Abstract
Aims: Human liver cirrhosis is commonly associated with increased fasting and glucose induced insulin concentrations. However, whether the hyperinsulinaemia is a consequence of increased pancreatic insulin secretion, decreased hepatic insulin removal, or impaired feedback regulation of insulin secretion is still doubtful. To investigate these issues, insulin secretion—during 24 hours of standardised living conditions—insulin sensitivity, and hepatic insulin extraction were assessed in cirrhotic patients compared with matched healthy subjects.
Patients: Nine Child’s disease grade B cirrhotic patients and seven healthy volunteers, participated in the study. The subjects were studied on two separate days, one for the assessment of insulin secretion during a standardised 24 hour life period (calorimetric chamber), and one for the determination of insulin sensitivity.
Methods: Insulin secretion rates were reconstructed from plasma C peptide concentrations by deconvolution, and indices of β cell function were derived using a mathematical model describing the functional dependence of insulin secretion on plasma glucose concentrations. Insulin sensitivity was determined using the euglycaemic hyperinsulinaemic clamp technique.
Results: Cirrhotic patients showed a marked hypersecretory response, both in absolute terms (mean (SEM) 295 (53) versus 138 (11) nmol/m2, p<0.02), and in relation to glucose (175 (26) versus 57 (5) pmol/min/m2, p<0.02). In particular, the β cell dose-response function was shifted upward compared with controls. The sensitivity of insulin secretion to the rate of glucose change was also increased. Insulin sensitivity, markedly reduced in cirrhosis (157 (10) versus 296 (30) ml/min/m2, p<0.002), was strongly inversely correlated (r=0.89, p<0.002) in these patients with insulin secretion at 5 mM glucose. Insulin clearance and hepatic insulin extraction were not reduced. A frank hypermetabolism with increased lipid oxidation was found in this series.
Conclusions: This study suggests that hyperinsulinaemia, at least in Child’s disease grade B cirrhotic patients, is the consequence of increased β cell sensitivity to glucose, while hepatic insulin extraction does not seem to play a significant part.
Keywords: insulin secretion, insulin clearance, insulin sensitivity, C peptide deconvolution, mathematical model
It is commonly found that liver human cirrhosis is associated with increased fasting and glucose induced insulin levels.1–9 However, these patients mostly have normal or increased blood glucose concentrations. Coexistence of hyperinsulinaemia and normal or impaired glucose tolerance indicates the presence of insulin resistance,8–11 which has been confirmed by several studies using the euglycaemic hyperinsulinaemic clamp (EHC).8,12 Different explanations of the hyperinsulinaemia have been advanced by several authors. These include increased insulin secretion by the pancreas,1–9,13–15 decreased insulin removal by the cirrhotic liver,16–18 escape of insulin from liver degradation because of portal systemic shunting,19–21 and impaired feedback regulation of insulin secretion.22 Thus, peripheral hyperinsulinaemia in cirrhosis does not necessarily imply increased insulin secretion.
As, in contrast with insulin, C peptide is not significantly extracted by the liver some investigators have used peripheral C peptide as an index of pancreatic insulin secretion. However, even using the above method, conflicting results about insulin secretion in cirrhosis23 have been reported. The insulin response to different stimuli, such as arginine,4 tolbutamide,24 intravenous glucose bolus injection,25 and oral glucose ingestion26,27 has been reported to be decreased,4,24,25 normal,25 or increased.26,27
In this study, for the first time, we have investigated these issues by assessing in cirrhotic and healthy subjects insulin secretion during 24 hours of standardised living instead of using acute phase response to a stimulus, such as intravenous or oral glucose load. Insulin sensitivity and hepatic insulin extraction were determined by means of the most reliable methods currently available. In particular, insulin secretion rates were reconstructed from plasma C peptide concentrations by deconvolution, and indices of β cell function were derived using a mathematical model describing the functional dependence of insulin secretion on plasma glucose concentrations. Insulin sensitivity was measured using the EHC.
METHODS
Study protocol
Subjects
Nine cirrhotic patients, with biopsy confirmed liver post-hepatitis (HCV) cirrhosis, and seven healthy control subjects, participated to the study. Table 1 ▶ shows details of the participants.
Table 1.
Subjects’ characteristics
| Mean 24 hour | ||||||
| Age (y) | Sex (M/F) | BMI (kg/m2) | Glucose (mM) | Insulin (pM) | FFA (mM) | |
| Controls | 55 (4) | 3/4 | 26 (0.4) | 5.7 (0.2) | 88 (4) | 0.33 (0.04) |
| Cirrhotics | 52 (1) | 4/5 | 26 (0.9) | 4.8 (0.2)* | 145 (9)* | 0.57 (0.03)* |
*p<0.02 or less versus control subjects. Data shown as mean (SEM).
