Abstract
Background: Patients with cholestatic liver function tests and histological features of primary sclerosing cholangitis (PSC) but a normal cholangiogram are considered to have small duct PSC. The natural history of this condition is unknown.
Methods: Thirty three patients with small duct PSC were identified among patients admitted for diagnostic workup of cholestatic liver function tests in one centre in the UK (Oxford) and one centre in Norway (Oslo). A total of 260 patients with large duct PSC were compared, and prognosis in terms of death, cholangiocarcinoma, biochemical features, histological features, and symptoms analysed.
Results: Mean age at diagnosis was 38 years and 39 years in small duct and large duct PSC, respectively. Mean follow up was 106 months in small duct and 105 months in large duct patients. Four patients originally considered to have small duct developed large duct PSC. Two of these underwent liver transplantation during follow up. Of the remainder who did not develop large duct PSC, two patients died during follow up: one of liver failure and the other of cardiac death unrelated to her liver disease. A total of 122 (47%) large duct patients either required liver transplantation (34 patients) or died (88 patients). Small duct patients had a significantly better survival compared with large duct patients. Among small duct patients, none developed cholangiocarcinoma compared with 28 of 260 (11%) large duct patients.
Conclusions: Patients with small duct PSC seem to have a good prognosis in terms of survival and development of cholangiocarcinoma. Small duct PSC progresses to large duct PSC in a small proportion of patients.
Keywords: primary sclerosing cholangitis, cholangiocarcinoma, liver transplantation, inflammatory bowel disease
Primary sclerosing cholangitis (PSC) is a chronic cholestatic liver disorder with fibrosis of the intrahepatic and/or extrahepatic bile ducts. PSC is characterised by periductal concentric obliterative fibrosis and bile duct strictures. Its course is very variable and can in individual cases follow a benign course, but for the most part PSC is a progressive disease which leads eventually to cirrhosis and is strongly associated with the development of cholangiocarcinoma. It is associated with inflammatory bowel disease (IBD) in the majority of cases. No specific disease marker has however been observed and the diagnosis is based on typical cholangiographic and histological findings, with exclusion of secondary sclerosing cholangitis.1
The clinical course of PSC is highly variable. Patients with IBD may be found to have abnormal liver function tests on routine follow up or patients may present with symptoms of PSC such as fatigue, pruritus, abdominal pain, or fever.2–6 The natural history of PSC is quite well characterised. Median survival to death or liver transplantation has been estimated to be 12 years in studies from Sweden,3 as well as from the USA4 and England.5 In the largest study,3 66% of patients were symptomatic at diagnosis and 26% died of cholangiocarcinoma which was diagnosed after a median time of 32 months after diagnosis. Some patients have cholestatic liver function tests and typical histological features of PSC but a normal cholangiogram. These patients with a clinical diagnosis of PSC but without the typical intra- and/or extrahepatic cholangiographic changes have been identified as having small duct PSC.7–9 Only a small number of patients with small duct PSC have so far been reported10–11 and the natural history of patients fulfilling the criteria for small duct PSC has been largely unexplored.11 It has been proposed that patients with small duct PSC would eventually develop large duct PSC.8 However, to date there is only anecdotal evidence for this suggestion and no systematic evaluation has been reported on this condition.9 The aim of the current study was to assess the clinical features at presentation and disease course in patients with small duct PSC compared with a large cohort of patients with large duct PSC.
