Abstract
Background: Injection of water into the pharynx at a threshold volume induces vocal cord adduction—the pharyngoglottal closure reflex (PGCR). This reflex together with other supraoesophageal reflexes may be helpful in preventing aspiration. Cigarette smoking has an adverse affect on the pharyngo-upper oesophageal sphincter contractile reflex and reflexive pharyngeal swallow. The effect of smoking on PGCR has not been studied previously.
Aims: To elucidate the effect of chronic and acute cigarette smoking on PGCR.
Subjects: We studied 10 chronic smokers and 10 non-smokers before and after real/simulated smoking, respectively.
Methods: Using concurrent recordings, glottal function was monitored by video endoscopy, swallowing by electromyography, and PGCR was triggered by rapid and slow pharyngeal water injections.
Results: The threshold volume to trigger PGCR during rapid injection was significantly higher in chronic smokers (non-smoker 0.20 (SEM 0.02) ml, smoker 0.36 (0.02) ml; p<0.001). In six of 10 smokers, acute smoking abolished this reflex during slow water injection.
Conclusions: Smoking adversely affects stimulation of PGCR. This finding may have implications in the development of reflux related respiratory complications in smokers.
Keywords: smoking, supraoesophageal reflexes, pharyngoglottal closure reflex, reflexive pharyngeal swallow, airway protection, gastro-oesophageal reflux
Several reflexes have been proposed to protect the airways during retrograde transit of gastric contents.1–13 For example, distention of the oesophagus can trigger the oesophago-upper oesophageal sphincter (UOS) contractile reflex1–3 and the resulting enhanced UOS pressure may prevent entry of oesophageal contents into the pharynx. Entry of refluxate into the pharynx can also enhance UOS pressure by triggering the pharyngo-UOS contractile reflex4–6 and this in turn may protect against further oesophagopharyngeal reflux. At another level, fluid in the pharynx can trigger closure of the tracheal introitus by stimulating the pharyngoglottal closure reflex (PGCR)7 and the reflexive pharyngeal swallow. Reflexive pharyngeal swallow will not only close the tracheal introitus but also clear the pharynx of any residual fluid.8–11 Distention of the oesophagus also results in adduction of the vocal cords, the oesophagoglottal closure reflex.12,13 Studies have shown that cigarette smoking or nicotine can alter lower oesophageal sphincter pressure, gastric emptying, and oesophageal acid clearance.14–22 These effects of smoking/nicotine on upper gastrointestinal functions may predispose to gastro-oesophageal reflux disease. Integrity of the supraoesophageal reflexes may be important to protect the airways against aspiration injury in smokers. In an earlier study, we showed that cigarette smoking adversely affected triggering of the pharyngo-UOS contractile reflex and reflexive pharyngeal swallow.23 The aim of the present study was to elucidate the effect of smoking on PGCR.
METHODS
We studied 10 healthy non-smokers (mean age 38 (SD 8) years; five male) and 10 healthy chronic smokers (39 (9) years; six male) in the sitting upright position. The Human Research Review Committee of the Medical College of Wisconsin approved the protocol, and subjects gave informed consent before their studies. Smokers were defined as those with a history of smoking one or more packs of cigarettes per day for at least two years. Non-smokers were those who never smoked or occasionally smoked but stopped over two years ago. Smokers were instructed to abstain from smoking for 12 hours prior to the study, following which 5 ml of blood were drawn to measure serum nicotine levels to ascertain compliance. Serum nicotine concentrations were determined using a modification of the gas chromatography-mass spectrometry method described by Feyerbend and Russel.24 Smokers were then studied before and 10 minutes after smoking two cigarettes (tar yield of 16 per cigarette). The 10 minute interval after smoking was given to allow the pharyngeal temperature to return to baseline. Elicitation of PGCR with incremental volumes of water injections (rapid and slow) until the end point (reflexive pharyngeal swallow) usually took approximately 30–40 minutes. Non-smokers were studied before and 10 minutes after simulated smoking of two unlit cigarettes. Duration of real/simulated smoking in each volunteer was kept to 15 minutes.