Cirrhotic patients belonged to Child’s disease grade B status (score 7.61 (0.73), mean (SEM)) according to the Child-Pugh classification. No evidence of significant spontaneous portal systemic shunting was detected by either ultrasonography or endoscopy. Drug treatment was suspended during the five days preceding the study. On examination, the cirrhotic subjects were in a stable clinical state: they were neither septic, nor febrile and suffered from no complications such as encephalopathy, acid base imbalance, diabetes mellitus, or renal failure. None had a history of thyroid dysfunction, neoplasm, or other acute or chronic diseases. All patients were on a weight maintaining diet with the following average composition: 70% carbohydrates, 30% fat, and 1 g of protein per kg of body weight. This dietary regimen was maintained for one week before the study.
Control subjects were healthy volunteers as indicated by physical examination and laboratory tests. None received any medication. All had followed their usual unrestricted diets for several months before the study.
The nature and purpose of the investigation were explained to each subject before they agreed to participate in the study, which followed the guidelines of the hospital ethics committee.
The subjects were studied on two separate days, one for the assessment of insulin secretion during a standardised 24 hour life period, and one for the determination of insulin sensitivity with the glucose clamp method.
24 Hour studies
The subjects spent a day (starting at 8 00 am) in the respiratory chamber (volume 23.6 m3) at the Metabolism Unit of the Catholic University School of Medicine in Rome. The characteristics of the device have been previously described.28–30
During the study day, all subjects were assigned a diet with an energy content of 30 kcal per kg of fat free mass consisting of 55% carbohydrate, 30% fat, and 15% protein. This amount was divided as follows: 20% at breakfast, 40% at lunch, 10% as an afternoon snack, and 30% at dinner. The four meals served in the chamber were prepared by a dietitian using common foods such as meat, fish, vegetables, bread, fruit, etc. The food given and returned was weighed to the nearest gram on precision scales (KS-01, Rowenta, Berlin, Germany). The nutrient content of all food items was calculated by using computerised tables (Food Processor II, Hesha Research, Salem, OR, modified according to the food tables of the Istituto Nazionale di Nutrizione, Italy). The energy content of food was computed as follows: 4.3 kcal/g for protein, 4.2 g for starch (or starch equivalent), and 9.3 kcal/g for fat.31
At 4 00 pm, the subjects performed a physical exercise session on the motorised treadmill by walking for 30 minutes at a constant speed of 3 km/h up a 10% grade.
Hourly blood samples were drawn from a central venous catheter derived outside the chamber through long plastic tubing for the measurement of glucose, insulin, C peptide, and free fatty acids (FFA) concentration.
Glucose clamp
Insulin sensitivity was determined using the EHC method.32 Whole body glucose uptake (M value in μmol/min per m2 body surface area) was determined during a primed constant infusion of insulin (at the rate of 6 pmol/min/1 kg) after an overnight fast. Glucose and insulin measurements were made on arterialised blood samples. At the time of the clamp studies, subjects were on a weight maintaining diet consisting of at least 250 g of carbohydrate a day for at least one week before each study. The clamp derived insulin sensitivity index (ISI, ml/min/m2) was the estimate of glucose clearance during the clamp, calculated as the ratio of the M value to the average insulin concentration during the clamp.
Analytical methods
Serum samples were stored at −70°C for an average duration of six months. These samples were not thawed until hormone assays were performed. Serum glucose was measured by the glucose oxidase method (Beckman, Fullerton, CA). Serum insulin was assayed by microparticle enzyme immunoassay (Abbott, Pasadena, CA). Serum C peptide was measured by the ELISA method (Chematil, Scafati, Italy). FFA were determined by enzymatic colorimetric method (Boehringer Mannheim, Mannheim, Germany). All assays were performed in duplicate.
Mathematical modelling
Model of β cell function
The model used for assessing insulin secretion33 is a combination of a β cell model, which relates insulin secretion to glucose concentration, and a model of C peptide kinetics.
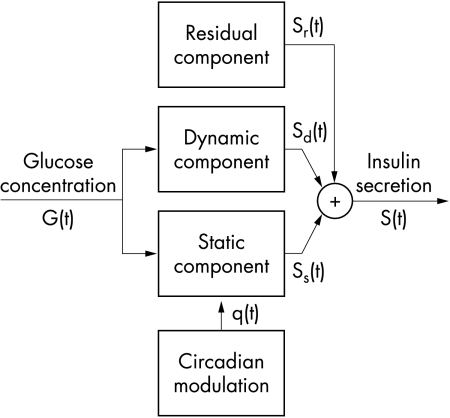
In the β cell model (fig 1 ▶), insulin secretion (S(t)) is expressed as the sum of three components. The first component (Ss(t)) expresses a static relation between insulin secretion and glucose concentration—that is, it embodies a β cell dose response function. The β cell dose-response function is assumed to be modulated by a circadian oscillation, represented by a sinusoidal function with a 24 hour period:
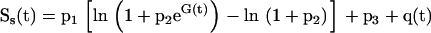 |
1a |
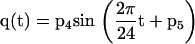 |
1b |
where G (mM) is the glucose concentration, t is time (hours), and p1-p5 are parameters. The term q(t) represents the circadian modulation. When the modulating term q(t) is zero, the dose-response function (equation 1a) is a curvilinear, convex function. The parameter p3 represents the intercept for G=0, p1 is the slope of the curve for high G values, and p2 determines the curvature—that is, for high (with respect to 1) values of p2 the dose-response function is quasi-linear, whereas for low values of p2 the dose-response curve exhibits a appreciable convexity. The parameters p4 and p5 are the amplitude and phase (0=p5<2π) of the 24 hour oscillation, respectively.