METHODS
Patients
Patients with small duct PSC were identified among those admitted for a diagnostic workup of cholestatic liver function tests (LFTs) in one centre in the UK (Oxford) and one in Norway (Oslo). Both are tertiary referral centres with a longstanding interest in PSC.2,6,11 This was a retrospective analysis of patients, covering the past 20 years. Among patients with a diagnosis of PSC, 22 patients were identified in Oxford and 11 in Oslo who fulfilled the diagnostic criteria for small duct PSC. The diagnosis of small duct PSC was based on cholestatic LFTs not explained by other cholestatic liver disorders other than PSC, typical histological changes identified in PSC, assessed by pathologists with a longstanding experience in this field (KF and OPC), and a normal cholangiogram. Age at diagnosis is the age at which the first endoscopic retrograde cholangiopancreatography (ERCP) was performed with a normal cholangiogram. All patients were investigated with ERCP and only patients with good quality imaging were included. Good filling of the intrahepatic biliary system and no intra- and/or extrahepatic changes were present in all patients, as evaluated by two experienced endoscopists (RWC and OF). All patients, symptomatic as well as asymptomatic, were investigated by colonoscopy for evidence for IBD. For comparison, we used 100 patients with large duct PSC from Oxford and 160 from Oslo. These patients had been hospitalised previously or were followed up at these two centres and were already on a database in both hospitals.
Diagnostic approach
In patients with large duct PSC, the radiological diagnosis was based on typical cholangiographic findings, with bile duct irregularities, strictures, or local narrowing of bile ducts.12 Standard histological techniques were applied to prepare the liver biopsy specimens obtained in every patient included in the study. Specimens were stained with haematoxylin, eosin, Goermoris and Sweet’s silver method for reticulin, Van Gieson or Sirius red for fibrosis, Pearl stain for iron, and orcein for copper associated protein. Biopsies were classified using Ishak inflammation and fibrosis scores as well as Ludwig’s score of fibrosis.
Efforts were made to exclude all other causes of liver disease. Apart from routine LFTs, autoantibodies (antimitochondrial antibody, smooth muscle antibody, and antinuclear antibody) were measured in all patients as well as electrophoresis and serology for hepatitis A, B, and C. Thus evidence of other liver diseases such as primary biliary cirrhosis, autoimmune hepatitis, as well as viral aetiology and drug induced liver disease were excluded by appropriate serological tests and clinical evaluation. The following variables were available in both groups: sex, follow up in months, age at diagnosis and at follow up, symptoms at diagnosis and follow up, occurrence and type of IBD, and information on colectomy, cholangiocarcinoma, liver transplantation, and survival. The specific symptoms recorded in both groups were jaundice (defined as bilirubin levels above 40 μmol/l), abdominal pain, fever, itching, fatigue, and weight loss. The following biochemical test were measured and compared between groups: haemoglobin, bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), albumin, prothrombin complex in the early part of the study and later international normalised ratio (INR). Information on the use of ursodeoxycholic acid (Ursofalk) was available only for patients from Oxford and was compared between the respective groups.
Patients were followed until 1 June 2001 and end points were liver transplantation and death. Findings from both centres were entered into a new database.
Statistical methods
The Student’s t test was used for continuous variables to compare duration of follow up, age at diagnosis and follow up, as well as results of LFTs. Fisher’s exact test was applied for comparison between symptoms at diagnosis and at follow up, and occurrence of cholangiocarcinoma, liver transplantation, and death. The χ2 test was used for comparison of the prevalence of subtypes of IBD. The correlation between biochemical parameters and symptoms between groups was calculated using the permutation test.13 Results are expressed as mean (SEM). The level of significance was 5%.
RESULTS
General features
Patients with small duct PSC represented 33 of 293 patients with PSC (11%). Among the 33 patients with small duct PSC, 19 were male (58%) and 14 were female (42%). In the large duct group, 188 (72%) were male and 72 (28%) were female (NS). Mean age at diagnosis was 38 (3) years in patients with small duct PSC which was similar to the mean age in the large duct group (39 (1) years) (p>0.3). Mean age at follow up was also similar (47 (3) and 48 (1) years in small duct and large duct PSC patients, respectively). Mean follow up time was 106 (12) months in small duct and 105 (5) months in large duct patients (p>0.3). Among those who were originally considered to have small duct PSC, four (12%) developed large duct PSC. The first ERCP was normal but because of clinical worsening of the liver disorder, another ERCP was performed which showed cholangiographic changes intra- and/or extrahepatically. Among the 33 patients with small duct PSC, 29 (88%) had concomitant IBD; 20 (61%) were found to have ulcerative colitis (UC), seven (21%) had Crohn’s disease, all with colitis and one also with small bowel involvement, and two (6%) had indeterminate colitis. Two patients did not have IBD at diagnosis but developed IBD during follow up. IBD was detected among 218 (83%) of 260 large duct patients; 172 patients had UC (66%), 25 (9.6%) had Crohn’s disease, and 21 (8%) indeterminate colitis. No statistically significant differences were observed in the prevalence of IBD among the small and large duct PSC patients, and the proportion of UC and indeterminate colitis, respectively, was similar in both groups. However, Crohn’s disease was more common in small duct (21%) than in large duct (9.6%) patients (p<0.05). Among the British patients with small duct PSC, 31% were treated with ursodeoxycholic acid for more than three years of follow up whereas 47% of large duct patients received ursodeoxycholic acid (Ursofalk) for more than three years of follow up (NS).
