractures were younger than 30 years. Lumbar bone mineral density was significantly reduced in patients with fractures compared with those without (T score −2.50 (0.88) v −2.07 (0.66); p<0.025) but not at the hip (−2.0 (1.1) v −1.81 (0.87); p=0.38). In subgroups analyses, no significant differences were observed.
Conclusions: In patients with Crohn’s disease and reduced bone mineral density, the prevalence of vertebral fractures—that is, manifest osteoporosis—was strikingly high at 22%, even in those aged less than 30 years, a problem deserving further clinical attention.
Keywords: Crohn’s disease, bone mineral density, osteoporosis, vertebral fracture
Patients with inflammatory bowel disease (IBD) are at high risk of low bone mineral density (BMD), especially in Crohn’s disease (CD). The increased prevalence of osteopenia and osteoporosis is a significant complication in both children1–3 and adults.4–12 Bone loss rates have been shown to be increased in patients with IBD.13–16 Other series have not reported continuous bone loss during follow up.17–20 The pathogenesis and risk factors are not fully understood. Endocrine, metabolic, genetic, nutritional, and inflammatory factors contribute to IBD associated osteoporosis.3–9,11–13,17,19,21–25
Osteoporosis is associated with a high risk of fractures of the spine, hip, and radius resulting in significant morbidity and mortality.26–28 Clinical problems of vertebral fractures include severe and chronic back pain, height loss, spinal deformity, and disability.29 New vertebral fractures are associated with substantial increases in back pain and increasing functional limitation, even those not recognised clinically,30 as well as being predictive of further fractures.26
The clinical relevance of osteopenia in IBD will remain unclear until sufficient data on the risk and prevalence of fractures in these patients are available. In CD, several small series of patients with vertebral fractures have been documented.4,6,7,21,31 Even in paediatric patients, vertebral fractures developed.1,2 More recent data on IBD related fractures were based on either databases32 or questionnaires,33 showing an increased incidence of fractures among patients with IBD,32 in particular an increased risk of low energy fractures in female patients with CD.33
However, the prevalence of vertebral fractures in CD patients with reduced BMD (T score <−1) is not known. Patients with CD have a normal life expectancy. Among these predominantly young patients, vertebral fractures may represent a serious lifelong handicap.
AIMS
We conducted a prospective study to assess the prevalence of vertebral fractures in CD using dual energy x ray absorptiometry (DXA) and detailed morphological analysis (quantitative morphometry (QM)) of x rays of the thoracic and lumbar spine (T4-L4).
MATERIAL AND METHODS
Patients
A total of 293 consecutive CD patients referred to our gastroenterological unit between January 1998 and January 2001 underwent osteodensitometry by DXA of the lumbar spine (L1-L4) and the proximal right femur. For assessment of vertebral fractures, anteroposterior and lateral x ray examinations of the thoracic and lumbar spine were performed in 156 patients (84 female) with lumbar osteopenia or osteoporosis. Patient characteristics are shown in table 1 ▶.
Table 1.
Baseline characteristics of 122 patients without compared with 34 patients with osteoporotic vertebral fractures
| 122 patients without fractures | 34 patients with fractures | |
| Sex (M/F) | 56/66 | 16/18 |
| Age (y) (mean (SD)) | 35.25 (11.67) | 38.45 (13.66) |
| Duration of disease (y) | 8.31 (7.81) | 7.43 (4.79) |
| BMI (kg/m2) (mean (SD)) | 21.85 (3.96) | 22.96 (3.87) |
| Nicotine abuses | 59 (48.4%) | 12 (35.3%) |
| Milk intolerance | 36 (29.5%) | 13 (38.2%) |
| Extent of disease: | ||
| Ileal disease | 43 (35.2%) | 9 (26.5%) |
| Colonic disease | 19 (15.6%) | 7 (20.6%) |
| Ileocolonic disease | 60 (49.2%) | 18 (52.9%) |
| Bowel resection | ||
| No previous bowel resection | 67 (55.0%) | 21 (61.7%) |
| Ileal bowel resection | 32 (26.2%) | 8 (23.5%) |
| Colonic bowel resection | 7 (5.7%) | 1 (2.9%) |
| Ileocolonic bowel resection | 16 (13.1%) | 4 (11.9%) |
| Corticosteroids | ||
| No previous use of steroids | 12 (9.8%) | 2 (5.9%) |
| Cumulative dose <10 g | 81 (66.5%) | 25 (73.5%) |
| Cumulative dose >10 g | 29 (23.7%) | 7 (20.6%) |
No significant differences between the two groups.