Figure 1.
Model of insulin secretion. See eqations 1 to 3.
The second insulin secretion component (Sd(t)) expresses a dynamic dependence of insulin secretion on the rate of change of glucose concentration. Sd(t) is proportional to the derivative of glucose concentration when the derivative is positive, and is zero otherwise:
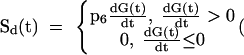 |
2 |
The third insulin secretion component represents a residual secretion term (Sr(t)), which accounts for the possibility that the secretory controls represented in equations 1 and 2 are not exactly modelled, or that other secretion components are present. Sr(t) is modelled in discrete form as a generic piece-wise linear function over 20 minute intervals, with zero mean. Because Sr(t) may take on both positive and negative values, it represents an additive correction term rather than a real secretion component.
Total insulin secretion is the sum of the three components described above:
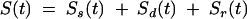 |
3 |
Total insulin secretion is calculated every 20 minute for the whole 24 hour period. Insulin secretion and its components are normalised to body surface area, and expressed in pmol/min/m2.
The model for C peptide kinetics is the two exponential model proposed by Van Cauter et al,34 in which the model parameters are determined in each individual on the basis of the subject’s sex, weight, height, and age. C peptide concentration (C(t)) is the convolution between the individualised, two exponential C peptide impulse response and C peptide secretion (S(t), equation 3).
The model parameters p1-p6 and Sr(t) (equations 1 to 3) were determined by fitting the model to the glucose and C peptide data using least squares techniques, as previously described.29
From the estimated model parameters, other parameters useful for describing the β cell dose response characteristics were calculated. From equation 1a, by letting G=5 and q(t)=0, the insulin secretion value corresponding to the fixed glucose concentration of 5 mM (ISR5, pmol/min/m2) was calculated. This parameter quantifies insulin secretion around the normal basal glucose value. The slope of the dose response function at 5 mM glucose concentration (Slope5, pmol/min/m2/mM) was also obtained from equation 1a. This parameter quantifies the sensitivity of β cells to glucose concentration changes around 5 mM. A measure of the average amplitude of the residual insulin secretion component (SDr, pmol/min/m2) was obtained from the standard deviation of Sr(t). Total 24 hour insulin secretion (ISRtot, nmol/m2) was calculated as the integral of total insulin secretion over the 24 hours.
Insulin clearance and hepatic extraction
Peripheral insulin clearance was calculated as the ratio of insulin infusion to average insulin concentration during the last 40 minutes of the clamp. Endogenous insulin clearance was calculated as the ratio of the average insulin secretion to the average insulin concentration over the whole 24 hour period.
Hepatic insulin fractional extraction (E) was calculated from the equation
 |
This equation yields the correct hepatic insulin extraction value under the hypothesis that hepatic and extrahepatic insulin clearance are the same during the clamp and the 24 hour experiments.
Statistical analysis
Data and results are presented as mean (SEM). Differences between groups were tested the with Mann-Whitney U test. Linear regression analysis was performed using standard methods.
RESULTS
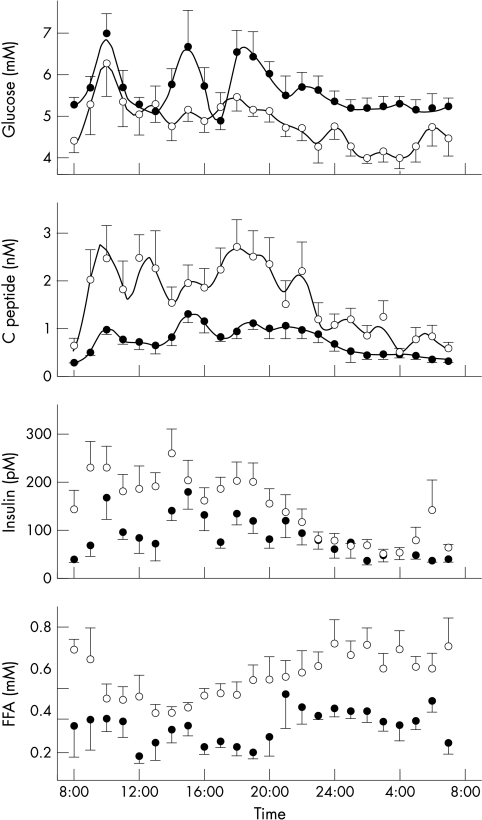
Figure 2 ▶ shows the 24 hour profiles of glucose, C peptide, insulin, and FFA. Mean insulin and FFA concentrations were higher in cirrhotic patients, while glucose concentration was lower (table 1 ▶).
