Abstract
Background: Circulating levels of endotoxin (or lipopolysaccharide (LPS)) and anti-endotoxin antibodies are increased in patients with inflammatory bowel disease, supporting the hypothesis of a role for endogenous bacterial products in the pathogenesis of these disorders.
Aim: The aim of this study was to analyse the direct effects of LPS on intestinal epithelial cell turnover.
Methods and Results: LPS significantly inhibited growth of the human non-transformed immature crypt cell line (HIEC), whereas IEC-6 cell proliferation was stimulated by LPS. As LPS is a physiological inducer of tumour necrosis factor α (TNFα) in various cell systems and this cytokine exerted similar anti-proliferative (HIEC) or growth stimulatory (IEC-6 cells) effects, the study thus tested the hypothesis that endogenously produced TNFα in response to LPS mediates this growth modulatory effect in an autoparacrine/paracrine way. Therefore, during LPS stimulation, the biological activity of TNFα was blocked using neutralising anti-TNFα antibodies, as well as inhibitory, antagonistic antibodies directed against the p55 TNF receptor, signalling the antimitotic TNFα effect in HIEC. Both experimental approaches completely abolished the growth modulatory effects of LPS in HIEC/IEC-6 cells. Production and secretion of TNFα by HIEC/IEC-6 cells in response to LPS was confirmed on mRNA and protein level by reverse transcription polymerase chain reaction (RT-PCR) and enzyme linked immunosorbent assay. LPS signalling was independent of CD14 in HIEC, as these cells lack this receptor. However, HIEC expressed TLR4 and MD2 resulting in a fully functional signalling complex as demonstrated by RT-PCR, western blot, and immunofluorescence analyses.
Conclusion: These results support the hypothesis that LPS induced changes of intestinal epithelial cell turnover may directly contribute to the pathogenesis of inflammatory epithelial cell lesions by endogenous TNFα production by enterocytes.
Keywords: enterocytes, lipopolysaccharide, proliferation, toll-like receptors, tumour necrosis factor α
The host’s endogenous bacterial flora is known to play an important part as trigger in the pathogenesis of inflammatory bowel disorders (IBD), as shown in various experimental models of genetically engineered “knockout” animals. T cell receptor-αβ deficient mice fail to develop colitis in the absence of a microbial environment.1 Similarly, interleukin (IL) 2 or IL10 deficient mice do not react with colitis, when kept in a germ free environment.2,3 The importance of the enteric flora and its products in inducing and perpetuating colitis is also well recognised in humans.4 Diversion of the faecal stream can induce remission in Crohn’s colitis, whereas ileocolonic anastomosis results in the rapid recurrence of colitis.5 Furthermore, systemically circulating endotoxin and increased titres of anti-endotoxin antibodies are found in patients with Crohn’s disease (CD) or ulcerative colitis (UC).6 These data as well as the beneficial effects of antibiotics in treating IBD patients support the hypothesis that bacterial compounds are implicated in the pathogenesis of IBD.
Endotoxin or lipopolysaccharide (LPS) refers to a glycolipid present in the outer membrane of Gram negative bacteria. LPS is a strong stimulator of the immune system, capable of activating neutrophils, lymphoytes, monocytes, and particularly macrophages. Upon this interaction immune competent cells release a great variety of immune mediators, such as cytokines or nitrous oxide.7–9 Evidence was put forward showing that the pathological uptake of such bacterial products not only activates cells of the immune system, but may also directly affect intestinal epithelial cell functions.10,11 Contact with bacterial products such as LPS, induces enterocytes to release several proinflammatory cytokines and chemokines, such as IL6 or IL8.12–14 This results in the recruitment of further immune competent cells into the intestinal mucosa, increasing the inflammatory cascade. LPS induced activation of immune competent cells is mainly mediated through the membrane receptor CD14.15 However, intestinal epithelial cells lack this receptor. Recently, it was proposed that the family of the newly discovered toll-like receptors (TLR) also serves as membrane receptors for LPS.16,17
In this study, we aimed to determine whether LPS directly changes normal enterocyte turnover, thereby contributing to pathological epithelial cell functions seen in IBD. We therefore studied the expression of CD14 and TLR as well as the effect of LPS on non-transformed human intestinal crypt cell (HIEC) and IEC-6 cell proliferation and apoptosis. In a second step, we attempted to elucidate the mechanisms involved in LPS induced changes in enterocyte turnover. We identified TNFα, produced by intestinal epithelial cells (IEC) in response to LPS, as the mediator responsible for these effects.
METHODS
Cell culture and reagents
HIEC, human small intestinal crypt cells of fetal origin18 were cultured at 37°C in a humidified atmosphere of 10% carbon dioxide in Dulbeco’s modified Eagle medium (DMEM) supplemented with 5% heat inactivated fetal calf serum (FCS) (Gibco, Karlsruhe, Germany), 1 mM sodium pyruvate and 1% penicillin/streptomycin. In addition, 1% glutamine and glutamax (Gibco) were added for HIEC, as previously described.19 IEC-6 cells (ATCC) were cultured under standard conditions.20 Protein free LPS, highly purified by chromatography, (from E coli 026:B6, Sigma, Munich, Germany) was used at concentrations between 0.1–100 μg/ml in DMEM containing FCS. Recombinant human TNFα was obtained from Genzyme (Russelsheim, Germany), the antihuman p55-TNF receptor antagonistic antibodies from R+D (Wiesbaden, Germany). Neutralising rabbit antihuman TNFα (50 μg/ml) was purchased from Peprotech (London, UK).