BMI, body mass index.
All patients had a diagnosis of CD established on histological, endoscopic, radiological, or clinical criteria. None of the patients was pregnant. All participants gave informed consent.
Measurement of bone mineral density
BMD of the lumbar spine (L1-L4) was assessed by DXA (Hologic QDR 1000; Hologic Inc., Waltham, Massachusetts, USA). At the proximal right femur, four sites (femoral neck, trochanter and intertrochanteric area, and Ward’s triangle) were measured; an average (total femur) was obtained from the first three sites. Average BMD values for L1-L4 and total femur were used for calculations. Normal values were supplied by Hologic Inc. Assessment of BMD was performed according to the manufacturer’s instructions.
BMD results were expressed in absolute values (g/cm2) and as number of standard deviations (SD) from the peak bone mass of a young adult sex matched reference population (T score). According to the WHO recommendations for postmenopausal women, osteopenia was defined as a T score <−1 SD, osteoporosis as a T score <−2.5 SD, and manifest osteoporosis as a T score <−1.0 with prevalent vertebral fractures.34 Patients with major sclerosis of the aorta, osteophytes, or scoliosis on x ray precluding accurate measurements of lumbar BMD by DXA were excluded (n=1).
In patients with vertebral fractures of the vertebral bodies L1-L4, average BMD values for L1-L4 were recalculated excluding the fractured vertebra.
Assessment of fractures: quantitative morphometry (QM)
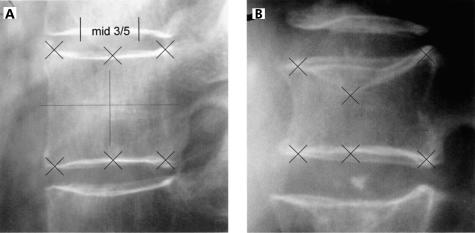
Morphometric methods have been developed for standardised assessment of vertebral deformities in studies of spinal osteoporosis.35,36 The use of a fixed percentage reduction in vertebral height is the simplest and most practical method of studying vertebral deformities.37 In this study, visual reading of x rays and QM of the vertebral bodies were standardised according to the criteria of the European Vertebral Osteoporosis Study (EVOS)38; only the threshold value was changed from 25% to 20%. QM was performed using six point digitisation to calculate the anterior (Ha), mid (Hm), and posterior (Hp) height of the vertebral bodies T4-L4 (fig 1 ▶). A vertebra was classified as having a prevalent deformity if at least one ratio (Ha/Hp, Hm/Hp, Hp/Hp up, or Hp/Hp low) was below the threshold value. For every vertebra considered as deformed quantitatively, a radiological differential diagnosis was performed for the aetiology of the deformation, distinguishing between osteoporotic, degenerative, traumatic, and other reasons. Differential diagnosis prevents overestimation of the prevalence of osteoporotic fractures due to deformations of other aetiology as 45.9% and 30.9% of spinal deformities in men and women, respectively, are reported to be of non-osteoporotic origin.39 For osteoporotic fractures, differentiation between fx. (osteoporotic fracture with visible fracture line running into the vertebral body and/or change of outer shape) and fxd. (osteoporotic fracture with change of outer shape but without visible fracture line) fractures was performed.
Figure 1.