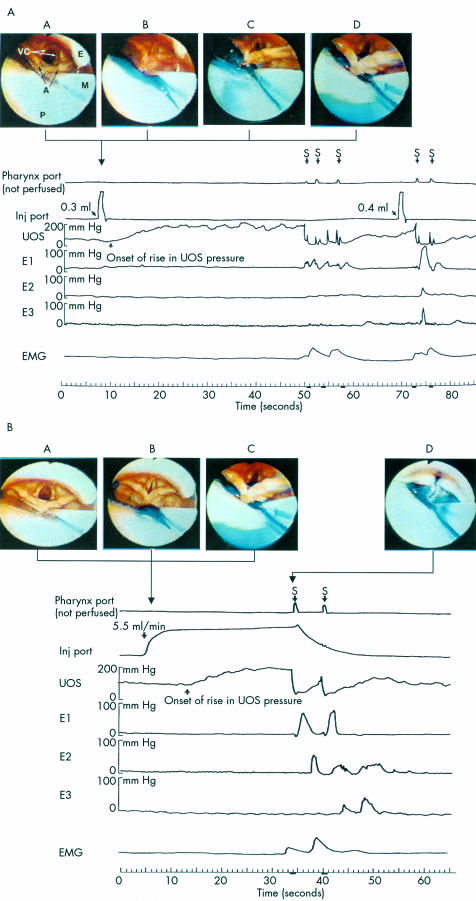
Glottal function and its response to pharyngeal water stimulation was monitored using a 5.3 mm diameter endoscope (N30; 100 degree angle of vision; Olympus, Lake Success, New York, USA) that was passed transnasally and positioned behind the uvula just above the isthmus where the oral cavity joins the pharynx. In this position, the base of the tongue, epiglottis, vallecula, pyriform sinuses, laryngeal vestibule, vocal cords, arytenoids, and hypopharynx were visualised (fig 1A ▶, B). Endoscopic images were recorded on a super-VHS tape at 60 fields/30 frames/second using a super VHS video recorder (Sony, Tokyo, Japan) for subsequent analysis in real time, slow motion, and frame by frame. To prevent the possibility of anaesthetising the pharynx, the nasal passage was lubricated by a non-anaesthetic jelly (Surgilube, E Fougera & Co., Atlanta Inc.; Melville, New York, USA) applied with a cotton swab.
Figure 1.
(A) Example of a pharyngoglottal closure reflex during rapid pharyngeal water injection. E, posterior aspect of the epiglottis; VC, vocal cords; A, arytenoids; P, posterior pharyngeal wall; M, manometry catheter; E1, E2, E3, perfusion ports 0, 7, and 14 cm below the upper oesophageal sphincter (UOS) sleeve, respectively; S, swallow; EMG, submental surface electromyography (EMG) recording. Video frame A: Vocal cord position immediately prior to rapid intrapharyngeal injection of water. Video frame B: Injection of 0.3 ml of water resulted in an abrupt complete closure of the vocal cords together with more than 100% increase in UOS pressure over baseline (pharyngo-UOS contractile reflex). Video frame C: Vocal cord partially abducted. Video frame D: Vocal cords returning to resting position 0.5 seconds after injection. These responses were not associated with any EMG activity of the submental muscles. Injection of 0.4 ml of water into the pharynx resulted in a reflexive pharyngeal swallow. (B) Example of pharyngoglottal closure reflex during slow pharyngeal water injection. Video frame A: Vocal cord position immediately prior to slow intrapharyngeal injection of water. Video frame B: Intrapharyngeal injection of water at the slow rate of 5.5 ml/min resulted in incomplete closure of the vocal cords together with more than 100% increase in UOS pressure over baseline. Video frame C: Vocal cord partially abducted. Similar to rapid water injection, these responses were not associated with any EMG activity of the submental muscles. Video frame D: Reflexive pharyngeal swallow was triggered 27 seconds after the onset of pharyngeal water perfusion.