Proliferation assays
Proliferation of HIEC and IEC-6 cells was monitored by 3H-thymidine incorporation into DNA, as previously described.19 Briefly, cells were allowed to attach over night in 24 multiwell plates (Costar, Germany). After a 24 hour stabilisation period without FCS addition, the cells were incubated with 0.1–10 μg/ml LPS in complete medium (DMEM+1%FCS) alone or in combination with anti-TNFα or anti-p55 TNF receptor antibodies for 22 hours. The last two to four hours, 3H-thymidine was added. In parallel, similar experiments with recombinant human IL6 (0.1–500 ng/ml, Genzyme), IL8 (0.1–500 ng/ml, Genzyme), and TNFα (0.01–10 ng/ml) were performed. In addition, proliferation of HIEC or IEC-6 cells was quantified by cell counts over a 48 hour stimulation period in the presence of LPS, TNFα alone, or in combination with anti-TNFα or antihuman p55 TNF receptor antibodies.
Apoptosis assay
Subconfluent HIEC or IEC-6 cells were cultured as above, after various time intervals (6–72 hours) of LPS or TNFα stimulation cells were harvested and stained with propidium iodide (PI, 10 μg/ml) and annexinV. Apoptosis was monitored by flow cytometry (FACScan, Becton Dickinson, Heidelberg, Germany), as previously described.19 1×104 cells were analysed and the apoptosis rate calculated as percentage of total cells. Immunofluorescence studies after staining of the nuclei with the DNA-dye HOECHST 33342 were performed using a Leica immunofluorescence microscope (Leica, Bernstein, Germany).
RNA preparation and reverse transcription polymerase chain reaction (RT-PCR)
mRNA was isolated from HIEC and IEC-6 cells at different time points (one to nine hours) after LPS stimulation using a Quickprep mRNA micro purification kit (Amersham Pharmacia Biotech, Freiburg, Germany). Integrity and purity of isolated RNA were assessed by electrophoresis on a 1.2% agarose gel before generation of cDNA using reverse transcription. PCR amplification was performed with Taq polymerase (Perkin Elmer) for 38 cycles at 95°C for 45 seconds, at 54°C for one minute, and at 72°C for one minute (for TLR2 and TLR4), for 38 cycles at 94°C for 45 seconds, at 55°C for one minute, and at 72°C for one minute (for MD2), for 33 and 30 cycles at 94°C for one minute, at 57°C for one minute, and at 72°C for one minute (for human and rat TNFα, respectively). As housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (G3PDH) was used in both human and rat intestinal epithelial cells. The oligonucleotide primers used for RT-PCR were: human TNFα sense 5’-GAG TGA CAA GCC TGT AGC CCA TGT TGT AGCA-3’ and antisense 5’-GCA ATG ATC CCA AAG TAG ACC TGC CCA GACT-3’, yielding a 441 base product, rat TNFα, sense: 5’-AAA GAC AAC CAA CTG GTG GTA CCA-3’ and antisense 5’-GAC TCC GTG ATG TCT AAG TAC TTG-3’, yielding a 308 base product. The sequences used for human G3PDH were: sense: 5’-TGA AGG TCG GAG TCA ACG GAT TTG GT-3’ and antisense: 5’-CAT GTG GGC CAT GAG GTC CAC CAC-3’, yielding a 983 base product and for rat G3PDH: sense: 5’-CCA TGG AGA AGG CTG GGG-3’ and antisense: 5’-GAG CCC TTC CAC GAT GCC-3’, yielding a 753 base product. The primer sequences for TLR2 and TLR4 yielding in 347 and 548 base products, respectively, were recently published by Faure et al.21 The primers sequences for MD2 were recently published by Abreu et al.22 Samples without cDNA were included to control external contamination during preparation for PCR. After RT-PCR, the TLR2, TLR4, and MD2 amplicons were purified and subsequently sequenced using an automated DNA sequencer (ABI Prism 3100 Genetic Analyzer, Applied Biosystems, France) to confirm the identity of the fragments.