Height measurement: quantitative x ray evaluation (quantitative morphometry) using six point digitisation to calculate the anterior (Ha), mid (Hm), and posterior (Hp) height of a vertebral body. (A) Normal shaped vertebra without fracture. (B) Osteoporotic fractured vertebra with concave deformation.
Statistics
Continuous data were expressed as mean (SD) (range). When data were non-normally distributed, Wilcoxon’s non parametric rank sum test was used. Two tailed tests for significance were used in all statistical analyses and p=0.05 was considered statistically significant. Correlation analysis was performed using Spearman’s rank correlation test. Two tailed Fisher’s exact test was used to determine associations between categorical variables. The Statistical Package SAS V6.11 was used for analysis.
RESULTS
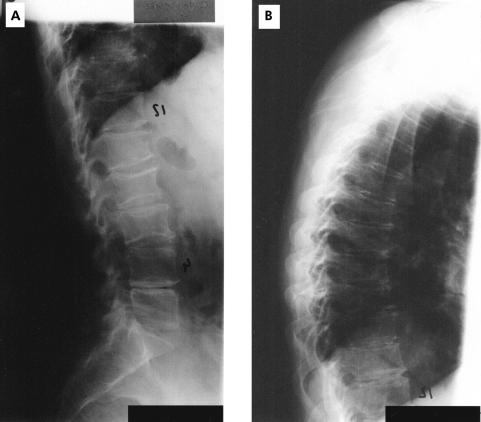
A total of 293 CD patients underwent DXA of the lumbar spine (L1-L4) and proximal right femur. In 156 patients (mean age 36 years (range 18–68), 84 female) with lumbar osteopenia (n=111) or osteoporosis (n=45) x ray examinations of the thoracic and lumbar spine were performed (fig 2 ▶).
Figure 2.
Vertebral column of a female patient with Crohn’s disease with multiple vertebral fractures at the lumbar and thoracic spine. (A) Lumbar spine; (B) thoracic spine.
Of the 156 patients, 34 (21.8%; 18 female) had one or more osteoporotic vertebral fractures—that is, manifest osteoporosis. QM and visual reading of x rays demonstrated a total of 63 fractures, 50 fx. in 25 (16%, 15 female) patients and 13 fxd. in nine (5.8%, three female) patients (table 2 ▶).
Table 2.
Number (percentage) of patients with vertebral fractures according to age and sex (34/156 patients (21.8%; 18 female) had one or more vertebral fractures) and number of vertebral fractures (fxd./fx.) at the lumbar or thoracic spine according to the age of the patients (nine patients aged 21–30 years had 24 vertebral fractures (4 fxd./20 fx.))
| Age (y) | |||||||
| <21 | 21–30 | 31–40 | 41–50 | 51–60 | 61–70 | Total | |
| No of patients | 15 | 40 | 60 | 16 | 19 | 6 | 156 |
| No of patients with vertebral fractures | |||||||
| n female | 2 | 5 | 6 | 1 | 3 | 1 | 18 |
| n male | 1 | 4 | 5 | 2 | 2 | 2 | 16 |
| Total (%) | 3 (20) | 9 (22.5) | 11 (18) | 3 (18.7) | 5 (26.3) | 3 (50) | 34 (21.8) |
| No of fractures (fxd) | |||||||
| fxd. thoracic | 2 | 3 | 4 | 1 | 10 | ||
| fxd. lumbar | 1 | 2 | 3 | ||||
| Total fxd. | 2 | 4 | 6 | 1 | 13 | ||
| No of fractures (fx.) | |||||||
| fx. thoracic | 2 | 15 | 7 | 4 | 5 | 2 | 35 |
| fx. lumbar | 5 | 3 | 5 | 2 | 15 | ||
| Total fx. | 2 | 20 | 10 | 4 | 10 | 4 | 50 |
fx., osteoporotic fracture with visible fracture line running into the vertebral body and/or change of outer shape; fxd., osteoporotic fracture with change of outer shape but without visible fracture line.