Figure 2.
Mean (SEM) glucose, C peptide, insulin, and free fatty acids concentrations. Closed circles: control subjects; open circles: cirrhotic patients. The solid lines in the two top panels represent the model fit.
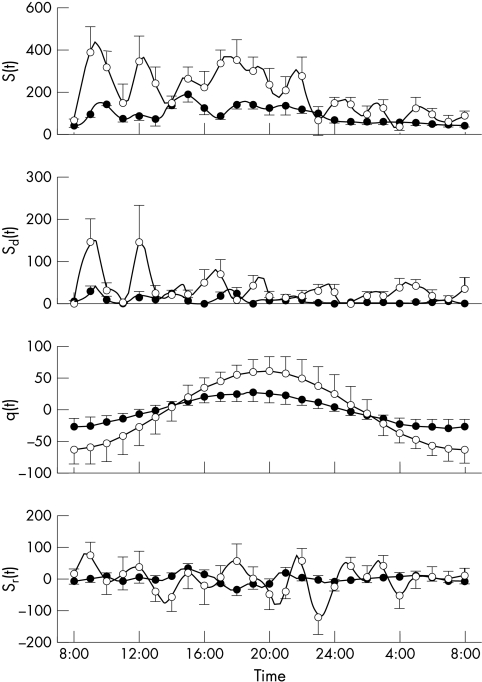
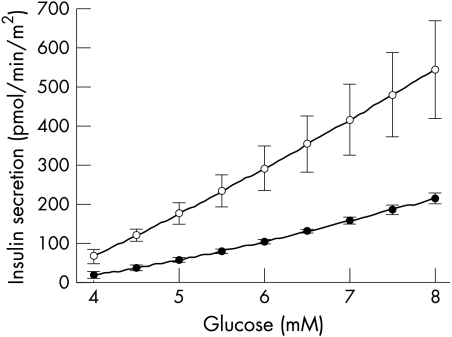
Figure 3 ▶ shows insulin secretion and its components, calculated using the model. The parameters of β cell function are shown in table 2 ▶. Cirrhotic patients showed a marked hypersecretory response, not only in absolute terms (total 24 hour insulin secretion, ISRtot), but also in relation to glucose. In particular, the β cell dose response function (fig 4 ▶) was shifted upward, and its slope increased, albeit the increase fell short of statistical significance. The parameter expressing the sensitivity of insulin secretion to the glucose rate of change (p6) was increased.
Figure 3.
Mean (SEM) insulin secretion and its components (all expressed in pmol/min/m2) calculated using the model. S(t), total insulin secretion; Sd(t), dynamic component; q(t) circadian modulation of the dose-response; Sr(t), residual component. See eqations 1 to 3. Closed circles: control subjects; open circles: cirrhotic patients. Insulin secretion and its components are calculated every 20 minutes, but the error bars are shown every hour for clarity.
Table 2.
Parameters of β cell function
| ISR5 (pmol min/m2) | Slope5 (pmol min/m2/ mM) | p6 (pmol m2/ mM) | p4 (pmol min/m2) | SDr (pmol min/m2) | ISRtot (nmol/m2) | |
| Controls | 57 (5) | 43 (6) | 1327 (425) | 35 (11) | 32 (6) | 138 (11) |
| Cirrhotics | 175 (26)* | 112 (34)† | 5385 (1586)* | 84 (27)† | 108 (21)* | 295 (53)* |
ISR5, insulin secretion at 5 mM glucose; Slope5, of the dose-response at 5 mM glucose; p6, parameter of the derivative component (eq 2); p4, amplitude of the 24 hour modulation (eq 1); SDr, standard deviation of the residual insulin secretion component (Sr(t), Eq. 3); ISRtot, 24 hour integral of insulin secretion. *p<0.02 or less versus control subjects. †0.08<p<0.12 versus control subjects.
Figure 4.
Mean (SEM) dose-response (equation 1a with q(t)=0). Closed circles: control subjects; open circles: cirrhotic patients. Error bars are shown at 1 mM increments.