Biochemical and histological findings
Biochemical results in patients with small duct and large duct PSC are compared in table 1 ▶. At diagnosis, serum levels of albumin were significantly higher in patients with small duct PSC. There was also a clear trend towards a lower INR in small duct patients (p=0.05) and lower ALP values compared with large duct patients (p=0.08). Serum bilirubin, ALT, and AST were not significantly different between the groups at diagnosis (table 1 ▶). No significant differences were observed between the groups for any of the measured biochemical variables at follow up, except for serum albumin which was higher in the small duct group (table 2 ▶). In four patients, original liver biopsies were not available for reevaluation (one in Oxford and three in Oslo). However, experienced pathologists had made the histological diagnosis of PSC previously and they were therefore included in the study. At diagnosis, none of the small duct patients had cirrhosis, 69% of small duct patients were classified as stage I on Ludwig’s fibrosis score, and 31% were stages II–III (table 4 ▶). Ishak inflammation and fibrosis scores as well as Ludwig’s fibrosis score are shown in tables 3 and 4 ▶ ▶.
Table 1.
Biochemical findings in patients with small duct and large duct primary sclerosing cholangitis at diagnosis
| Small duct | Large duct | p Value | |
| Bilirubin | 24 (8) | 50 (6) | NS |
| ALP | 674 (83) | 959 (58) | NS |
| AST | 124 (33) | 116 (9) | NS |
| ALT | 228 (77) | 181 (19) | NS |
| PT | 1 (0.04) | 1.1 (0.2) | NS |
| Albumin | 42 (1) | 39 (0.3) | 0.02 |
| Hb | 13.3 (0.3) | 13.2 (0.1) | NS |
ALP, alkaline phosphatase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; PT, prothrombin time; Hb, haemoglobin.
Table 2.
Biochemical findings in patients with small duct and large duct primary sclerosing cholangitis at follow up
| Small duct | Large duct | p Value | |
| Bilirubin | 34 (18) | 33 (8) | NS |
| ALP | 499 (148) | 587 (52) | NS |
| AST | 48 (11) | 62 (6) | NS |
| ALT | 64 (24) | 181 (19) | NS |
| PT | 1.1 (0.5) | 1.16 (0.7) | NS |
| Albumin | 41 (0.9) | 39 (0.7) | 0.02 |
| Hb | 13.3 (0.3) | 13 (0.2) | NS |
ALP, alkaline phosphatase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; PT, prothrombin time; Hb, haemoglobin.
Table 4.
Histological features of patients with small duct primary sclerosing cholangitis according to the Ishak fibrosis score as well as Ludwig’s score of fibrosis. Distribution of scores within Ishak and Lundwig’s categories are demonstrated
| No of cases in each category | |||||||||||
| Ishak fibrosis score | Ludwig fibrosis score | ||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 0 | 1 | 2 | 3 | 4 |
| 1 | 16 | 9 | 3 | 0 | 0 | 0 | 0 | 20 | 6 | 3 | 0 |
Table 3.