There were no vertebral fractures in 122 patients (78.2%, 66 female) with osteopenia or osteoporosis. However, the prevalence of vertebral fractures was higher in osteoporosis (28.9%) compared with osteopenia (18.9%; p=0.2) (table 3 ▶).
Table 3.
Prevalence of vertebral fractures (fxd./fx.) in osteopenia (T score <−1) and osteoporosis (T score <−2.5)
| 111 patients with osteopenia | 71.1% | ||
| 90 patients without vertebral fractures | 81.1% | ||
| 7 patients with fxd. | 6.3% | } | |
| 14 patients with fx. | 12.6% | 18.9%* | |
| 45 patients with osteoporosis | 28.9% | ||
| 32 patients without vertebral fractures | 71.1% | ||
| 2 patients with fxd. | 4.4% | } | |
| 11 patients with fx. | 24.5% | 28.9%* |
*p=0.2 (NS).
In four female patients the fractures were clinically evident with severe back pain and disability (representing 14 fractures). The fractures in the other 30 patients were clinically undetected.
Lumbar bone mineral density was significantly reduced in patients with fractures compared with those without (T score −2.07 (0.66) v −2.50 (0.88); p<0.025). At the hip, no significant difference was observed (T score −1.81 (0.87) v −2.0 (1.1), respectively; p=0.38). Lumbar BMD was not significantly different in the nine patients with fxd. compared with the 25 patients with fx. fractures, nor was there a significant difference at the hip (lumbar T score −2.12 (0.47) v −2.64 (0.95) (p=0.19); hip T score −1.44 (0.99) v −2.21 (0.99), respectively (p=0.09)) (table 4 ▶).
Table 4.
Bone mineral density (BMD), age, and sex in patients with or without vertebral fractures (fx./fxd.)
| 156 patients | 122 patients without fractures | Versus | 34 patients with fractures | 9 patients with fxd. | Versus | 25 patients with fx. |
|
| Age (y) (mean (SD)) | 35.95 (12.2) | 35.25 (11.67) | NS | 38.45 (13.66) | 34.70 (9.91) | NS | 39.79 (14.54) |
| Sex (M/F) | 72/84 | 56/66 | 16/18 | 6/3 | 10/15 | ||
| Lumbar BMD | |||||||
| T score | −2.16 (0.73) | −2.07 (0.66) | p<0.025 | −2.50 (0.88) | −2.12 (0.47) | NS | −2.64 (0.95) |
| g/cm2 | 0.83 (0.08) | 0.84 (0.07) | p<0.001 | 0.79 (0.09) | 0.84 (0.05) | NS | 0.77 (0.10) |
| Total femur BMD | |||||||
| T score | −1.85 (0.91) | −1.81 (0.87) | NS | −2.0 (1.1) | −1.44 (0.99) | NS | −2.21 (0.99) |
| g/cm2 | 0.79 (0.12) | 0.79 (0.12) | NS | 0.77 (0.13) | 0.86 (0.14) | p<0.05 | 0.74 (0.12) |
fx., osteoporotic fracture with visible fracture line running into the vertebral body and/or change of outer shape; fxd., osteoporotic fracture with change of outer shape but without visible fracture line.
No significant difference in age was observed between the 45 patients with osteoporosis and the 111 patients with osteopenia (40.46 (14.51) v 34.12 (10.60) years; NS) and the 122 patients without versus the 34 patients with vertebral fractures (35.25 (11.67) v 38.45 (13.66) years; p=0,30). Twelve patients with vertebral fractures were <30 years old (table 2 ▶).
No correlation between age and BMD was found in any subgroup. In patients with one fracture (n=20) versus those with two or more fractures (n=14), no significant differences in BMD at any site were demonstrated. There was no correlation between the total number of vertebral fractures per patient and age or femoral or lumbar BMD (data not shown).
Regarding other disease related risk factors such as disease extent, previous bowel resections, or use of steroids, no significant differences or correlations in any subgroup analysis were found (table 1 ▶).