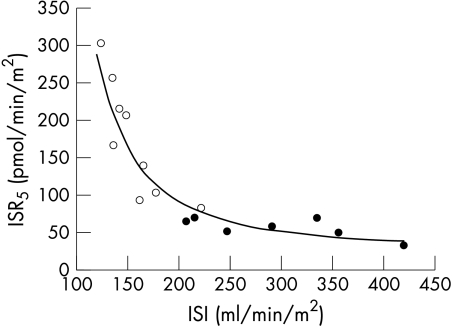
Insulin sensitivity was markedly reduced in cirrhotic patients (table 3 ▶). Insulin sensitivity (ISI) was strongly inversely correlated with insulin secretion at 5 mM glucose (ISR5) (fig 5 ▶, r=0.91, p<0.0001, control and cirrhotic subjects pooled, variables log transformed). The correlation was highly significant in cirrhotic subjects (r=0.89, p<0.002), and borderline in control subjects (r=0.70, p=0.08). As figure 5 ▶ shows, a simple function can describe the relation between ISI and ISR5 in both groups.
Table 3.
Insulin sensitivity and insulin clearance
| Insulin sensitivity (ml/min/m2) | Peripheral insulin clearance (ml/min/m2) | Endogenous insulin clearance (ml/min/m2) | Hepatic insulin extraction (%) | |
| Controls | 296 (30) | 484 (13) | 1096 (86) | 54 (4) |
| Cirrhotic patients | 157 (10)* | 531 (10) | 1480 (299) | 59 (6)† |
*p<0.02 or less versus control subjects. †One cirrhotic patient with a negative value of hepatic insulin extraction (−13%) was excluded from the mean. With that subject included, hepatic insulin extraction was 51 (10), with no change in statistical significance.
Figure 5.
Relation between β cell sensitivity to glucose (as represented by ISR5) and insulin sensitivity (ISI). Closed circles: control subjects; open circles: cirrhotic patients. The solid line represents the equation ISR5=e340/ISI.
Insulin clearance and hepatic insulin extraction were not reduced in cirrhotic patients (table 3 ▶). Indeed, insulin clearance, as calculated from the glucose clamp, was 10% higher in cirrhotic patients.
FFA concentration was not correlated either with the parameters of β cell function or with insulin sensitivity when correlation was tested in the individual groups.
Twenty four hour energy expenditure was 8.38 (0.71) kJ/d in cirrhotic patients and 7.26 (0.37) kJ/d in controls (p<0.01). The resting energy expenditure was also significantly (p<0.01) higher in cirrhotic patients than in controls (7.03 (0.82) versus 5.96 (0.49)). A prevalent lipid oxidation was observed in cirrhotic patients (24 h RQ=0.77 (0.03) versus 0.83 (0.04); p<0.01).
DISCUSSION
Insulin secretion, insulin sensitivity, and hepatic insulin extraction have been studied extensively in cirrhotic patients, but conflicting results, in part because of inadequate methodologies, have been reported.1–27,35–39 Another important problem in the interpretation of metabolic alterations in cirrhosis is attributable to the heterogeneity of patients regarding both the aetiology of the liver disease and the clinical stage. However, a large consensus has been progressively reached on the lack of significant relation between insulin resistance, or impaired glucose tolerance, and the aetiology of liver cirrhosis, liver function, or the clinical or nutritional status of the patients.11,36,39–41 In patients with alcoholic cirrhosis an insulin hypersecretion was observed, while estimated hepatic insulin extraction was reduced in only the most advanced stages of the disease.6 Using the deconvolution of plasma C peptide concentrations the insulin secretion rate was found to be doubled in alcoholic cirrhotic patients, while the insulin clearance was 40% reduced.16 To our knowledge no data are reported in the literature about the insulin secretion rate in pure post-HCV cirrhosis. Therefore, this represent the first report in which the insulin secretion was assessed in a group of patients suffering with HCV related liver cirrhosis.
The value of this study is that it uses accurate methods for the assessment of these variables in physiological conditions. Firstly, the experimental design simulates normal living, with a 24 hour observation period and the administration of mixed meals and exercise. Secondly, calculation of insulin secretion is based on the C peptide deconvolution approach, which yields true β cell output and is not affected by hepatic insulin extraction. Thirdly, insulin secretion is assessed not only in absolute terms, but also in relation to the glucose stimuli by means of a model that gives multiple parameters of β cell function. Fourthly, insulin sensitivity is determined with the EHC glucose clamp method, which is the gold standard for insulin sensitivity.42 Fifthly, the role of hepatic insulin extraction is studied with different methods—that is, from insulin infusion and concentration during the glucose clamp, from the 24 hour C peptide and insulin profiles using the model, and from the combination of the two.
