Abstract
Background: Faecal incontinence occurs in over one third of patients with systemic sclerosis. The aetiology is multifactorial. Conventional treatment is often unsuccessful. Sacral nerve stimulation is a new effective treatment for resistant faecal incontinence.
Aims: To evaluate sacral nerve stimulation in patients with systemic sclerosis.
Patients: Five women, median age 61 years (30–71), with scleroderma associated faecal incontinence were evaluated. All had failed maximal conventional treatment. Median number of preoperative weekly episodes of incontinence was 15 (7–25), median duration of incontinence was five years (5–9), and scleroderma 13 years (4–29).
Methods: All patients were screened with temporary stimulation. Those who benefited underwent permanent implantation. At baseline and after stimulation a bowel diary, the SF-36 quality of life assessment, endoanal ultrasound, and anorectal physiology were performed.
Results: Four patients were continent at a median follow up of 24 months (6–60). One patient failed temporary stimulation and was not permanently implanted. The weekly episodes of incontinence decreased from 15, 11, 23, and 7 to 0. Urgency resolved (median time to defer <1 minute (0–1) v 12.5 minutes (5–15)). Quality of life, especially social function, improved. Endoanal ultrasound showed an atrophic internal anal sphincter (median width 1.0 mm (0–1.6)). Anorectal physiology showed an increase in median resting pressure (37 pre v 65 cm H2O post) and squeeze pressure (89 v 105 cm H2O). Stimulation produced enhanced rectal sensitivity to distension. There were no major complications.
Conclusions: Sacral nerve stimulation is a safe and effective treatment for resistant faecal incontinence secondary to scleroderma. The benefit is maintained in the medium term.
Keywords: sacral nerve stimulation, faecal incontinence, systemic sclerosis
Systemic sclerosis (scleroderma) is a multisystem disease of unknown cause. Although relatively uncommon with an incidence of 14 per million population per year1 patients can be severely incapacitated. It may be localised to the skin but more commonly involves other organs. The gastrointestinal tract is often involved, with over 90% of patients experiencing upper gastrointestinal symptoms2 and up to 38% of patients complaining of faecal incontinence.3
The aetiology of scleroderma associated incontinence is multifactorial. Diarrhoea may relate to small and large bowel disease with dilatation, impaired motility, and bacterial overgrowth leading to malabsorption. Collagenous replacement of the muscularis propria and smooth muscle of the sigmoid colon and rectum4 leads to decreased rectal capacity and compliance.5 Fibrous replacement of the internal anal sphincter, demonstrated on endoanal ultrasound or magnetic resonance imaging, produces a thin weakened muscle promoting the development of passive faecal leakage.6 In addition, the autonomic nervous system, controlling rectal motility and internal sphincter function, may be impaired in up to 80% of patients.7
Current treatment for scleroderma associated incontinence is mainly medical with antidiarrhoeal drugs and behavioural therapy (biofeedback) but results are often unsatisfactory. If conservative treatment fails there are few proven therapies. Procedures such as dynamic graciloplasty or artificial bowel sphincter involve major surgery and are associated with considerable morbidity and a substantial failure rate.8,9 This may be potentiated in scleroderma patients who have high associated levels of comorbidity. A permanent stoma can relieve symptoms but is not an attractive option. The value of sphincter bulking agents in scleroderma is not known.
An alternative approach is to influence the nervous system controlling bowel motility, anal sphincter, and pelvic floor function. Sacral nerve stimulation has been demonstrated to be effective in patients with resistant incontinence.10 It involves the placement of temporary or permanent electrodes through the sacral foramen to modulate the nerves of the sacral plexus. In our initial experience two patients with scleroderma benefited from stimulation.11
There has been no study to date evaluating its use specifically in patients with scleroderma. We report the treatment of five patients with scleroderma associated faecal incontinence where conventional treatment had failed.
PATIENTS AND METHODS
Entry criteria included severe incontinence for liquid or solid stool, at least three episodes per week, and failure of traditional treatment, including antidiarrhoeal medication and behavioural therapy (biofeedback).
Five patients (four female), median age 61 years (range 30–71), with scleroderma associated incontinence were evaluated for this treatment and underwent temporary stimulation. Those who demonstrated benefit were implanted with a permanent device. The median number of preoperative weekly incontinent episodes was 15 (range 7–25), median duration of incontinence was five years (5–9), and of scleroderma 13 years (4–29).
Prior to stimulation all patients completed a three week bowel diary, the SF-36 quality of life assessment,12 an endoanal ultrasound,13 and anorectal physiological testing. Anal manometry used an eight channel water perfused system (MMS, Enschede, Holland) with a stationary pull through technique; squeeze pressure was the maximum voluntary increment above resting pressure.14 Rectal sensation was to balloon distension to air at threshold, urge, and maximal tolerated volume. Barium enema was performed if felt necessary. The diary, quality of life assessment, and anorectal physiology were completed at baseline, and after temporary and permanent stimulation.