DISCUSSION
We found a high prevalence of osteoporotic vertebral fractures in patients with CD and decreased BMD (T score <−1). The prevalence of vertebral fractures—that is, manifest osteoporosis—was 21.8%. In 34 of 156 CD patients, we demonstrated one or more vertebral fractures. A total of 63 fractures, 50 fx. in 25 patients (16%, 15 female) and 13 fxd. in nine patients (5.8%, three female) were found.
CD patients are at high risk for low BMD but the prevalence of vertebral fractures of clinical relevance has not previously been thoroughly assessed. The few data on IBD related vertebral fractures were based on self administered questionnaires,33 databases,32 or case reports.2,4,6,7,21,31 Bernstein and colleagues32 demonstrated a 40% greater incidence of fractures at any site among patients with IBD than in the general population, with an age specific incidence rate ratio of 1.54 for spine fractures in CD, indicating an increased incidence of vertebral fractures of approximately 50%. Vestergaard and colleagues33 showed an increased risk of low energy fractures in female patients with CD.
Our data represent the first prospective study investigating the prevalence of vertebral fractures based on a standardised evaluation regimen of x rays of the thoracic and lumbar spine, analogous to the criteria of EVOS.38 The EVOS study was an epidemiological study that investigated the radiographic prevalence of vertebral fractures in men and women aged 50 years and over.40 In our study, the prevalence of vertebral fractures (21.8%) was higher compared with the mean centre prevalence in EVOS (12%) and compared with the prevalence in the EVOS study centre in Heidelberg (16.4%), located in southern Germany in common with our study centre.40 This difference is interesting due to the older age of the patients in the EVOS study (64.8 v 35.9 years in our study) and the fact that in EVOS, no differential diagnosis of fractures (osteoporotic v others) was done. In the European Prospective Osteoporosis Study (EPOS),39 the overall prevalence of osteoporotic fractures was 10.6%, and the prevalence in the Heidelberg study centre was 11.5%, making the difference between their results and our results even more remarkable.
The association between postmenopausal osteoporosis and the development of vertebral compression fractures is well described.26 Each SD decline in BMD possibly increases the fracture risk approximately twofold.41–43 The prevalence of vertebral fractures increases the risk of developing further fractures by up to 10-fold,26 with a positive correlation with the number of prevalent fractures.44 This risk is extremely high in new incident fractures.45 Our data showed a higher prevalence of fractures in patients with osteoporosis (29%) compared with osteopenia (19%; NS). Lumbar BMD was significantly higher in the 122 patients without vertebral fractures, supporting the hypothesis that decreased BMD is a risk factor for vertebral fractures. By subgroup analysis of patients with one versus those with two or more fractures, no significant differences were found regarding age, or femoral or lumbar BMD. Whether there is a significant prevalence of fractures in CD patients with a normal BMD (T score >−1) cannot be answered by this study.
Even though the number of vertebral fractures was negatively correlated with vertebral and femoral bone density reported by Mann and colleagues,46 no correlation between the number of vertebral fractures per patient and BMD at any site was found in our study. Patients with osteoporosis were slightly older than patients with osteopenia, and the 34 patients with vertebral fractures were only a little older than the 122 patients without vertebral changes, suggesting a weak correlation with age. However, there were a larger number of young patients aged <30 years with vertebral fractures.
Our data showed a high rate of clinically silent fractures in CD patients with manifest osteoporosis, according to the criteria of the WHO.34 The fractures were clinically evident only in four patients. Severe clinical osteoporosis, including back pain, height loss, spinal deformity, and disability was already present in these young patients. The fractures in the other 30 patients were not evident clinically. These data are consistent with other reports that only one third of x ray assessed vertebral fractures are clinically detected.26
We demonstrated that reduced BMD, a common problem in patients with CD, is not only a surrogate marker with no clinical relevance but is associated with a high prevalence of vertebral fractures. This implies that it is worthwhile paying even more attention to this extraintestinal complication of CD, especially among young patients. Although hormone replacement therapy,47 vitamin D,48 bisphosphonates (Alendronate),20 sodium fluoride,49 and a simple schedule of regular physical activity50 reduces the rate of bone loss or increases BMD in patients with CD, the optimum strategy for prevention and therapy has yet to be established.