Western blotting
The expression of the TLR2 and TLR4 was determined by western blotting. HIEC lysates were prepared using an ice cold lysis buffer (50 mM TRIS, 150 mM NaCl, 10 mM EDTA, 1% Triton) supplemented with a mixture of protease inhibitors (Boehringer, Mannheim, Germany). Equivalent protein samples were resolved on 10% SDS-polyacrylamide gels and transferred to nitrocellulose membranes (Bio-Rad). For immunodetection, the membranes were incubated overnight with anti-TLR2 (1:500), or TLR4 antibody (1:500, all Biocarta, Hamburg, Germany) in TRIS buffered saline/Tween 20-1% milk powder, followed by incubation with the hoseradish peroxidase conjugated antimouse-IgG (1:5000, Biosource). To confirm the presence of both receptors and the specificity of the anti-TLR2 and TLR4 mouse IgG antibodies, a second set of anti-TLR2 and TLR4 antibodies (polyclonal goat IgG, Santa Cruz) was used. In addition, control experiments with highly specific blocking peptides were performed. The bands were read by enhanced chemiluminescence (ECL-kit, Amersham). Caco-2 cell lysates were used as positive control.
CD14 and TLR expression
Unstimulated and LPS or TNF stimulated HIEC as well as normal human macrophages (from a healthy donor) were stained with an anti-CD14-FITC labelled antibody (DAKO, Hamburg, Germany) for 30 minutes at 37°C. Monocytes, serving as positive control, were isolated from PBL by isotonic density gradient centrifugation (Ficoll, Becton Dickinson) followed by subsequent cultivation on plastic dishes permitting the separation of attached cells (monocyte/macrophage fraction) from the non-attached leucocytes. After intensive washing, the surface expression of CD14 was analysed by flow cytometry. In parallel, immunostaining for TLR2 and TLR4 was performed. Therefore, HIEC were incubated with highly specific antibodies directed against TLR2 or TLR4 (1:1000 each, all Biocarta) for 60 minutes at room temperature. Thereafter, a secondary FITC labelled antimouse antibody was used (30 minutes at room temperature) to permit visualisation and subsequent quantification of the receptor expression by immunofluorescence and flow cytometry. The effect of LPS (0.1–10 μg/ml, 24–48 hours) on TLR2 and TLR4 expression was quantified by flow cytometry, as described above.
Statistical analysis
Results are reported as the mean (SEM) of triplicate samples. Significance was established at 95%, and determined by Student’s t test for non-paired values, and the Mann-Whitney U test for non-parametric values.
RESULTS
Effect of LPS on IEC growth
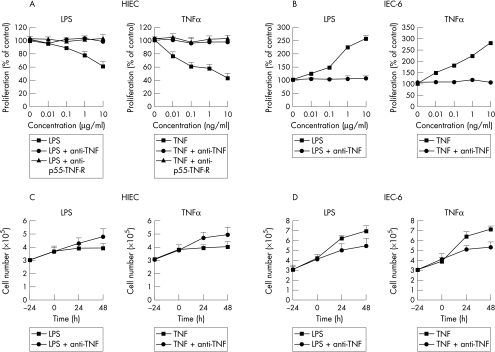
LPS proved to be a potent inhibitor of HIEC growth in a dose dependent manner. After a 22 hour stimulation period, HIEC proliferation rate was maximally reduced to 62 (7)% in comparision with control wells at 10 μg/ml LPS, with a plateau effect at higher concentrations (fig 1A ▶). Cell count analyses confirmed the anti-proliferative effect of LPS in HIEC (fig 1B and C ▶). In contrast with this growth inhibitory effect on the HIEC line, the same LPS preparation significantly stimulated proliferation in the rat cell line IEC-6 (fig 1B and D ▶). This effect was dose dependent with a maximal proliferation rate of 256 (12)% compared with unstimulated control cells.
Figure 1.
Effect of LPS and TNFα on HIEC and IEC-6 cell proliferation, analysed by 3H-thymidine incorporation (A) and (B) and cell counts (C) and (D). LPS significantly inhibited HIEC growth in a dose dependent manner, similar to TNFα. Blockade of the p55-TNF receptor with an antagonistic antibody completely reversed the antiproliferative effect of TNFα as well as LPS. In addition, neutralisation of biological active TNFα with a specific anti-TNFα antibody totally inhibited the antiproliferative effect of LPS. In contrast, IEC-6 cell growth was significantly stimulated by LPS, similar to TNFα. Neutralisation of the biological active TNFα with a highly specific rat anti-TNFα antibody completely reversed this growth stimulation by LPS, similar to the effect in HIEC. These data clearly demonstrate that TNFα is an important mediator of IEC growth modulation by LPS. Basal proliferation rate in HIEC were 1214 (49) cpm (=100 (4)% control), in IEC-6 cells: 6873 (344) cpm (=100 (5)% control). Experiments are mean of four similar experiments in triplicates. Cell counts experiments were repeated twice and the means are shown.