Histological features of patients with small duct primary sclerosing cholangitis according to the Ishak inflammation score
| No of cases in each category | |||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | |
| Ishak inflammation score | |||||||
| A | 14 | 13 | 1 | 0 | 1 | ||
| B | 28 | 0 | 1 | 0 | 0 | 0 | 0 |
| C | 10 | 15 | 2 | 2 | 0 | ||
| D | 4 | 13 | 11 | 1 | 0 | ||
Ishak inflammation score: A, interface hepatitis; B, confluent necrosis; C, lobular inflammation; D, portal inflammation.
Symptoms and signs
Among patients with small duct PSC, 11 of 33 (30%) were symptomatic at diagnosis in comparison with 139 (53%) of 260 large duct patients (p<0.01). The most common symptoms in small duct patients were fatigue and abdominal pain whereas weight loss, itching, jaundice were the most common symptoms in large duct patients at diagnosis (table 5 ▶). Symptoms and signs at follow up are shown in table 6 ▶. During follow up, among the 23 patients who were asymptomatic at diagnosis, eight (35%) developed symptoms and in three (30%) of 10 symptomatic patients, symptoms disappeared. In large duct patients, 18% of those who were asymptomatic at diagnosis developed symptoms but in 30% of symptomatic patients symptoms disappeared during follow up. The occurrence of symptoms at diagnosis did not predict poor outcome in the small duct group. The four patients who developed large duct disease were all asymptomatic at diagnosis and the patient who died of liver related disease was also asymptomatic.
Table 5.
Symptoms and signs in patients with small duct and large duct primary sclerosing cholangitis at diagnosis
| Small duct | Large duct | p Value | |
| Jaundice | 6% | 25% | <0.05 |
| Abdominal pain | 15% | 15% | NS |
| Fever | 7% | 9% | NS |
| Itching | 12% | 27% | NS |
| Fatigue | 18% | 22% | NS |
| Weight loss | 0% | 29% | <0.01 |
Table 6.
Symptoms and signs in patients with small duct and large duct primary sclerosing cholangitis at follow up
| Small duct | Large duct | p Value | |
| Jaundice | 12% | 9% | NS |
| Abdominal pain | 13% | 12% | NS |
| Fever | 3% | 6% | NS |
| Itching | 16% | 18% | NS |
| Fatigue | 14% | 24% | NS |
| Weight loss | 0% | 4% | NS |
Among small duct patients, no correlation was observed between biochemical findings and the occurrence of symptoms at diagnosis or with jaundice (except for bilirubin), abdominal pain, fever, itching, fatigue, or weight loss. However, among large duct patients, a significant association was observed between symptoms at diagnosis and bilirubin (p<0.01), ALP (p<0.01), AST (p<0.05), and haemoglobin (p<0.01). Jaundice at diagnosis correlated significantly with serum bilirubin (p<0.01), ALP (p<0.01), AST (p<0.01), and albumin (p<0.01). Itching at diagnosis correlated positively with serum bilirubin (p<0.01), and the occurrence of fatigue was correlated with serum bilirubin (p<0.01).
Follow up
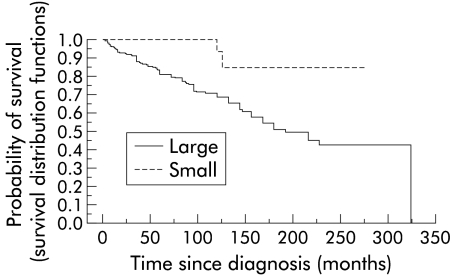
ERCP had been repeated in 19 of 33 patients (58%). Four patients originally considered to have small duct PSC developed large duct PSC, with typical intra- and extrahepatic changes on ERCP. Two of these underwent liver transplantation during follow up. In the two other patients the disease course was uneventful during the follow up period. Of the rest who did not develop large duct PSC, two patients died during follow up. One died of liver failure and the other of cardiac death unrelated to liver disease. Another patient developed signs of portal hypertension with small oesophageal varices which did not bleed during follow up. In comparison, 9% of large duct patients had a variceal bleed during follow up. None of the small duct patients developed cholangiocarcinoma. Among patients with large duct PSC, 28 (11%) of 260 developed cholangiocarcinoma (p=0.056 compared with small duct). All of the patients with cholangiocarcinoma died during follow up. Comparison of survival among the two groups revealed that small duct patients had a significantly better survival than patients with large duct PSC (fig 1 ▶). Two small duct patients died during follow up compared with 88 of 260 large duct patients. Among the large duct patients, 34 of 260 (13%) underwent liver transplantation during follow up which was not significantly different from two of 33 in the small duct group. Thus four of 33 (12%) small duct patients either required liver transplantation or died. However, 122 (47%) large duct patients either required liver transplantation or died during a similar period of follow up (p<0.05 compared with the small duct group). Among those who underwent liver transplantation for large duct disease, 13 died during follow up.