CONCLUSIONS
In patients with CD and low BMD (T score <−1), the prevalence of vertebral fractures—that is, manifest osteoporosis—was 22%. Characterisation of risk factors for low BMD and associated fractures in CD patients needs further attention. BMD measurement and x ray examinations of the spine should be performed in patients with CD to identify those with reduced BMD in order to initiate treatment to reduce fractures. However, the optimum prevention and therapy of osteopenia and fractures have yet to be established.
Abbreviations
BMD, bone mineral density
CD, Crohn’s disease
DXA, dual energy x ray absorptiometry
EVOS, European Vertebral Osteoporosis Study
EPOS, European Prospective Osteoporosis Study
fx., osteoporotic fracture with visible fracture line running into the vertebral body and/or change of outer shape
fxd., osteoporotic fracture with change of outer shape but without visible fracture line
IBD, inflammatory bowel disease
QM, quantitative morphometry
REFERENCES
- 1.Cowan FJ, Parker DR, Jenkins HR. Osteopenia in Crohn’s disease. Arch Dis Child 1995;73:255–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Semeao EJ, Stallings VA, Peck SN, et al. Vertebral compression fractures in pediatric patients with Crohn’s disease. Gastroenterology 1997;112:1710–13. [DOI] [PubMed] [Google Scholar]
- 3.Gokhale R, Favus MJ, Karrison Th, et al. Bone mineral density in children with inflammatory bowel disease. Gastroenterology 1998;114:902–11. [DOI] [PubMed] [Google Scholar]
- 4.Compston JE, Judd D, Crawley EO, et al. Osteoporosis in patients with inflammatory bowel disease. Gut 1987;28:410–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vogelsang H, Ferenci P, Woloszeznk W, et al. Bone disease in vitamin D deficient patients with Crohn’s disease. Dig Dis Sci 1989;34:1094–9. [DOI] [PubMed] [Google Scholar]
- 6.Pigot F, Roux C, Chaussade S, et al. Low bone mineral density in patients with inflammatory bowel disease. Dig Dis Sci 1992;37:1396–403. [DOI] [PubMed] [Google Scholar]
- 7.Abitbol V, Roux C, Chaussade S, et al. Metabolic bone assessment in patients with inflammatory bowel disease. Gastroenterology 1995;108:417–22 [DOI] [PubMed] [Google Scholar]
- 8.Bernstein CN, Seeger LL, Sayre JW, et al. Decreased bone density in inflammatory bowel disease is related to corticosteroid use and not disease diagnosis. J Bone Min Res 1995;10:250–6. [DOI] [PubMed] [Google Scholar]
- 9.Silvennoinen JA, Karttunen TJ, Niemelä SE, et al. A controlled study of bone mineral density in patients with inflammatory bowel disease. Gut 1995;37:71–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bjarnason I, Macpherson A, Mackintosh C, et al. Reduced bone density in patients with inflammatory bowel disease. Gut 1997;40:228–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schulte C, Dignass AU, Mann K, et al. Reduced bone mineral density and unbalanced bone metabolism in patients with inflammatory bowel disease. Inflamm Bowel Dis 1998;4:268–75. [DOI] [PubMed] [Google Scholar]
- 12.v. Tirpitz Ch, Pischulti G, Klaus J, et al. Pathological bone density in chronic inflammatory bowel disease—prevalence and risk factors. Z Gastroenterol 1999;37:5–12. [PubMed] [Google Scholar]
- 13.Motley RJ, Crawley EO, Evans C, et al. Increased rate of spinal trabecular bone loss in patients with inflammatory bowel disease. Gut 1988;29:1332–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clements D, Motley RJ, Evans WD, et al. Longitudinal study of cortical bone loss in patients with inflammatory bowel disease. Scand J Gastroenterol 1992;27:1055–60. [DOI] [PubMed] [Google Scholar]