The procedure and equipment for temporary electrode and permanent implantation have previously been described.15 Modifications of the original technique are as follows.
Temporary stimulation
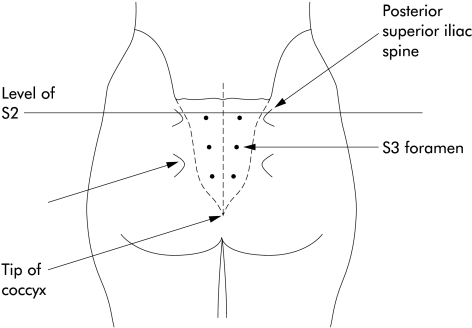
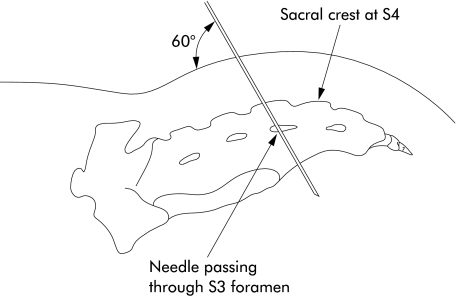
Temporary stimulation was performed in the jack-knife position under general anaesthesia as a day case. A 20 gauge, 9 cm, insulated spinal needle was inserted bilaterally into the S2, S3, and S4 sacral foramina, identified using bony landmarks (figs 1, 2 ▶ ▶). Stimulation was supplied with a portable stimulator (Medtronic 3625), pulse width 210 μs, frequency 15 Hz, continuous mode. The optimal foramen, eliciting the strongest visual and electromyographic response from the pelvic floor and great toe, was identified using high amplitude stimulation.
Figure 1.
Operative position of patient showing bony landmarks and position of the sacral foramen.
Figure 2.
Lateral view of the sacrum and bony landmarks.
A helical percutaneous wire (Medtronic 3057) was inserted, replacing the needle. Voltage was adjusted to just above the sensory threshold, usually a “tingling” or “pulsating” sensation in the perineum. A much lower voltage than during needle placement was used for chronic stimulation, during both temporary and permanent treatment.
After three weeks of temporary stimulation, if there was a reduction of greater than 50% in incontinent episodes and no serious complications, a permanent system was implanted.
Permanent stimulation
Under general anaesthesia the same foramen was exposed through a 6 cm midline incision and the needle replaced with a permanent electrode (Medtronic 3080). A subcutaneous pocket was prepared in the ipsilateral buttock for the permanent stimulator (Medtronic 3023) and the electrode tunnelled subcutaneously and connected. Parameters were set as for temporary stimulation.
The Harrow research ethics committee approved the study and all patients gave informed consent. Due to the small number of patients in the study the results are presented in full.
RESULTS
Temporary stimulation was performed in five patients and was successful in four (duration 21 days). Screening failed in one patient due to premature lead dislodgement after 24 hours. In successful patients, weekly incontinent episodes decreased from 15, 11, 23, and 7 to 0, 0, 2, and 0 during temporary stimulation.
In one patient, on steroids, there was a superficial skin infection during temporary stimulation that resolved on removal of the temporary electrode.
Permanent implantation was performed one month after temporary stimulation. Unilateral S3 stimulation was used in all patients; amplitude 2.6, 0.1, 3.9, and 3.8 volts. Inpatient stay was 4–6 days. There were no perioperative complications and there have been no side effects due to chronic stimulation.
With permanent implantation at the longest follow up of 60, 36, 24, and 6 months, weekly incontinent episodes decreased from 15, 11, 23, and 7 to 0. Stimulation abolished urgency, median time to defer <1 minute (range 0–1) compared with 15, 10, 15, and 5 minutes.
The different subscales of the SF36 questionnaire showed a variable improvement, physical function and social function being the most consistent.
The internal anal sphincter was atrophic in all patients (median width 1.0 mm (range 0–1.6); normal range 2.4–3.4). The external anal sphincter was normal in all patients with no evidence of obstetric trauma.
Anorectal physiological testing showed an increase in the maximal anal resting pressure (median 37 cm H2O (range 16–39) pre v 65 (47–85) post), and maximal squeeze pressure (89 cm H2O (40–120) pre v 105 (43–204) post). The rectum became more sensitive to distension at threshold volume (median 53 ml air (range 45–80) pre v 33 (20–65) post), urge volume (83 ml air (65–100) pre v 58 (30–90) post), and maximum tolerated volume (143 ml air (100–145) pre v 75 (40–120) post).
DISCUSSION
This study has shown that sacral nerve stimulation is an effective treatment for patients with scleroderma associated faecal incontinence when other therapies have failed. Stimulation had an immediate beneficial clinical effect that was maintained with time. Urgency and urge incontinence were abolished. All implanted patients were fully continent at the longest follow up.