Figure 1.
Kaplan-Meier estimated survival curves for patients with small duct and large duct primary sclerosing cholangitis (p<0.01).
DISCUSSION
The natural history of classical large duct PSC has been well characterised in several studies. However, some patients with cholestatic LFTs have a typical PSC histology but a normal cholangiogram and these are classified as having small duct PSC. The natural history of this condition has been largely unexplored. In the current study, we found that patients with small duct PSC had a favourable long term prognosis in terms of death or liver transplantation compared with those with large duct PSC. Mean age at diagnosis and the period of follow up were very similar in the two groups. Thus small duct PSC does not seem to be an early stage of PSC in the majority of cases. Furthermore, patients with small duct PSC rarely progressed to large duct PSC. Only 12% of patients originally diagnosed as having small duct PSC developed large duct PSC. However, ERCP was not repeated in all patients with small duct PSC but only when it was clinically motivated and hence we cannot exclude the fact that more patients developed large duct PSC.
The prognosis for patients with small duct PSC has not previously been known and it is important to investigate this subgroup of PSC patients further to obtain more understanding of PSC in general. The importance of extrahepatic changes in PSC is controversial as cholestasis is better correlated with intrahepatic than extrahepatic cholangiographic changes in patients with large duct PSC.14 Furthermore, it is not known whether large duct PSC is a prerequisite for the development of cholangiocarcinoma or whether disease of the small bile ducts is associated with this severe cancer. The nature of abdominal pain in PSC is also unknown and the importance of extrahepatic biliary strictures in the causation of this clinical feature is not fully understood as these are often asymptomatic.15 The most common criteria for diagnosis of small duct PSC that have been proposed are occurrence of biochemical features of chronic cholestasis, liver histology compatible with PSC, normal cholangiogram, and concomitant IBD. The prevalence of IBD in patients with PSC has been reported as ranging from 71%4,5 to 81%,3 and as not all patients with large duct PSC have concomitant IBD, it could be argued that patients with typical histological features, although not having concomitant IBD, should also be diagnosed as having small duct PSC. In the classic paper by Ludwig and colleagues,8 it is argued that there is no valid scientific reason why small duct PSC could not occur without large duct PSC or concomitant IBD. Our clinical impression in the past is along these lines and we also chose to include patients without IBD but fulfilling all other criteria for this condition.
Only a few cases of small duct PSC have been reported in the literature8,11,16 and therefore information has been largely lacking on the clinical course and prognosis of small duct patients. Previous studies have revealed that inflammatory changes in the liver are quite mild.8 Results from the histological evaluation in the current study support these early observations, demonstrating mild inflammation in the majority of cases. Periductal fibrosis without inflammation is a common finding in liver diseases other than PSC. In the current study, portal inflammation was evident with typical histological changes in almost all patients. Other differential diagnoses were ruled out, with negative markers for primary biliary cirrhosis, autoimmune hepatitis, and viral hepatitis, and patients did not fulfill histopathological criteria for the rare entity of idiopathic adulthood ductopenia.17–19 Thus very strict criteria were applied to support the criteria for small duct PSC.