A key method of this study is the model of β cell function. This model builds on the classic C peptide deconvolution approach developed by others,34 the application of which to cirrhotic patients is expected to be accurate, as C peptide clearance in cirrhosis is normal.7 The purpose of the model is to assess β cell sensitivity to glucose by taking into consideration the main known determinants of the β cell response to glucose. The model in fact represents the β cell response as the sum of three components: the first is a quasi-linear function of glucose concentration (the dose-response function), modulated by a night-day fluctuation, the second is a response to the rate of glucose change, and the third is a secretion component that is apparently unrelated to glucose (residual component, Sr(t)). While the dose-response and the derivative component are present in previous models of insulin secretion, the night-day modulation and the residual component are specific to the model, and are essential to explain the observed insulin secretion changes.33 Thus, within the limitations that any mathematical model inevitably has, our approach describes all the features of the relation between glucose concentration and insulin secretion observed in our experiments.
One main finding is that cirrhotic patients are hypersecretory, not only in absolute terms, as expressed by the total 24 hour secretion, but also in relation to the glucose stimulus, as expressed by the model parameters of β cell function, which were all increased in cirrhotic patients, albeit not all to a statistically significant extent. In particular, in cirrhotic patients the β cell response to a fixed 5 mM glucose level (ISR5) was almost threefold, the sensitivity to the glucose derivative (p6) was fourfold, and standard deviation of the residual component (SDr) more than threefold. These results, while are in agreement with the majority of the studies on insulin secretion,1–19,13–15,26,27 add the notion that hypersecretion in cirrhosis originates from an increased β cell sensitivity to glucose, which seem to concern most, if not all, of the mechanisms by which the β cells respond to glucose stimuli.
In agreement with previous findings,8–13,27,33,43 our results show that cirrhotic patients were markedly insulin resistant. Because compensation mechanisms exist between insulin sensitivity and β cell sensitivity to glucose, β cell sensitivity may be higher in cirrhotic patients solely because they are insulin resistant. Unfortunately, there is no exact way to normalise β cell sensitivity to insulin sensitivity, in order to assess if the compensation between the two is normal. Because there is almost no overlap of the insulin sensitivity indices in control and cirrhotic subjects (fig 5 ▶), it is not possible to predict safely by extrapolation what would be β cell sensitivity in normal subjects in presence of a severe insulin resistance such as that observed in cirrhotic patients. Figure 5 ▶ shows that there is no discontinuity in the increase in the β cell sensitivity, and thus adaptation of β cell sensitivity to insulin resistance in cirrhotic patients may be normal. Whether increased β cell sensitivity is the cause or the consequence of insulin resistance cannot be obviously established from the present data.
We did find increased FFA concentrations in our cirrhotic patients similar to other investigators.44–47 However, the average 24 hour FFA concentration was not correlated with the parameters of β cell function and total insulin secretion, either in control or in cirrhotic subjects. Average FFA concentrations were also not correlated with insulin sensitivity in the two groups.
As far as the energy expenditure and the respiratory quotient measured in the respiratory chamber is concerned, this study confirms a previous report30 in which we found that Child’s disease grade B, post-hepatitis cirrhotic patients show a hypermetabolism and a preferential lipid oxidation.
In conclusion, this study clearly shows that hyperinsulinaemia in our cirrhotic patients is entirely the consequence of increased β cell sensitivity to glucose, while hepatic insulin extraction does not play a part. That insulin clearance and hepatic extraction are not decreased in cirrhotic patients is shown by three concordant figures—that is, insulin clearance calculated from insulin infusion and concentration during the clamp, insulin clearance calculated from average insulin secretion and concentration in the 24 hour experiments, and hepatic insulin extraction calculated from these clearance values.
Abbreviations
EHC, euglycaemic hyperinsulinaemic clamp
FFA, free fatty acid
ISI insulin sensitivity index
REFERENCES
- 1.Samaan NA, Stone DB, Eckardt RD. Serum glucose, insulin and growth hormone in chronic hepatic cirrhosis. Arch Intern Med 1969;124:149–52 [PubMed] [Google Scholar]
- 2.Sestoft L, Rehfeld JF. Insulin and glucose metabolism in liver cirrhosis and in liver failure. Scand J Gastroenterol 1970;7 (suppl):133–6. [PubMed] [Google Scholar]
- 3.Greco AV, Ghirlanda G, Patrono C, et al. Behavior of pancreatic glucagon, insulin and HGH in liver cirrhosis after arginine and i.v. glucose. Acta Diabetol Lat 1974;11:330–9. [DOI] [PubMed] [Google Scholar]