- 15.Motley RJ, Clements D, Evans WD, et al. A four-year longitudinal study of bone loss in patients with inflammatory bowel disease. Bone Miner 1993;23:95–104. [DOI] [PubMed] [Google Scholar]
- 16.Roux C, Abitbol V, Chaussade S, et al. Bone loss in patients with inflammatory bowel disease: a prospective study. Osteoporosis Int 1995;5:156–60. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh S, Cowen S, Hannan WJ, et al. Low bone mineral density in Crohn’s disease, but not in ulcerative colitis, at diagnosis. Gastroenterology 1994;107:1031–9. [DOI] [PubMed] [Google Scholar]
- 18.Staun M, Tjellesen L, Thale M, et al. Bone mineral content in patients with Crohn’s disease. Scand J Gastroenterol 1997;32:226–32. [DOI] [PubMed] [Google Scholar]
- 19.Schulte C, Dignass AU, Mann K, et al. Bone loss in patients with inflammatory bowel disease is less than expected: a follow-up study. Scand J Gastroenterol 1999;34:696–702. [DOI] [PubMed] [Google Scholar]
- 20.Haderslev KV, Tjellesen L, Sorensen HA, et al. Alendronate increases lumbar spine bone mineral density in patients with Crohn’s disease. Gastroenterology 2000;119:639–46. [DOI] [PubMed] [Google Scholar]
- 21.Bischoff SC, Herrmann A, Goke M, et al. Altered bone metabolism in inflammatory bowel disease. Am J Gastroenterol 1997;92:1157–63. [PubMed] [Google Scholar]
- 22.Jahnsen J, Falch JA, Aadland E, et al. Bone mineral density is reduced in patients with Crohn’s disease, but not in ulcerative colitis. A population based study. Gut 1997;40:313–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pollak RD, Karmeli F, Eliakim R, et al. Femoral neck osteopenia in patients with inflammatory bowel disease. Am J Gastroenterol 1998;93:1483–90. [DOI] [PubMed] [Google Scholar]
- 24.Schoon EJ, Blok BM, Geerling BJ, et al. Bone mineral density in patients with recently diagnosed inflammatory bowel disease. Gastroenterology 2000;119:1203–8. [DOI] [PubMed] [Google Scholar]
- 25.Schulte CMS, Dignass AU, Goebell H, et al. Genetic factors determine extent of bone loss in inflammatory bowel disease. Gastroenterology 2000;119:909–20. [DOI] [PubMed] [Google Scholar]
- 26.Cooper C, O’Neill T, Silman A on behalf of the European Vertebral Osteoporosis Group. The epidemiology of vertebral fractures. Bone 1993;14:89–97. [DOI] [PubMed] [Google Scholar]
- 27.Kanis JA, Melton LJ, Christiansen C, et al. The diagnosis of osteoporosis. J Bone Miner Res 1994;9:1137–41. [DOI] [PubMed] [Google Scholar]
- 28.Melton LJ. How many women have osteoporosis? J Bone Miner Res 1995;10:175–7. [DOI] [PubMed] [Google Scholar]
- 29.Ettinger B, Black DM, Nevitt MC, et al. Contribution of vertebral deformities to chronic back pain and disability. J Bone Miner Res 1992;7:449–56. [DOI] [PubMed] [Google Scholar]
- 30.Nevitt C, Ettinger B, Black DM, et al. The association of radiographically detected vertebral fractures with back pain and function: a prospective study. Ann Intern Med 1998;128:793–800. [DOI] [PubMed] [Google Scholar]
- 31.Neef B, Horing E, Maier KE, et al. Schwere Osteoporose bei einer jungen Patientin mit Morbus Crohn. Dtsch Med Wochenschr 1991;116:1055–60. [DOI] [PubMed] [Google Scholar]
- 32.Bernstein CN, Blanchard JF, Leslie W, et al. The incidence of fracture among patients with inflammatory bowel disease. A population-based cohort study. Ann Intern Med 2000;133:795–9. [DOI] [PubMed] [Google Scholar]