The prevalence of small duct patients in the total PSC population was found to be 11% in the current study which is in agreement with the 9% value reported from the Mayo Clinic.10 The prevalence of IBD among the two groups was very similar in the present study (88% in small and 83% in large duct patients). However, the proportion of patients with Crohn’s disease in small duct patients was much higher than that previously reported in large duct PSC.3,5 In the present study, Crohn’s disease was present in 21% of our patients, which is the highest prevalence of Crohn’s disease reported in patients with PSC. In comparison, in 305 Swedish patients,3 Crohn’s disease was detected in 7% and in a recent Japanese study, Crohn’s disease was found in only 5% of PSC patients.20 Thus it seems that patients with Crohn’s disease are more often associated with small duct PSC than with large duct disease. A study of hepatobiliary dysfunction in patients with Crohn’s disease demonstrated that the most common liver disease associated with Crohn’s disease was PSC.21 Moreover, the majority of patients had evidence of small duct PSC and it was suggested that PSC associated with Crohn’s disease was a milder liver disease than PSC associated with ulcerative colitis.21 In the current study, all patients with Crohn’s disease had a very mild liver disease, with Ludwig’s fibrosis stages I–II in all patients, and none showed progression to cirrhosis or had any known complications of liver disease, although the duration of follow up was similar to the rest of the small duct group.
The clinical course of large duct PSC is highly variable and cases have been reported with no symptoms of liver disease who have been followed for up to 15 years.22 However, in most cases, PSC is a progressive disease even in those who are asymptomatic at diagnosis.3,22 A study from the Mayo Clinic revealed that 76% of 45 asymptomatic patients followed for a mean period of 75 months had clinical and biochemical progression of their liver disease and one third of these patients developed liver failure.23 In the present study, our mean duration of follow up is the longest reported in PSC patients that we are aware of, with a follow up time of 106 and 105 months in small duct and large duct PSC, respectively. Despite the lengthy follow up, only three small duct patients (9%) underwent liver transplantation or developed liver failure. In comparison, 47% of large duct patients either died or underwent liver transplantation. It has also been demonstrated that if clinical deterioration occurs in patients with PSC, this seems to occur within the first eight years after clinical presentation15 and in the present study the majority of patients with small duct disease were followed for more than eight years (mean follow up 8.3 years).
An important part of the mortality in PSC is due to cholangiocarcinoma which is detected in 10–20% of patients.3,24 None of the small duct patients in the present study developed cholangiocarcinoma whereas 11% of large duct patients developed this cancer. This could be an underestimate as cholangiocarcinoma has been reported to occur in 35% of histological examinations of the explanted livers of transplant recipients,3 information which was not available in the current study. As far as we are aware, cholangiocarcinoma has not been reported in patients with small duct PSC.21,24,25 The results of our study support the lack of association between cholangiocarcinoma and small duct PSC. This suggests that cholangiocarcinoma in PSC is a disease of large duct epithelium and not of small duct epithelium. In other words, this epithelium is susceptible to the carcinogenetic agent(s) of PSC (whatever it is) while the small epithelium seems to be resistant. Understanding of the nature of this resistance could be of potential therapeutic use. Conversely, another explanation is that the pathogenesis of small duct PSC is different from that of large duct PSC, and in particular does not include a carcinogenetic component.