TNFα as mediator of LPS growth modulation
LPS is known to be a strong inducer of proinflammatory cytokines and chemokines, which can interfere with the regulation of intestinal epithelial cell growth. Therefore, we tested the hypothesis that the growth modulatory effects of LPS in HIEC and IEC-6 cells was mediated by one of these cytokines induced by LPS. In previous studies,23 we observed a potent growth stimulatory effect of TNFα in IEC-6 cells. In contrast, HIEC growth was significantly inhibited by TNFα even at concentrations as low as 0.01 ng/ml (fig 1 ▶). Receptor analyses with blocking antibodies revealed that this growth inhibitory effect on HIEC was mediated by the p55-TNF receptor (fig 1 ▶). Given the similarities of the effects of LPS and TNFα on HIEC (anti-proliferative) and IEC-6 cells (growth stimulatory), it is very probable that these growth modulatory effects of LPS were mediated by LPS inducible TNFα in an autoparacrine/parcrine manner. To confirm this hypothesis, the biological activity of TNFα was neutralised with highly specific antibodies. As shown in figure 1 ▶, in the presence of a neutralising anti-TNFα antibody the growth inhibitory effect of LPS on HIEC was completely abolished. Using rat specific anti-TNFα antibodies, similar results were obtained with IEC-6 cells (fig 1 ▶). As complementary approach, the p55-TNF receptor was selectively blocked, using an antagonistic antibody before the stimulation with LPS. As shown in figure1 (A) and (B), the antiproliferative LPS effect in HIEC was completely blocked after inhibition of this TNF receptor.
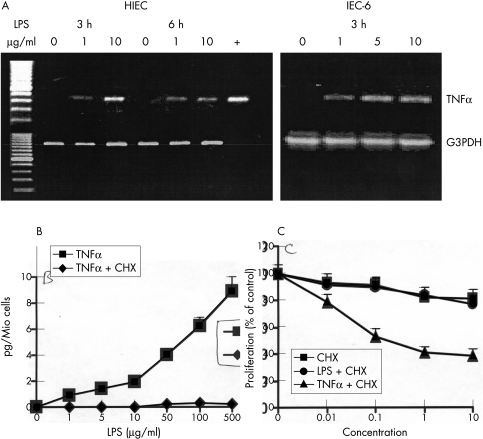
In the next step, to further confirm these findings, we wanted to know if IEC really produce TNFα in response to LPS stimulation. No TNF transcripts were detectable in unstimulated HIEC or IEC-6 cells. However, LPS rapidly induced mRNA expression of TNFα, achieving highest levels within three hours of its addition to HIEC (fig 2 ▶). Similarly, TNFα mRNA was inducible in IEC-6 cells, and it remained upregulated up to six hours after LPS stimulation. Subsequently, after translation into its protein product, TNFα was secreted in the cell culture medium and it became detectable by ELISA (fig 2B ▶). No production of TNFα was observed in response to LPS when HIEC were treated with low doses (1–10 mg/ml) of the protein synthesis inhibitor cycloheximide (CHX). Therefore, we used this experimental setting to further confirm the role of TNF as mediator of LPS in the growth modulation of IEC. The proliferation rate of HIEC was analysed after stimulation with low doses of CHX and LPS. CHX alone moderately inhibited HIEC growth (81 (7)% compared with 100 (6)% in control wells). However, LPS did not further down-regulate HIEC proliferation in the presence of CHX, whereas addition of recombinant TNFα inhibited HIEC growth for an additional 43% (fig 2C ▶). Experiments with CHX were not performed in IEC-6 cells, as they rapidly died, even in the presence of low doses of CHX. Additional experiments with recombinant IL6 or IL8, also produced by IEC-6 and HIEC after stimulation with LPS, showed in both models that none of these two cytokines was able to modulate intestinal epithelial cell growth (data not shown).
Figure 2.
Stimulation of HIEC/IEC-6 cells with LPS induced TNFα, as demonstrated by RT-PCR (A). Already after a stimulation period of three hours TNFα mRNA transcripts were observed in both IEC models. The expression of G3PDH was used as house keeping gene. The translation into the protein product and subsequent secretion of TNFα into the culture medium was analysed in HIEC using a high sensitivity ELISA (B). TNFα was produced in a dose dependent manner after stimulation with LPS. CHX treatment completely suppressed the secretion of TNFα. In keeping, functional experiments with CHX showed in HIEC that the proliferation rate after LPS stimulation remained unchanged once TNFα production was suppressed (C). On the other hand, addition of TNFα in the presence of CHX was still able to suppress HIEC proliferation. Concentrations (x axis) for CHX or LPS in μg/ml, for TNFα in ng/ml. The concentrations of CHX used together with LPS or TNFα were 1 μg/ml.
Effect of LPS on HIEC/IEC-6 cell apoptosis
At all concentrations tested, LPS failed to induced HIEC or IEC-6 cell apoptosis or necrosis. Morphological analysis revealed a normal nuclear and cellular morphology in LPS treated cells even after prolonged incubation intervals for 48 hours. No expression of phosphatidylserine on the outer leaflet of the membrane was detectable, a sign of early apoptosis. In contrast, TNFα was a weak inducer of HIEC or IEC-6 cell apoptosis, at concentrations of 10 ng/ml and higher, as we recently showed.19,20 After TNF treatment, typical morphological signs of apoptosis, such as nuclear condensation, fragmentation, and the formation of apoptotic bodies as well as the expression of phosphatidylserine were detectable.