To stimulate the pharynx, we used the water injection method, as described previously.7,23 An UOS sleeve assembly (6×0.6×0.4 cm) (Dentsleeve, Adelaide, Australia) was used that had an injection port located 2 cm proximal to the sleeve device and recording ports at the proximal and distal ends of the sleeve for manometric positioning. It also incorporated two oesophageal ports located 7 and 14 cm below the UOS. The sleeve assembly was passed transnasally and positioned within the UOS such that the injection port (the distal pharyngeal port, immediately above the sleeve segment) was oriented posteriorly and situated 2 cm above the UOS high pressure zone. This posterior orientation was verified using the endoscopic views during injections. Our previous study had shown7 that water injected at this location does not splash into the larynx. As the UOS sleeve conforms to that of the UOS opening, once in position, the sleeve prevents axial rotation of the UOS catheter. As a result, the pharyngeal water injection port is always maintained in the posterior orientation. The pharyngeal, oesophageal, and sleeve ports were connected to pressure transducers in line with a minimally compliant pneumohydraulic perfusion system (Arndorfer Medical Specialties, Greendale, Wisconsin, USA). With this arrangement, the onset and offset of water injection, UOS pressure, and oesophageal pressures were recorded on a chart paper that was run at a speed of 50 mm/s. After the sleeve assembly was positioned, to avoid stimulation of swallowing, pharyngeal ports (including the top of the sleeve) were not perfused. Studies were then performed following a 10 minute adaptation period.
As described in our previous study,23 the pharynx was stimulated by injecting graded volumes of water at room temperature through the injection port. To simulate gastro-oesophageal refluxate entering the pharynx either rapidly or slowly,25 we tested two modes of fluid delivery into the pharynx: rapid water injection and slow continuous water injection. Rapid water injection was performed by rapidly injecting water using a hand held syringe attached to the injection port. We started with 0.05 ml, followed by 0.1 ml of water, and then increased the volume by 0.1 ml increments until an irrepressible swallow occurred (reflexive pharyngeal swallow, fig 1A ▶). Slow continuous perfusion was performed at a rate of 5.5 ml/minute using a Harvard infusion pump (model N0975; Harvard Apparatus Co. Inc., Dover, Massachusetts, USA) until an irrepressible swallow occurred (fig 1B ▶). Prior to each injection (rapid or slow), subjects were asked to swallow to clear the pharynx and then withhold swallowing until an irrepressible urge induced swallowing. Each injection was performed after the UOS pressure stabilised at baseline and at mid inspiration. Each volume was repeated three times. For better endoscopic visualisation, we added blue food dye to the water.
A swallow occurrence was documented by submental surface EMG of the mylohyoid/geniohyoid muscle group, characteristic UOS deglutitive relaxation and, in addition, subjects signalled swallowing using a hand held marker that marked the chart paper when activated. All of the above were concurrently recorded on the polygraph (Grass Instrument, Quincy, Massachusetts, USA) chart paper. Video endoscopic and manometric recordings were synchronised using a specially designed timer (Thalner Electronics, Ann Arbor, Michigan, USA) that encoded digital time in hundredths of a second on each video image and simultaneously marked the polygraph paper tracing at one second intervals. The timing of events was determined by direct reading of the digital time displayed on each video frame and the one second timer marks on the polygraph paper that was run at the speed of 50 mm/s, providing an equivalent of 0.02 seconds for each 1 mm distance on the paper.
Using the above technique the following observations were made: (1) onset and offset of pharyngeal water injection, (2) distribution of the injected water within the pharynx, (3) contact or lack thereof of the injected water with the laryngeal structures, (4) movement of the vocal cords prior, during, and following pharyngeal water injection, (5) onset of vocal cord adduction, (6) maximal vocal cord adduction, (7) duration of vocal cord adduction, and (8) onset of vocal cord abduction and their return to the resting position. In each volunteer, we determined, prior to and after real/simulated smoking: (1) presence or absence of PGCR, (2) the smallest volume that consistently triggered PGCR on rapid and slow injections, (3) latent period, defined as the time in seconds from onset of pharyngeal water injection that triggered PGCR to the onset of adduction of the vocal cords, and (4) duration of vocal cord closure.
Within and between groups comparisons were done using paired and unpaired t tests. For data that did not pass the normality test, the Mann-Whitney rank sum test was used. Values are given as mean (SEM) unless stated otherwise.
RESULTS
Video recording analysis in real time, slow motion, and frame by frame revealed that exit of coloured water from the injection port and its distribution within the pharyngeal cavity could be adequately visualised. Onset of entry of coloured water into the pharynx was simultaneous with the onset of injection signal recorded on the chart paper. Coloured water did not make contact with any laryngeal structures during rapid or slow pharyngeal injections.