The end points in the present study—severity of liver disease, survival, and cholangiocarcinoma—differed markedly between small duct and large duct PSC. However, biochemical findings did not differ at diagnosis or follow up between the two groups, except for lower serum albumin values at diagnosis and follow up in the large duct group. This supports the concept that cholestasis in PSC is not a result of large duct disease.14 Unlike primary biliary cirrhosis, the clinical course and biochemical findings in PSC are characteristically unpredictable in individual patients.26 Patients with PSC often have fluctuations in bilirubin levels, and periods of clinical and cholestatic relapses follow periods of clinical remission with less cholestasis.16,27 Thus a high bilirubin level at one period in time in patients with PSC is unreliable for predicting the prognosis in this condition. Even more uncertain is evaluation of symptoms for the development of liver disease in the individual PSC patient.26 The survival rate in asymptomatic PSC patients has been shown to be significantly higher compared with patients who present with symptoms at diagnosis.3 In the present study, symptoms at diagnosis were significantly more prevalent in the large duct group. However, this difference was mainly due to differences in the prevalence of jaundice and itching in the large duct compared with the small duct group. The prevalence of abdominal pain and fatigue, which were the most common symptoms in the small duct patients, were very similar in the two groups. Abdominal pain was present in 15% of patients in both groups. Thus strictures of the extrahepatic bile ducts are unlikely to explain the abdominal pain in the majority of PSC cases with large duct disease as abdominal pain was as frequent in the small duct group. Additionally, the finding of typical large duct PSC histological features in small duct PSC, albeit with less fibrotic distortion of the liver structure, suggests that the changes in periduct fibrosis, cholestasis, etc., are not a result of large duct strictures and associated obstructive damage but reflect intrinsic damage to the biliary epithelium, supporting the concept of a “primary” sclerosing cholangitis.
In conclusion, patients with small duct PSC seem to have a good prognosis in terms of risk of progression of liver disease and cholangiocarcinoma. Small duct PSC rarely progresses to large duct PSC.
Acknowledgments
This study was supported by the Swedish Medical Research Council (grant Nos 8288 and 13409) and by the Faculty of Medicine, University of Gothenburg.
Abbreviations
PSC, primary sclerosing cholangitis
IBD, inflammatory bowel disease
UC, ulcerative colitis
CD, Crohn’s disease
LFTs, liver function tests
AST, aspartate aminotransferase
ALT, alanine aminotransferase
ALP, alkaline phosphatase
ERCP, endoscopic retrograde cholangiopancreatography
INR, international normalised ratio
REFERENCES
- 1.Ludwig J. Histopathology of primary sclerosing cholangitis. In: Manns M, Chapman RW, Stiehl A, et al, eds. Primary sclerosing cholangitis. London: Kluwer Academic publishers, 1998:14–21.
- 2.Chapman RW, Arborgh BM, Rhodes JM, et al. Primary sclerosing cholangitis: a review of its clinical features, cholangiography and hepatic histology. Gut 1980;21:1870–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broomé U, Olsson R, Lööf L, et al. Natural history and prognostic factors in 305 patients with primary sclerosing cholangitis. Gut 1996;38:610–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiesner TH, Grambsch PB, Dickson ER, et al. Primary sclerosing cholangitis: natural history, prognostic factors, and survival analysis. Hepatology 1989;10:430–6. [DOI] [PubMed] [Google Scholar]
- 5.Farrant JM, Hayllar KM, Wilkinson ML, et al. Natural history and prognostic variables in primary sclerosing cholangitis. Gastroenterology 1991;100:1710–17. [DOI] [PubMed] [Google Scholar]
- 6.Aadland E, Schrumpf E, Fausa O, et al. Primary sclerosing cholangitis: a long term follow-up study. Scand J Gastroenterol 1987;22:655–64. [DOI] [PubMed] [Google Scholar]
- 7.Wee A, Ludwig J. Pericholangitis in chronic ulceratice colitis: primary sclerosing cholangitis of the small bile ducts? Ann Intern Med 1985;102:581–7. [DOI] [PubMed] [Google Scholar]
- 8.Ludwig J. Small duct primary sclerosing cholangitis. Semin Liver Dis 1991;11:11–17. [DOI] [PubMed] [Google Scholar]
- 9.Kim WR, Ludwig J, Lindor K. Variant forms of cholestatic diseases involving small bile ducts in adults. Am J Gastroenterol 2000;95:1130–8. [DOI] [PubMed] [Google Scholar]
- 10.Angulo P, Maor-Kendler Y, Donlinger JJ, et al. Small-duct primary sclerosing cholangitis: prevalence and natural history. Gastroenterology 2000;120:A33. [Google Scholar]
- 11.Boberg KM, Schrumpf E, Fausa O, et al. Hepatobiliary disease in ulcerative colitis. An analysis of 18 patients with hepatobiliary lesions classified as small-duct primary sclerosing cholangitis. Scand J Gastroenterol 1994;29:744–52. [DOI] [PubMed] [Google Scholar]
- 12.MacCarty RL, LaRusso NF, Wiesner RH, et al. Primary sclerosing cholangitis; findings on cholangiography and pancreatography. Radiology 1983;149:39–44. [DOI] [PubMed] [Google Scholar]
- 13.Good P. Permutation tests. In: A practical guide to resampling methods for testing hypotheses. New York: Springer Inc, 2000:36–7.