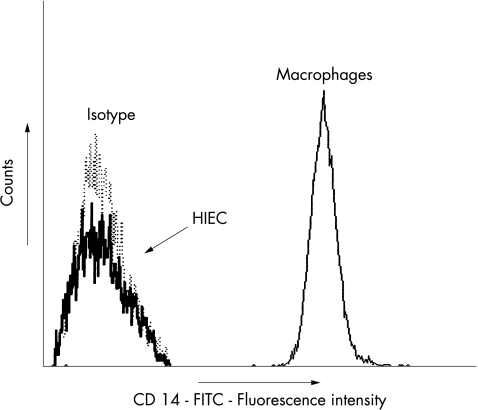
HIEC express no CD14, but TLR2, TLR4 and MD2
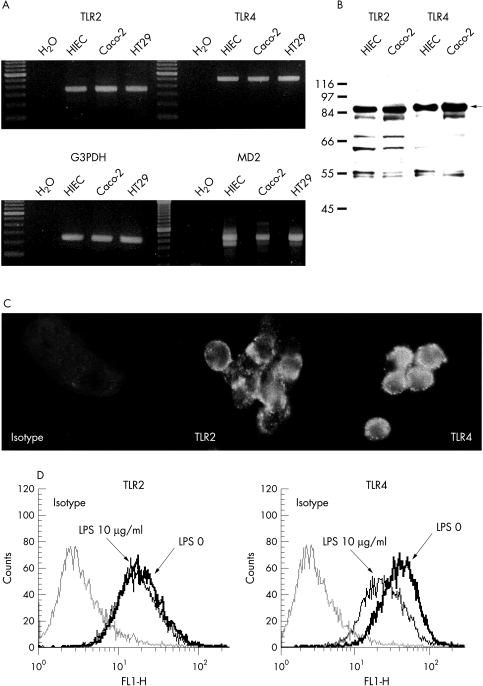
Immunofluorescence studies revealed that in contrast with immune competent cells, HIEC do not express the LPS receptor CD14 (fig 3 ▶). No induction or upregulation of this membrane receptor by LPS or TNFα was observed under all experimental conditions. Recently, with the discovery of the TLR family, a new class of receptors for LPS was proposed.24 In particular, the receptor TLR4 (and less convincingly TLR2) is believed to bind LPS and activate the intracellular signalling cascade.16,17,24 RT-PCR (fig 4A ▶) clearly showed TLR2 and TLR4 expression in HIEC. Similarly, in the human intestinal cancer cell lines Caco-2 and HT-29, mRNA transcripts for both receptors were detected. In HIEC, both PCR products were completely sequenced confirming that sequences were identical to TLR2 and TLR4, respectively. In the next step, the translation into the corresponding protein product was analysed using western blot analysis. HIEC, as well as Caco-2 cells clearly expressed TLR2 and TLR4 (fig 4B ▶). In addition, surface expression of TLR2 and TLR4 on HIEC was confirmed by immunofluorescence (fig 4C ▶). Constitutive expression of TLR4 was higher compared with TLR2 in HIEC. Analysis of the receptor expression revealed, that LPS stimulation did not change TLR2 expression in HIEC. However, a significant downregulation of TLR4 occurred to 71 (3)% compared with 100 (4) in control cells (fig 4D ▶). Activation of TLR4 by LPS requires the presence of additional molecules: MD2, a recently described secretory protein, seems to play an important part to obtain a functional TLR4 signalling complex.24 As shown in figure 4A ▶ HIEC clearly expressed MD2, indicating a potentially fully functional TLR4 signalling complex in IEC.
Figure 3.
Flow cytometric analysis revealed no surface expression of CD 14 on HIEC in contrast with normal human macrophages. One of three representative experiments is shown. The x axis is an arbitrary log scale of CD14 fluorescence.
Figure 4.
TLR expression in HIEC: RT-PCR analysis clearly showed TLR2 and TLR4 mRNA transcripts in unstimulated HIEC as well as Caco-2 and HT29 cells (A). Subsequent isolation and sequencing confirmed the identity of TLR2 and TLR4. In addition, all cell lines expressed MD2, a costimulatory molecule associated with TLR4. Western blot analysis (B) confirmed that HIEC express TLR2 (86 kDa) as well as TLR4 (88 kDa). Caco-2 cells served as positive control. In addition, immunofluorescence analysis (C) revealed a clear signal of membrane expressed TLR2 and TLR4 on native HIEC. The regulation of the expression of TLR2 and TLR4 by LPS was analysed by flow cytometry (D). TLR4 expression was higher in unstimulated HIEC compared with TLR2. LPS (10 μg/ml, 48 hours) significantly reduced TLR4 expression without changing TLR2 expression in HIEC. One of three representative experiments is shown.