Pharyngoglottal closure reflex: rapid pharyngeal water injection
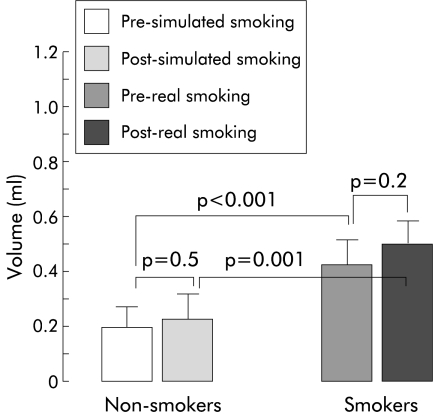
At a threshold volume, rapid injection of water into the pharynx, directed posteriorly, resulted in complete adduction of the vocal cords in all non-smokers and smokers. However, the threshold volume to trigger PGCR by rapid pharyngeal water injection was significantly higher in chronic smokers compared with non-smokers (non-smoker 0.20 (SEM 0.02) ml, smoker 0.36 (0.02) ml; p<0.001) (fig 2 ▶). Acute smoking of two cigarettes by smokers and simulated smoking of unlit cigarette by non-smokers did not significantly increase the threshold volume any further.
Figure 2.
Threshold volume (mean (SEM)) to trigger the pharyngoglottal closure reflex during rapid intrapharyngeal injection of water was significantly higher in chronic smokers compared with non-smokers. The threshold volume to trigger this reflex did not change significantly after acute smoking of two cigarettes.
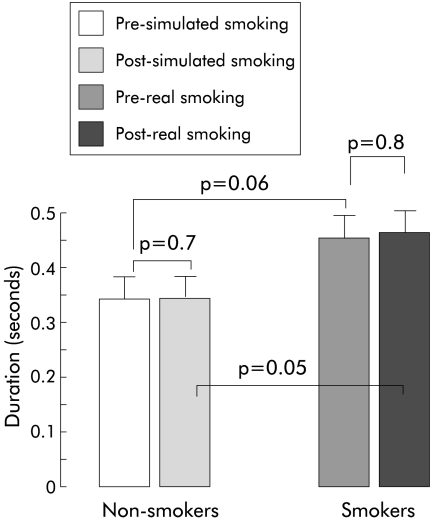
The duration between onset of rapid pharyngeal water injection and onset of vocal cord adduction—that is, latent period—did not change significantly after real or simulated smoking compared with pre-smoking values (fig 3 ▶). However, the latent period was significantly longer after real smoking in the smoker group compared with after simulated smoking by non-smokers (p=0.05) (fig 3 ▶).
Figure 3.
Duration (latent period) in seconds (mean (SEM)) between onset of rapid intrapharyngeal water injection and onset of vocal cord adduction.
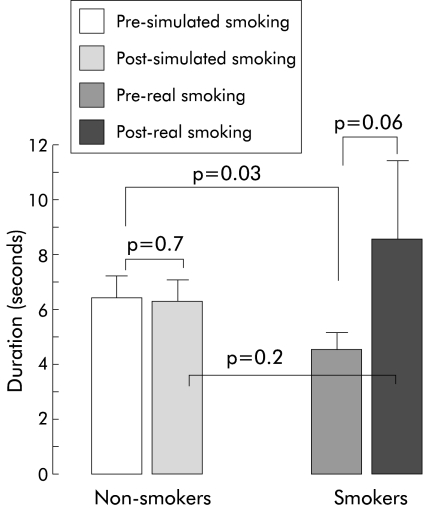
Duration of complete vocal cord adduction elicited by rapid pharyngeal water injections was similar before and after simulated smoking by non-smokers (0.04 (SEM 0.006) seconds and 0.05 (0.004) seconds, respectively; p=0.7). Similarly, there was no difference in this duration before and after real smoking by smokers (0.06 (SEM 0.006) seconds and 0.07 (0.02) seconds, respectively; p=0.5). The difference in duration of complete vocal cord adduction elicited by rapid pharyngeal water injection in smokers and non-smokers did not reach statistical significance (before real/simulated smoking p=0.1, after real/simulated smoking p=0.2).
Pharyngoglottal closure reflex: slow pharyngeal water injection
In both non-smokers and smokers, adduction of the vocal cords elicited by slow pharyngeal water injection was incomplete and the respiratory movements of the cords were preserved. The vocal cords remained partially adducted until a reflexive pharyngeal swallow was elicited.