- 33.Vestergaard P, Krogh K, Rejnmark L, et al. Fracture risk is increased in Crohn’s disease, but not in ulcerative colitis. Gut 2000;46:176–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.WHO Study Group. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. WHO Technical Report Series 843. Geneva, Switzerland: WHO, 1994. [PubMed]
- 35.Eastell R, Cedal SL, Wahner HW, et al. Classification of vertebral fractures. J Bone Miner Res 1991;6:207–15. [DOI] [PubMed] [Google Scholar]
- 36.McCloskey EV, Spector TD, Eyres KS, et al. The assessment of vertebral deformity. A method for use in population studies and clinical trials. Osteoporosis Int 1993;3:138–47. [DOI] [PubMed] [Google Scholar]
- 37.Black DM, Palermo L, Nevitt MC, et al. Defining incident vertebral deformity: a prospective comparison of several approaches. J Bone Miner Res 1999;14:90–101. [DOI] [PubMed] [Google Scholar]
- 38.Felsenberg D, Wieland E, Gowin W, et al. Morphometric analysis of spine x-rays for diagnosis of osteoporotic fractures. Med Klin 1998;93:(suppl II):26–30. [DOI] [PubMed] [Google Scholar]
- 39.Felsenberg D, Armbrecht G, Khorassani A. Europäische Prospektive Osteoporosestudie (EPOS). Förderungsprojekt des Bundesministeriums für Bildung, Wissenschaft, Forschung und Technologie (BMBF). Förderkennzeichen 01 KM 9402/3.
- 40.O’Neill TW, Felsenberg D, Varlow J, et al. The prevalence of vertebral deformity in european men and women: the European Vertebral Osteoporosis Study. J Bone Miner Res 1996;11:1010–18. [DOI] [PubMed] [Google Scholar]
- 41.Cummings SR, Black DM, Nevitt MC, et al. Bone density on various sites for prediction of hip fractures. Lancet 1993;341:72–5. [DOI] [PubMed] [Google Scholar]
- 42.Marshall, D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 1996;312:1254–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Legrand E, Chappard D, Pascaretti C, et al. Bone mineral density and vertebral fractures in men. Osteoporosis Int 1999;10:265–70. [DOI] [PubMed] [Google Scholar]
- 44.Black DM, Arden NK, Palermo L, et al. Prevalent vertebral deformities predict hip fractrues and new vertebral deformities but not wrist fractures. J Bone Miner Res 1999;14:821–8. [DOI] [PubMed] [Google Scholar]
- 45.Lindsay R, Watts N, Roux C. Increased risk of new vertebral fracture within 1 year of an incident vertebral fracture. Osteoporosis Int 2000;10:S209. [Google Scholar]
- 46.Mann T, Oviatt SK, Wilson D, et al. Vertebral deformity in men. J Bone Min Res 1992;7:1259–65. [DOI] [PubMed] [Google Scholar]
- 47.Clements D, Compston JE, Evans WD, et al. Hormone replacement therapy prevents bone loss in patients with inflammatory bowel disease. Gut 1993;34:1543–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vogelsang H, Ferenci P, Resch H, et al. Prevention of bone mineral loss in patients with Crohn’s disease by long-term oral vitamin D supplementation. Eur J Gastroenterol Hepatol 1995;7:609–14. [PubMed] [Google Scholar]
- 49.von Tirpitz C, Klaus J, Bruckel J, et al. Increase of bone mineral density with sodium fluoride in patients with Crohn’s disease. Eur J Gastroenterol Hepatol 2000;12:19–24. [DOI] [PubMed] [Google Scholar]
- 50.Robinson RJ, Krzywicki T, Almond L, et al. Effect of a low-impact exercise program on bone mineral density in crohn’s disease: a randomised controlled trial. Gastroenterology 1998;115:36–41. [DOI] [PubMed] [Google Scholar]