DISCUSSION
There is increasing evidence that bacterial toxins and antigens such as LPS (or endotoxin) play an important role in the pathogenesis of IBD. Previous studies have concentrated on the effects of bacterial products on immune cells. In this study, we show for the first time that LPS is a direct modulator of intestinal epithelial cell turnover. LPS significantly inhibited HIEC proliferation without inducing apoptosis or necrosis. However, the effect on the rat intestinal cell line IEC-6 cells, was in clear contrast with a marked growth stimulation in response to LPS. As demonstrated by functional assays, on RNA and protein levels, endogenously produced TNFα is an autopanacrine/paracrine mediator of this LPS action. This is in concert with our previous observation that TNFα stimulates IEC-6 cell proliferation,23 whereas HIEC growth is inhibited by TNF. The different growth pattern observed between the human HIEC and the rat IEC-6 crypt cells may reflect, besides the species difference, a differing degree of maturation of either cell line. This indicates that depending on the position along the crypt to villus axis, the same cytokine can exert different biological effects on IEC, potentially resulting in a more growth stimulatory effect on immature cells. This could be one of the mechanisms leading to crypt hyperplasia seen in IBD.25
In enterocytes, LPS induces a whole array of signalling events leading to the production of various cytokines and chemokines.12–14 However, in contrast with TNFα we did not observe any changes of enterocyte turnover by IL6 or IL8 despite the fact that HIEC produce up to 1000-fold higher concentrations of either factor compared with TNFα. All three factors are potent proinflammatory immune regulators in the intestinal mucosa. They help to recruit immune competent cells to the site of mucosal inflammation, greatly increasing the primary inflammatory reaction through chemoattractant effects. In the past, proinflammatory factors secreted from these newly recruited, activated immune competent cells were believed to cause the pathological intestinal epithelial cell turnover.25 In this study, we confirmed and extended this view, providing evidence that bacterial products such as LPS directly affect the intestinal epithelium further contributing to an impaired turnover state through autopancrine/paracrine effects.
Recently, Kim et al26 observed that infection of IEC with invasive bacteria induced apoptosis. This effect shown in HT-29 and Caco-2 tumour cells, was also—at least partially—mediated via endogenously produced TNFα, confirming the importance of this cytokine as mediator of LPS in other experimental models of inflammation. TNFα is known to induce apoptosis in many cell models, also in HIEC or IEC-6 cells, as we recently demonstrated.19,20 However, relatively high concentrations of TNFα (10–100 ng/ml) were required to induce IEC apoptosis. In this study, we did not observe any apoptosis in HIEC or IEC-6 cells after stimulation with LPS. This might be attributable to rather low levels of endogenously produced TNFα, (about 10pg/ml in our model compared with 110 pg/ml in the study of Kim et al26) insufficient to stimulate the apoptotic machinery, but high enough to change IEC growth. TNFα production and secretion of IEC in response to bacterial products seem to depend on the experimental conditions as well as the specific stimuli used. For instance, Panja et al27 did not observe any TNFα production in response to LPS in freshly isolated intestinal epithelial cells.
LPS is a very potent inducer of monocyte and mast cell TNFα production via CD 14, the receptor for LPS.15 In this study, we observed that immature IEC react in a very similar way to LPS as do immune competent cells with increased TNFα and other cytokine production. However, under basal conditions, IEC do not express CD14. Furthermore, we were not able to induce CD14 expression on HIEC after stimulation with LPS or TNFα. However, LPS exerted potent biological effects on enterocytes, as shown in this and other studies.26–28 These data are in concert with the previous observation of Pugin et al28 showing that soluble CD14 along with LPS binding protein, both present in serum, permit binding and uptake of LPS to CD14 negative cells, such as IEC. Recently, several TLR based on the homology to the Drosophila proteins, were described as membrane receptors to LPS.24 Cario et al29 reported that intestinal epithelial cells express the TLR2, TLR3, and TLR4 in a varying degree. After stimulation of the tumorous transformed IEC lines T84 and HT-29 activation of stress pathways MAPkinases, JNK, and p38 occurred in response to LPS. In addition a clear activation of the signalling pathway NFκB was observed, leading to an upregulation of various inflammatory genes such as IL6, IL8, or TNFα. In this study we were able to show that non-transformed IEC constitutively express TLR2 and TLR4. Whereas TLR2 is more likely to serve as receptor for mycobacterial antigens or peptidoglycans and lipoproteins present on Gram positive bacteria, there is increasing evidence that TLR4 is the sole LPS receptor.24,30,31 Besides CD14 and LPS binding protein, a fully functional TLR4 signalling complex requires the presence of an additional, novel protein, called MD2.32 MD2 is a secreted protein that binds to the extracellular domain of TLR4, thereby potentially stabilising the formation of TLR4 dimers. In addition, MD2 seems to facilitate LPS responsiveness. In contrast with the recent report of Abreu et al22using tumoral transformed IEC, we clearly observed MD2 expression in HIEC, indicating that this signalling pathway is fully functional in normal IEC. It is important to note that LPS downregulated its receptor TLR4 in HIEC. This observation is in keeping with the recent report of Nomura and coworkers33 who showed that the molecular mechanism of endotoxin tolerance in macrophages is via LPS induced downregulation of TLR4. Therefore, in vivo, because of a chronic exposure to LPS, TLR4 might be downregulated on enterocytes avoiding a pathological stimulation and inflammatory reaction. However, under specific pathological conditions, TLR4 might be upregulated (as we recently observed in human enterocytes stimulated with IL1β, unpublished data), leading to a markedly increased responsiveness of enterocytes to LPS and an activation of the inflammatory cascade.