- 4.Greco AV, Crucitti F, Ghirlanda G, et al. Insulin and glucagon concentrations in portal and peripheral veins in patients with hepatic cirrhosis. Diabetologia 1979;17:23–8. [DOI] [PubMed] [Google Scholar]
- 5.Shankar TP, Solomon SS, Duckworth WC, et al. Studies of glucose intolerance in cirrhosis of the liver. J Lab Clin Med 1983;102:459–69. [PubMed] [Google Scholar]
- 6.Marchesini G, Pacini G, Bianchi G, et al. Glucose disposal, beta-cell secretion, and hepatic insulin extraction in cirrhosis: a minimal model assessment. Gastroenterology 1990;99:1715–22. [DOI] [PubMed] [Google Scholar]
- 7.Kruszynska YT, Home PD, McIntyre N. Relationship beetwen insulin sensitivity, insulin secretion and glucose tolerance in cirrhosis. Hepatology 1991;14:103–11. [DOI] [PubMed] [Google Scholar]
- 8.Petrides AS, Vogt C, Schulze-Berge D, et al. Pathogenesis of glucose intolerance and diabetes mellitus in cirrhosis. Hepatology 1994;19:616–27. [DOI] [PubMed] [Google Scholar]
- 9.Greco AV, Rebuzzi AG, Altomonte L, et al. Glucose, insulin and somatostatin infusion for the determination of insulin resistance in cirrhosis. Horm Metab Res 1979;11:547–9. [DOI] [PubMed] [Google Scholar]
- 10.Petrides AS, Stanley T, Matthews DE, et al. Insulin resistance in cirrhosis: prolonged reduction of hyperinsulinemia normalizes insulin sensitivity. Hepatology 1998;28:141–9. [DOI] [PubMed] [Google Scholar]
- 11.Muller MJ, Willmann O, Rieger A, et al. Mechanism of insulin resistance associated with liver cirrhosis. Gastroenterology 1992;102:2033–41. [DOI] [PubMed] [Google Scholar]
- 12.Vannini P, Forlani G, Marchesini G, et al. The euglycemic clamp technique in patients with liver cirrhosis. Horm Metab Res 1984;16:341–3. [DOI] [PubMed] [Google Scholar]
- 13.Collins JR, Lacy WW, Stiel JN, et al. Glucose intolerance and insulin resistance in patients with liver disease. II. A study of etiologic factors and evaluation of insulin action. Arch Intern Med 1970;126:608–14. [PubMed] [Google Scholar]
- 14.Megyesi C, Samols E, Marks V. Glucose tolerance and diabetes in chronic liver disease. Lancet 1967;ii:1051–5. [DOI] [PubMed] [Google Scholar]
- 15.Proietto J, Alford FP, Dudley FJ. The mechanism of the carbohydrate intolerance of cirrhosis. J Clin Endocrinol Metab 1980;51:1030–6. [DOI] [PubMed] [Google Scholar]
- 16.Letiexhe MR, Scheen AJ, Gérard PL, et al. Insulin secretion, clearance, and action on glucose metabolism in cirrhotic patients. J Clin Endocrinol Metab 1993;77:1263–8. [DOI] [PubMed] [Google Scholar]
- 17.Johnston DG, Alberti KGMM, Faber OK, et al. Hyperinsulinism of hepatic cirrhosis: diminished degradation or hypersecretion? Lancet 1977;i:10–12 [DOI] [PubMed] [Google Scholar]
- 18.Iwasaki Y, Sato H, Ohkubo A, et al. Effect of spontaneous portal-systemic shunting on plasma insulin and aminoacid concentrations. Gastroenterology 1980;78:677–83. [PubMed] [Google Scholar]
- 19.Alberti KGMM, Johnston DG, Sutton G. Some aspects of carbohydrate metabolism in liver disease. In: Crepaldi G, Lefebvre PJ, Alberti KGMM, eds. Diabetes, obesity, and hyperlipidemies. London: Academic Press, 1977:29–39.
- 20.Smith-Laing G, Sherlock S, Faber OK. Effects of spontaneous portal-systemic shunting on insulin metabolism. Gastroenterology 1979;76:685–90. [PubMed] [Google Scholar]
- 21.Shurberg JL, Resnick RH, Coff RS, et al. Serum lipids, insulin and glucagon after portacaval shunt in cirrhosis. Gastroenterology 1977;72:301–4. [PubMed] [Google Scholar]
- 22.Cavallo-Perin B, Bruno A, Nuccio P, et al. Feedback inhibition of insulin secretion is altered in cirrhosis. J Clin Endocrinol Metab 1986;63:1023–7. [DOI] [PubMed] [Google Scholar]
- 23.Petrides AS, De Fronzo RA. Glucose metabolism in cirrhosis: a review with some perspectives for the future. Diabetes Metab Rev 1989;5:691–709. [DOI] [PubMed] [Google Scholar]
- 24.Pelkonen R, Kallio H, Suovanta H, et al. Plasma insulin, C-peptide, and blood glucose in portal, hepatic and peripheral veins in liver cirrhosis: effects of tolbutamide. Acta Endocrinol 1981;97:496–502. [DOI] [PubMed] [Google Scholar]
- 25.Magnusson J, Transberg KG. Impaired early response to intravenous glucose in alcoholic liver cirrhosis. Scand J Gastroenterol 1987;22:301–7. [DOI] [PubMed] [Google Scholar]
- 26.Riggio O, Merli M, Cangiano C, et al. Glucose intolerance in cirrhosis. Metabolism 1982; 31:627–34. [DOI] [PubMed] [Google Scholar]
- 27.Proietto J, Dudley FJ, Aitken P, et al. Hyperinsulinemia and insulin resistance of cirrhosis: the importance of insulin hypersecretion. Clin Endocrinol 1984;21:657–65. [DOI] [PubMed] [Google Scholar]
- 28.Greco AV, Tataranni PA, Mingrone G, et al. Daily energy metabolism in patients with type 1 diabetes mellitus. J Am Coll Nutr 1995;14:286–91. [DOI] [PubMed] [Google Scholar]
- 29.Tataranni PA, Mingrone G, Caradonna P, et al. Twenty-four-hour energy and nutrient balance in weight stable post-obese patients after biliopancreatic diversion. Nutrition 1996;12:239–44. [DOI] [PubMed] [Google Scholar]
- 30.Greco AV, Mingrone G, Benedetti G, et al. Daily energy and substrate metabolism in patients with cirrhosis. Hepatology 1998;27:346–50. [DOI] [PubMed] [Google Scholar]
- 31.Kaltenbach M. Manger correctement; mais comment? Zurich: Fédération des Cooperatives Migros, 1984.