There was variation in improvement in individual parameters of quality of life. The SF-36 measures a wide range of physical and emotional parameters and it might be expected with a progressive disease that continued symptoms would lead to impaired quality of life. In previous studies including patients with a broader range of conditions, quality of life improved with sacral nerve stimulation.10
Temporary percutaneous stimulation is a minor procedure with low morbidity that can be performed under local anaesthesia. However, we now perform this under general anaesthesia to eliminate discomfort. General anaesthesia does not affect the response to acute stimulation, providing no muscle relaxant has been used. Temporary screening for three weeks then provides an indication of success prior to surgery, especially advantageous in scleroderma patients with an increased perioperative risk.
Implantation of a permanent stimulator is also a relatively minor procedure causing minimal operative trauma. The operative site is distant from the bowel; previous anal procedures therefore do not complicate surgery, and the infection risk is low.
Manometric changes suggest an improvement in function of the internal and external anal sphincters. The effect on anal function with this procedure is controversial. Some studies have suggested enhanced function10 while others have not shown a consistent benefit.11 If enhanced function does occur it may be mediated by muscle hypertrophy, changes in fibre type, recruitment of redundant motor units, or a combination of these factors. The changes in rectal sensation suggest modification of the afferent sensory nerves. Which of these motor or sensory mechanisms is dominant in mediating improved continence and whether the mechanism for improvement is different in patients with scleroderma is unknown.
Physiological manipulation of the nervous system to produce a clinical effect is a new approach to treating faecal incontinence. In this series patients did not have evidence of secondary pseudo-obstruction, megacolon, or malabsorption, their major abnormality being a characteristic atrophic internal sphincter. In this restricted group of patients sacral nerve stimulation appears to offer a safe and effective therapy.
Acknowledgments
Financial support was received from Medtronic. However, the study design, performance, analysis, and reporting were conducted without the influence of Medtronic.
REFERENCES
- 1.Silman AJ. Epidemiology of scleroderma. Curr Opin Rheumatol 1991;3:967–72. [DOI] [PubMed] [Google Scholar]
- 2.Rees WD, Leigh RJ, Christofides ND, et al. Interdigestive motor activity in patients with systemic sclerosis. Gastroenterology 1982;83:575–80. [PubMed] [Google Scholar]
- 3.Trezza M, Krogh K, Egekvist H, et al. Bowel problems in patients with systemic sclerosis. Scand J Gastroenterol 1999;34:409–13. [DOI] [PubMed] [Google Scholar]
- 4.Engel AF, Kamm MA, Talbot IC. Progressive systemic sclerosis of the internal anal sphincter leading to passive faecal incontinence. Gut 1994;35:857–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leighton JA, Valdovinos MA, Pemberton JH, et al. Anorectal dysfunction and rectal prolapse in progressive systemic sclerosis. Dis Colon Rectum 1993;36:182–5. [DOI] [PubMed] [Google Scholar]
- 6.deSouza NM, Williams AD, Wilson HJ, et al. Fecal incontinence in scleroderma: assessment of the anal sphincter with thin-section endoanal MR imaging. Radiology 1998;208:529–35. [DOI] [PubMed] [Google Scholar]
- 7.Generini S, Fiori G, Moggi PA, et al. Systemic sclerosis. A clinical overview. Adv Exp Med Biol 1999;455:73–83. [PubMed] [Google Scholar]
- 8.Baeten CG, Bailey HR, Bakka A, et al. Safety and efficacy of dynamic graciloplasty for fecal incontinence: report of a prospective, multicentre trial. Dynamic Graciloplasty Therapy Study Group. Dis Colon Rectum 2000;43:743–51. [DOI] [PubMed] [Google Scholar]
- 9.Lehur PA, Roig JV, Duinslaeger M. Artificial anal sphincter: prospective clinical and manometric evaluation. Dis Colon Rectum 2000;43:1100–6. [DOI] [PubMed] [Google Scholar]
- 10.Rosen HR, Urbarz C, Holzer B, et al. Sacral nerve stimulation as a treatment for faecal incontinence. Gastroenterology 2001;121:536–41. [DOI] [PubMed] [Google Scholar]
- 11.Malouf AJ, Vaizey CJ, Nicholls RJ, et al. Permanent sacral nerve stimulation for fecal incontinence. Ann Surg 2000;232:143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jenkinson C, Coulter A, Wright L. Short form 36 (SF36) health survey questionnaire: normative data for adults of working age. BMJ 1993;306:1437–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Law PJ, Bartram CI. Anal endosonography: technique and normal anatomy. Gastrointest Radiol 1989;14:349–53. [DOI] [PubMed] [Google Scholar]
- 14.Gibbons CP, Bannister JJ, Trowbridge EA, et al. An analysis of anal sphincter pressure and anal compliance in normal subjects. Int J Colorectal Dis 1986;1:231–7. [DOI] [PubMed] [Google Scholar]
- 15.Bosch JL, Groen J. Sacral (S3) segmental nerve stimulation as a treatment for urge incontinence in patients with detrusor instability: results of chronic electrical stimulation using an implantable neural prosthesis. J Urol 1995;154:504–7. [DOI] [PubMed] [Google Scholar]