TNFα is considered to be a key mediator in the pathogenesis of IBD. This hypothesis is based on several in vitro models as well as increased TNFα mRNA and protein levels in the intestinal mucosa of IBD patients as well as in their stool compared with non-IBD and healthy controls.20,34–36 The encouraging positive results of two recent clinical trials with anti-TNF antibodies to treat relapsing CD patients further support the importance of this cytokine.37,38 However, the exact mechanisms by which TNFα participates in mucosal injury remain unclear.
The findings of this study add a novel aspect to the complex picture of immune cell-epithelial cell interactions in the intestinal mucosa. Enterocytes are known to react upon contact with bacterial products by secreting chemoattractant cytokines that may start and perpetuate the inflammatory response. Here, we demonstrate they are also capable of producing inflammatory cytokines, such as TNFα, which is attributed to immune competent cells. The data of this study led us to the hypothesis that the interaction of LPS with TLR4/MD2 contributes to the perpetuation of the inflammatory epithelial cell injury via TNFα induced alterations of enterocyte turnover in an autoparacrine/paracrine manner.
Acknowledgments
This study was supported by research grants from the Deutsche Forschungsgemeinschaft Bonn, Germany, (to FMR, Ru 694 3–1) and INSERM, Paris, France. We acknowledge the excellent technical assistance of B Begue, T Gross, T Rottmann, and S Schwartz.
Abbreviations
IBD, inflammatory bowel disease
CD, Crohn’s disease
UC, ulcerative colitis
LPS, lipopolysaccharide
IL, interleukin
TLR, toll-like receptors
IEC, intestinal epithelial cells
FCS, fetal calf serum
DNEM, Dulbecco’s modified Eagle’s medium
CHX, cycloheximide
REFERENCES
- 1.Dianda L, Hanby AM, Wright NA, et al. T cell receptor-alpha beta-deficient mice fail to develop colitis in the absence of a microbial environment. Am J Pathol 1997;150:91–7. [PMC free article] [PubMed] [Google Scholar]
- 2.Schultz M, Tonkonogy SL, Sellon RK, et al. IL-2-deficient mice raised under germfree conditions develop delayed mild focal intestinal inflammation. Am J Physiol 1999;276:G1461–72. [DOI] [PubMed] [Google Scholar]
- 3.Sellon RK, Tonkonogy S, Schultz M, et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun 1998;66:5224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology 1998;115:182–205. [DOI] [PubMed] [Google Scholar]
- 5.Rutgeerts P, Geboues K, Peeters M, et al. Effect of fecal stream diversion on recurrence of Crohn’s disease in the neoterminal ileum. Lancet 1991;ii:771–4. [DOI] [PubMed] [Google Scholar]
- 6.Gardiner KR, Halliday MI, Barclay GR, et al. Significance of systemic endotoxaemia in inflammatory bowel disease. Gut 1995;36:897–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Svanborg C, Godaly G, Hedlund M. Cytokine responses during mucosal infections: role in disease pathogenesis and host defense. Curr Oppin Microbiol 1999;2:99–105. [DOI] [PubMed] [Google Scholar]
- 8.Parrilo JE. Pathogenetic mechanisms of septic shock. N Engl J Med 1993;328:1471. [DOI] [PubMed] [Google Scholar]
- 9.Salkowski CA, Detore G, McNally R, et al. Regulation of inducible nitric oxide synthase messenger RNA expression and nitric oxide production by lipopolysaccharide in vivo. J Immunol 1997;158:905–12. [PubMed] [Google Scholar]
- 10.Kimura H, Sawada N, Tobioka H, et al. Bacterial lipopolysaccharide reduced intestinal barrier function and altered localization of 7H6 antigen in IEC6-rat intestinal crypt cells. J Cell Physiol 1997;171:284–90. [DOI] [PubMed] [Google Scholar]
- 11.Unno N, Wang H, Menconi MJ, et al. Inhibition of inducible nitric oxide synthase ameliorates endotoxin induced gut mucosal barrier dysfunction in rats. Gastroenterology 1997;113:1246–57. [DOI] [PubMed] [Google Scholar]
- 12.McGee DW, Bamberg T, Vitkus JD, et al. A synergistic relationship between TNF-α, IL-1β and TGF-β1 on IL-6 secretion by the IEC-6 intestinal epithelial cell line. Immunology 1995;86:6–11. [PMC free article] [PubMed] [Google Scholar]
- 13.Schuerer-Maly CC, Eckmann L, Kagnoff MF, et al. Colonic epithelial cell lines as a source of interleukin-8: stimulation by inflammatory cytokines and bacterial lipopolysaccharide. Immunology 1994;81:85–91. [PMC free article] [PubMed] [Google Scholar]
- 14.Stadnyk AW. Cytokine production by epithelial cells. FASEB J 1994;8:1041–7. [DOI] [PubMed] [Google Scholar]
- 15.Wright SD, Ramos RA, Tobias PS, et al. CD 14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 1990;249:1431–3. [DOI] [PubMed] [Google Scholar]