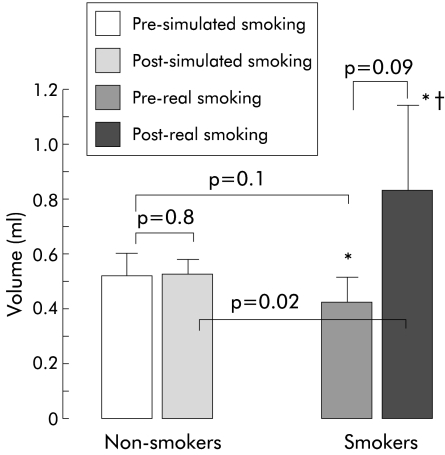
Pharyngoglottal closure reflex elicited by slow pharyngeal water injection was present in all non-smokers before and after simulated smoking. This reflex was absent in one smoker and real smoking of two cigarettes abolished this reflex in an additional six of the 10 smokers.
In those smokers in whom PGCR was elicited by slow water injection, the threshold volume to trigger this reflex was similar before and after real smoking (p=0.09) and there was no significant difference in the threshold volumes between non-smokers and smokers (p=0.1) (fig 4 ▶).
Figure 4.
Slow intrapharyngeal injection of water triggered the pharyngoglottal closure reflex in all non-smokers. This reflex was absent in one smoker pre- and post-smoking (*) and smoking two cigarettes abolished this reflex in an additional six of the 10 smokers (†). Values are mean (SEM).
Duration between onset of slow intrapharyngeal water injection and onset of vocal cord adduction is shown in fig 5 ▶.
Figure 5.
Duration (latent period) in seconds (mean (SEM)) between onset of slow intrapharyngeal water injection and onset of vocal cord adduction.
DISCUSSION
In this study, we determined that cigarette smoking adversely affected elicitation of the pharyngoglottal closure reflex. This finding is in agreement with our previous report on the deleterious effect of smoking on other reflexes originating from the pharynx—that is, pharyngo-UOS contractile reflex and reflexive pharyngeal swallow.23 Pharyngoglottal closure reflex is one of a number of supraoesophageal reflexes that have been proposed to help prevent aspiration of gastric content.
The effects of cigarette smoking/nicotine on parts of the upper digestive tract have been reported previously. These include delaying gastric emptying, decreasing lower oesophageal sphincter pressure, and impairing oesophageal acid clearance.14–22 These alterations may predispose to gastro-oesophageal reflux disease. Anatomical proximity of the inlets of the respiratory and digestive tracts along with the reciprocal interaction of the two tracts in the pharynx can predispose the airways to risks of aspiration when gastric refluxate reaches the pharynx. Although not clearly established, microaspiration is considered to be one of the mechanisms by which gastro-oesophageal reflux disease can cause chronic pulmonary and laryngeal disorders.26–28 Simultaneously monitoring oesophageal and tracheal pH in patients with asthma, Jack and colleagues26 showed that during a 24 hour recording period, of the 37 episodes of oesophageal reflux, each lasting longer that five minutes, five were associated with a decrease in tracheal pH. Peak expiratory flow rates decreased over 10 times more when both oesophageal acid and tracheal acid were present compared with when only oesophageal acid was present. In the presence of compromised pulmonary functions, integrity of the supraoesophaeal reflexes that potentially protect against aspiration may be crucial in smokers. In this study and in our previous study,23 we have now shown that three of these reflexes are adversely affected by smoking. Whether the higher prevalence of acute respiratory tract illness, longer duration of cough, and greater frequency of abnormal auscultatory findings in smokers compared with non-smokers29 is secondary to the direct effect of smoking on the respiratory system, due to microaspiration or both, needs further investigation.