- 14.Olsson R, Asztely MS. Prognostic value of cholangiography in primary sclerosing cholangitis. Eur J Gastroenterol Hepatol 1995;7:251–4. [PubMed] [Google Scholar]
- 15.Stockbrügger RW, Olsson R, Jaup B, et al. Forty-six cases patients with primary sclerosing cholangitis: radiological bile duct changes in relationship to clinical course and concomitant inflammatory bowel disease. Hepatogastroenterology 1988;35:289–94. [PubMed] [Google Scholar]
- 16.Chapman RW. Primary sclerosing cholangitis as an autoimmune disease: pros and cons. In: Manns MP, Paumgartner G, Leuschner U, eds. Immunology and liver. London: Kluwer Academic Publishers, 2000:279–87.
- 17.Ludwig J, Wiesner RH, LaRusso NF. Idiopathic adulthood ductopenia. A cause of chronic cholestatic liver disease and biliary cirrhosis. J Hepatol 1988;7:193–9. [DOI] [PubMed] [Google Scholar]
- 18.Casall AM, Carbone G, Cavalli G. Intrahepatic bile duct loss in primary sclerosing cholangitis: a quantitative study. Histopathology 1998;32:449–53. [DOI] [PubMed] [Google Scholar]
- 19.Khanlou H, Sass D, Rothstein K, et al. Idiopathic adulthood ductopenia: case report and review of the literature. Arch Intern Med 2000;160:1033–6. [DOI] [PubMed] [Google Scholar]
- 20.Takikawa H, Manabe T. Primary sclerosing cholangitis in Japan—analysis of 192 cases. J Gastroenterol 1997;32:134–7. [DOI] [PubMed] [Google Scholar]
- 21.Rasmussen HH, Fallingborg JF, Mortensen PB, et al. Hepatobiliary dysfunction and primary sclerosing cholangitis in patients with Crohn’s disease. Scand J Gastroenterol 1997;32:604–10. [DOI] [PubMed] [Google Scholar]
- 22.Chapman RWG, Burroughs AK, Bass NM, et al. Longstanding asymptomatic primary sclerosing cholangitis: report of three cases. Dig Dis Sci 1981;26:78–82. [DOI] [PubMed] [Google Scholar]
- 23.Dickson ER, Murtaugh PA, Wiesner RH, et al. Primary sclerosing cholangitis: refinement and validation of survival models. Gastroenterology 1992;103:1893–901. [DOI] [PubMed] [Google Scholar]
- 24.Riordan SM, Williams R. Risk of cholangiocarcinoma in primary aclerosing cholangitis. In: Manns MP, Chapman RW, Stiehl A, eds. Primary sclerosing cholangitis. London: Kluwer Academic Publishers, 1997;69–85.
- 25.Wiesner RH, LaRusso NF. Clinicopathologic features of the syndrome of primary sclerosing cholangitis. Gastroenterology 1980;79:200–6. [PubMed] [Google Scholar]
- 26.Chapman RW. The natural history of primary sclerosing cholangitis. In: Manns MP, Chapman RW, Stiehl A, eds. Primary sclerosing cholangitis. London: Kluwer Academic Publishers, 1997:55–68.
- 27.Björnsson E, Kilander A, Olsson R. CA 19-9 and CEA are unreliable markers for cholangiocarcinoma in patients with primary sclerosing cholangitis. Liver 1999;19:501–8. [DOI] [PubMed] [Google Scholar]