- 16.Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 1998;282:2085–8. [DOI] [PubMed] [Google Scholar]
- 17.Yang RB, Mark MR, Gray A, et al. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signaling. Nature 1998;395:284–8. [DOI] [PubMed] [Google Scholar]
- 18.Perreault N, Beaulieu JF. Use of the dissociating enzyme thermolysin to generate viable human normal intestinal epithelial cell cultures. Exp Cell Res 1996,224:254–64. [DOI] [PubMed] [Google Scholar]
- 19.Ruemmele FM, Russo P, Beaulieu J-F, et al. Susceptibility to FAS-induced apoptosis in human non-tumoral enterocytes - the role of co-stimulatory factors. J Cell Physiol 1999;181:45–54. [DOI] [PubMed] [Google Scholar]
- 20.Ruemmele FM, Dionne S, Levy E, et al. TNFα-induced IEC-6 cell apoptosis requires activation of ICE-caspases whereas complete inhibition of the caspase-cascade led to necrotic cell death. Biochem Biophys Res Commun 1999;260:159–66. [DOI] [PubMed] [Google Scholar]
- 21.Faure E, Equils O, Sieling PA, et al. Bacterial lipopolysaccharide activates NF-kappaB trough toll-like receptor 4 (TLR4) in cultured human dermal endothelial cells. J Biol Chem 2000;275:11058–63. [DOI] [PubMed] [Google Scholar]
- 22.Abreu A, Vora P, Faure E, et al. Decreased expression of toll-like eeceptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J Immunol 2001;167:1609–17. [DOI] [PubMed] [Google Scholar]
- 23.Dionne S, D’Agata ID, Ruemmele FM, et al. Tyrosine kinase and MAPK inhibiton of TNF alpha and EGF stimulated IEC-6 cell growth. Biochem Biophys Res Commun 1998;242:146–50. [DOI] [PubMed] [Google Scholar]
- 24.Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature 2000;406:782–7. [DOI] [PubMed] [Google Scholar]
- 25.Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology 1998;115:182–205. [DOI] [PubMed] [Google Scholar]
- 26.Kim JM, Eckmann L, Savidge TC, et al. Apoptosis of human intestinal epithelial cells after bacterial invasion. J Clin Invest 1998;102:1815–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panja A, Goldberg S, Eckmann L, et al. The regulation and functional consequence of pro-inflammatory cytokine binding on human intestinal epithelial cells. J Immunol 1998;161:3675–84. [PubMed] [Google Scholar]
- 28.Pugin J, Schürer-Maly C, Leturcq D, et al. Lipopolysaccharide activation of human endothelial and epithelial cells is mediated by lipopolysaccharide-binding protein and soluble CD14. Proc Natl Acad Sci USA 1993;90:2744–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cario E, Rosenberg IM, Brandwein Sl, et al. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J Immunol 2000;164:966–72. [DOI] [PubMed] [Google Scholar]
- 30.Tapping RI, Akashi S, Miyake K, et al. Toll-like receptor 4, but not tToll-like receptor 2, is a signaling receptor for Escherichia and Salmonella lipopolysaccharides. J Immunol 2000;165:5780–7. [DOI] [PubMed] [Google Scholar]
- 31.Beutler B. TLR4 : central component of the sole mammalian LPS sensor. Curr Opin Immunol 2000;12:20. [DOI] [PubMed] [Google Scholar]
- 32.Shimazu R, Akashi H, Ogata F, et al. MD-2, a molecule that confers lipopolysaccharide responsiveness in toll-like receptor 4. J Exp Med 1999;189:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nomura F, Akashi S, Sakao Y, et al. Cutting edge: endotoxin tolerance in mouse peritoneal macrophages correltes with down-regulation of surface toll-like receptor 4 expression. J Immunol 2000;164:3476–9. [DOI] [PubMed] [Google Scholar]
- 34.Stevens C, Walz G, Singavam C, et al. Tumor necrosis factor-alpha, interleukin-1 beta and interleukin-6 expression in inflammatory bowel disease. Dig Dis Sci 1992;37:818–26. [DOI] [PubMed] [Google Scholar]
- 35.MacDonald TT, Huchings P, Choy MY, et al. Tumor necrosis factor-alpha and interferon-gamma production measured at the single-cell level in normal and inflammed human intestine. Clin Exp Immunol 1990;81:301–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Braegger CP, Nicholls S, Murch SH, et al. Tumor necrosis factor alpha in stool as a marker of intestinal inflammation. Lancet 1992;339:89–91. [DOI] [PubMed] [Google Scholar]
- 37.van Dullemen H, van Deventer SJ, Hommes DW, et al. Treatment of Crohn’s disease with anti-tumor necrosis factor chimeric monoclonal antibody (cA2). Gastroenterology 1995;109:129–35. [DOI] [PubMed] [Google Scholar]
- 38.Targan SR, Hanauer SB, van Deventer SJ, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. N Engl J Med 1997;337:1029–35. [DOI] [PubMed] [Google Scholar]