The effect of cigarette smoking on the lower oesophageal sphincter and gastric emptying appears to be systemic in origin as the inhaled cigarette smoke does not come in direct contact with these organs. The mechanism(s) responsible for the deleterious effect of smoking on PGCR reported in this study and on pharyngo-UOS contractile reflex and reflexive pharyngeal swallow reported in our earlier study23 is currently not known. The effect of nicotine or some other component of cigarette smoke can be local or systemic in origin. It is unlikely that pharyngeal temperature changes during smoking could have caused the above effect as we allowed a 10 minute interval between completion of smoking and estimation of threshold volume. Similarly, it is unlikely that the post smoking absence of PGCR in six volunteers during slow pharyngeal water injection could be secondary to subjects being more relaxed or adapted to the manometric catheter during the second half of the study (namely, post smoking period) compared with the first half, as no significant difference in threshold volumes was noted before and after simulated smoking by non-smokers. It is possible that cigarette smoking may alter the concentration and/or function of the pharyngeal sensory nerve endings resulting in a higher threshold volume required to trigger PGCR. Similarly, pharyngeal epithelial changes secondary to the effect of cigarette smoke or its components could also lead to a higher threshold volume. Increased keratinisation can result from smoking and is thought to also explain the lower incidence of recurrent aphthous stomatitis in smokers.30 Nicotine can adversely affect the oesophageal mucosa by producing free radicals resulting in oxidative stress,31 and by inhibiting sodium transport.32 Cigarette smoking may have similar effects on the pharyngeal mucosa leading to alteration in the function of the pharyngeal sensory nerve endings.
In summary, smoking adversely affects the triggering of PGCR. This effect along with the previously observed deleterious effect of smoking on pharyngo-UOS contractile reflex and reflexive pharyngeal swallow23 can further weaken the airway protective mechanisms against aspiration. These findings may have implications in the pathogenesis of reflux related respiratory complications in smokers.
Acknowledgments
This work was supported in part by Merit Review Entry Program, Department of Veterans Affair, and the General Clinical Research Center, Medical College of Wisconsin. Part of this work was presented at Digestive Disease Week and abstracted in Gastroenterology.
Abbreviations
UOS, upper oesophageal sphincter
PGCR, pharyngoglottal closure reflex
EMG, electromyography
REFERENCES
- 1.Reynolds RPE, Effer GW, Bendeck MP. The upper esophageal sphincter in the cat: the role of central innervation assessed by transient vagal blockade. Can J Physiol Pharmacol 1987;65:96–9. [DOI] [PubMed] [Google Scholar]
- 2.Freiman JM, El-Sharkay TY, Diamant NE. Effect of bilateral vagosympathetic nerve blockade on response of the dog upper esophageal sphincter (UES) to intra esophageal distention and acid. Gastroenterology 1981;812:78–84 [PubMed] [Google Scholar]
- 3.Enzman DR, Harell GS, Zboralske FF. Upper esophageal sphincter response to intraluminal distention in man. Gastroenterology 1977;72:1292–8. [PubMed] [Google Scholar]
- 4.Medda BK, Lang IM, Layman RD, et al. Characterization and quantification of a pharyngo-UES contractile reflex in the cat. Am J Physiol 1994;267:G972–83. [DOI] [PubMed] [Google Scholar]
- 5.Ren J, Shaker R, Lang I, et al. Effect of volume, temperature and anesthesia on the pharyngo-UES contractile reflex in humans. Gastroenterology 1995;108:A677. [Google Scholar]
- 6.Shaker R, Ren J, Xie P, et al. Characterization of the pharyngo-UES contractile reflex in humans. Am J Physiol 1997;273:G854–8. [DOI] [PubMed] [Google Scholar]
- 7.Ren J, Shaker R, Dua K, et al. Glottal adduction response to pharyngeal water stimulation: evidence for a pharyngoglottal closure reflex. Gastroenterology 1994;106:A558. [Google Scholar]
- 8.Nishino T, Takizawa K, Yokokawa N, et al. Depression of the swallowing reflex during sedation and/or relative analgesia produced by inhalation of 50% nitrous oxide in oxygen. Anesthesiology 1987;67:995–8. [DOI] [PubMed] [Google Scholar]