- 32.De Fronzo RA, Tobin JD, Andres R. The glucose clamp technique: a method for the quantification of beta-cell sensitivity to glucose and of tissue sensitivity to insulin. Am J Physiol 1979;237:E214–23. [DOI] [PubMed] [Google Scholar]
- 33.Mari A, Camastra S, Toschi E, et al. A model for glucose control of insulin secretion during 24 h of free living. Diabetes 2001;50 (suppl 1):S164–8. [DOI] [PubMed] [Google Scholar]
- 34.Van Cauter E, Mestrez F, Sturis J, et al. Estimation of insulin secretion rate from C-peptide levels. Comparison of individuals and standard kinetic parameters for C-peptide clearance. Diabetes 1992;41:368–77. [DOI] [PubMed] [Google Scholar]
- 35.Taylor R, Heine RJ, Collins J, et al. Insulin action in cirrhosis. Hepatology 1985;5:64–71. [DOI] [PubMed] [Google Scholar]
- 36.Petrides AS, Groop L, Riely CA, et al. Effect of physiologic hyperinsulinemia on glucose and lipid metabolism in cirrhosis. J Clin Invest 1991;88:561–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohnishi K, Mishima A, Takashi M, et al. Effect of intra and extra hepatic portal systemic shunts on insulin metabolism. Dig Dis Sci 1983;28:201–6. [DOI] [PubMed] [Google Scholar]
- 38.Nygren A, Adner N, Sunblad L, et al. Insulin uptake by the human alcoholic cirrhotic liver. Metabolism 1985;34:48–52. [DOI] [PubMed] [Google Scholar]
- 39.Muller MJ, Fenk A, Lautz HU, et al. Energy expenditure and substrate metabolism in ethanol-induced liver cirrhosis. Am J Physiol 1991;260:E338–44. [DOI] [PubMed] [Google Scholar]
- 40.Shmueli E, Record CO, Alberti KGMM. Liver disease, carbohydrate metabolism and diabetes. Baillieres Clin Endocrinol Metab 1992;6:719–43. [DOI] [PubMed] [Google Scholar]
- 41.Shmueli E, Walker M, Alberti G, et al. Normal splanchnic but impaired peripheral insulin-stimulated glucose uptake in cirrhosis. Hepatology 1993;18: 6–95. [PubMed] [Google Scholar]
- 42.Fulcher GR, Walker M, Alberti KGMM. The assessment of insulin action in vivo. In: Alberti KGMM, De Fronzo RA, Keen H, eds. International textbook of diabetes mellitus. New York: Wiley, 1992:513–30.
- 43.Petrides AS, Riely CA, Groop LC, et al. The glucose fatty-acid cycle does not explain the insulin resistance of cirrhosis. [Abstract]. Gastroenterology 1987;92:1763. [Google Scholar]
- 44.Merli M, Leonetti F, Riggio O, et al. Resistance to insulin suppression of plasma free fatty acids in liver cirrhosis. J Endocrinol Invest 1990;13:787–95. [DOI] [PubMed] [Google Scholar]
- 45.Owen OE, Trapp VE, Reichard GA, et al. Nature and quantity of fuels consumed in patients with alcoholic cirrhosis. J Clin Invest 1983;72:1821–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riggio O, Merli M, Cantafora A, et al. Total and individual free fatty acid concentrations in liver cirrhosis. Metabolism 1984;33:646–51. [DOI] [PubMed] [Google Scholar]
- 47.Romijn JA, Endert E, Sauerwein HP. Glucose and fat metabolism during short term starvation in cirrhosis. Gastroenterology 1991;100:731–7. [DOI] [PubMed] [Google Scholar]