- 9.Nishino T. Swallowing as a protective reflex for the upper respiratory tract. Anesthesiology 1993;79:588–601. [DOI] [PubMed] [Google Scholar]
- 10.Shaker R, Ren J, Zamir Z, et al. Effect of aging, position, and temperature on the threshold volume triggering pharyngeal swallows. Gastroenterology 1994;107:396–402. [DOI] [PubMed] [Google Scholar]
- 11.Trifan A, Shaker R, Ren J, et al. Inhibition of resting lower esophageal sphincter pressure by pharyngeal water stimulation in humans. Gastroenterology 1995;108:441–6. [DOI] [PubMed] [Google Scholar]
- 12.Shaker R, Dodds WJ, Ren J, et al. Esophagoglottal closure reflex: a mechanism of airway protection. Gastroenterology 1992;102:857–61. [DOI] [PubMed] [Google Scholar]
- 13.Shaker R, Ren J, Medda B, et al. Identification and characterization of the esophagoglottal closure reflex in a feline model. Am J Physiol 1994;266:G147–53. [DOI] [PubMed] [Google Scholar]
- 14.Scott AM, Kellow JE, Shuter B, et al. Effect of cigarette smoking on solid and liquid intragastric distribution and gastric emptying. Gastroenterology 1993;104:410–16. [DOI] [PubMed] [Google Scholar]
- 15.Johnson RD, Horowitz M, Maddox AF, et al. Cigarette smoking and rate of gastric emptying: effect on alcohol absorption. BMJ 1991;302:20–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kahrilas PJ, Gupta RR. Mechanisms of acid reflux associated with cigarette smoking. Gut 1990;31:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chattopadhyay DK, Greaney MG, Irvin TT. Effect of cigarette smoking on the lower oesophageal sphincter. Gut 1977;18:833–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stanciu C, Bennett JR. Smoking and gastro-oesophageal reflux. BMJ 1972;3:793–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dennish GW, Castell DO. Inhibitory effect of smoking on the lower esophageal sphincter. N Engl J Med 1971;284:1136–7. [DOI] [PubMed] [Google Scholar]
- 20.Rahal PS, Wright RA. Transdermal nicotine and gastroesophageal reflux. Am J Gastroenterol 1995;90:919–21. [PubMed] [Google Scholar]
- 21.Kahrilas PJ, Gupta RR. The effect of cigarette smoking on salivation and oesophageal acid clearance. J Lab Clin Med 1989;114:431–8. [PubMed] [Google Scholar]
- 22.Rattan S, Goyal RK. Effect of nicotine on the lower esophageal sphincter. Gastroenterology 1975;69:154–9. [PubMed] [Google Scholar]
- 23.Dua K, Ren J, Sui Z, et al. Effect of chronic and acute cigarette smoking on the pharyngo-upper oesophageal sphincter contractile reflex and reflexive pharyngeal swallow. Gut 1998;43:537–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feyerabend C, Russel MAH. A rapid gas-liquid chromatographic method for the determination of cotinine and nicotine in biological fluids. J Pharm Pharmacol 1990;42:450–2. [DOI] [PubMed] [Google Scholar]
- 25.Shaker R, Dodds WJ, Hogan WJ, et al. Mechanisms of esophago-pharyngeal acid regurgitation. Gastroenterology 1991;100:A494. [Google Scholar]
- 26.Jack CI, Calverley PM, Donnelly RJ et al. Simultaneous tracheal and oesophageal pH measurements in asthamatic patients with gastro-oesophageal reflux. Thorax 1995;50:201–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hunt JF, Fang K, Malik R, et al. Endogenous airway acidification; implication for asthma pathophysiology. Am J Respir Crit Care Med 2000;161:694–9. [DOI] [PubMed] [Google Scholar]
- 28.Ruth M, Carlsson S, Mansson I, et al. Scintigraphic detection of gastro-pulmonary aspiration in patients with respiratory disorders. Clin Physiol 1993;13:19–33. [DOI] [PubMed] [Google Scholar]
- 29.Aronson MD, Weiss ST, Ben RL, et al. Association between cigarette smoking and acute respiratory tract illness in young adults. JAMA 1982;248:181–3. [PubMed] [Google Scholar]
- 30.Rogers RS. Recurrent aphthous stomatitis: clinical characteristics and associated systemic disorders. Seminars in cutaneous medicine and surgery, vol 16. Philadelphia: WB Saunders, 1997:278–83. [DOI] [PubMed]
- 31.Wetscher GJ, Bagchi D, Perdikis G, et al. In vitro free radical production in rat esophageal mucosa induced by nicotine. Dig Dis Sci 1995;40:853–8. [DOI] [PubMed] [Google Scholar]
- 32.Orlando RC, Bryson JC, Powell DW. Effect of cigarette smoke on esophageal epithelium of the rabbit. Gastroenterology 1986;91:1536–42. [DOI] [PubMed] [Google Scholar]