Abstract
Background: The hepatopulmonary syndrome (HPS) is defined as the triad of liver disease, arterial deoxygenation, and pulmonary vascular dilatation. The reported prevalence of HPS in cirrhotic patients varies between 4% and 19%, and various threshold values defining arterial deoxygenation have been used and recommended previously. However, it is not known how the prevalence of HPS differs using different cut off values for arterial deoxygenation.
Methods: We studied 127 patients for the presence of HPS using transthoracic contrast echocardiography for detection of pulmonary vasodilation, pulmonary function tests, and blood gas analysis.
Results: Ninety eight patients were included in the study, of whom 33 (34%) had a positive contrast echocardiography. Using an increased alveolar-arterial difference for the partial pressure of oxygen (AaDO2) as an indication of hypoxaemia, the prevalence of HPS was considerably higher (>15 mm Hg, 32%; >20 mm Hg, 31%; and >age related threshold, 28%) than using reduced partial pressure of arterial oxygen (PaO2) as a threshold (<80 mm Hg, 19%; <70 mm Hg, 15%; and <age related threshold, 15%). For AaDO2 as the cut off, the positive predictive value for a diagnosis of HPS was low (34%, 37%, and 53%, respectively). In contrast, PaO2 as a cut off had considerably higher positive predictive values (44%, 93%, and 94%, respectively). Introducing PaO2 <65 mm Hg as the cut off, the positive predictive value increased to 100%. Dyspnoea was more often present in patients with “clinically significant” HPS (57%) compared with “subclinical HPS” (8%), and patients without HPS (6%). The Child-Pugh score correlated significantly with the severity of HPS. Two of five liver transplanted patients with “subclinical HPS” had embolic brain infarcts, possibly induced by venous emboli passing through dilated intrapulmonary vessels.
Conclusions: Defining arterial hypoxaemia in HPS by different, previously used, cut off values for arterial oxygenation leads to a wide variation in the prevalence of HPS in the same sample of cirrhotic patients.
Keywords: liver cirrhosis, portal hypertension, hypoxaemia, pulmonary vasodilation, contrast echocardiography
The hepatopulmonary syndrome (HPS) is defined as the triad of liver disease, pulmonary gas exchange abnormalities leading to arterial deoxygenation, and widespread pulmonary vascular dilatation.1,2 Whereas both acute and chronic liver diseases have been associated with HPS, most commonly it presents in patients with cirrhosis. Portal hypertension seems to be the predominant factor related to this syndrome. The hallmark of pulmonary vascular changes in HPS are dilated vessels at the precapillary and capillary level and direct arteriovenous communications.1 This causes right to left shunting of blood flow, mismatch between ventilation and perfusion, and diffusion limitation.2 Pulmonary features include digital clubbing, cyanosis, dyspnoea, platypnoea, and orthodeoxia; the latter two are defined as dyspnoea and arterial deoxygenation induced by the upright position and relieved by recumbency.3
A diagnosis of HPS is established when the following three points are fulfilled.4
Chronic liver disease, usually complicated by portal hypertension.
Arterial hypoxaemia, defined by a reduced partial pressure of arterial oxygen (PaO2) or more accurately by an increased alveolar-arterial difference in the partial pressure of oxygen (AaDO2). The latter includes determination of the partial pressure of arterial carbon dioxide (PaCO2) which is often low in cirrhotic patients as a result of hyperventilation.2
Intrapulmonary vascular dilatation, detected either by two dimensional contrast echocardiography or macroaggregated albumin lung perfusion scan.3
In the literature, the prevalence of pulmonary vasodilation, detected by transthoracic contrast echocardiography, varies widely (5–47%)5–11 and a definitive diagnosis of HPS varies between 4% and 19%5–8,10–13 in cirrhotic patients. Various threshold values defining arterial hypoxaemia have been recommended and used in previous publications: PaO2 <70 mm Hg,5,7,8,11 PaO2 <80 mm Hg,14 AaDO2 >15 mm Hg,12,15 AaDO2 >20 mm Hg,16 and AaDO2 >age related threshold value.6,13 Consequently, these different threshold values may result in a different prevalence of HPS. Thus the aims of our study were: (1) to clarify how the prevalence of HPS in a large sample of cirrhotic patients varies according to previously used different cut off values for arterial hypoxaemia; and (2) to determine the predictive value of those cut off values in the diagnosis of HPS.
PATIENTS AND METHODS
Patients
The study protocol was approved by the institutional ethics committee of the University of Vienna and written informed consent was obtained from each patient. A total of 127 patients with biopsy proven cirrhosis, evaluated for liver transplantation or transjugular intrahepatic portosystemic shunt, underwent transthoracic contrast echocardiography, arterial blood gas analysis on room air, lung function tests, and chest radiograph. Nine patients were excluded because of inadequate echocardiographic image quality, 12 patients were excluded because of unfeasible lung function tests, and eight patients were excluded because of impaired lung function tests, defined as forced expiratory volume in one second (FEV1) or total lung capacity (TLC) <66% of predicted.5,17,18 Mean age of the remaining 98 patients was 56 years (range 33–82); 65 (66%) were men and 33 (34%) were women. No patient had evidence of portopulmonary hypertension, assessed by echocardiography,19 including Doppler measurements.20–22
Procedure
Contrast echocardiography
Agitated saline was used as a contrast medium which creates a stream of microbubbles after intravenous injection.1 In healthy individuals, these microbubbles, greater than 15 μm in diameter,16,23 opacify the right heart chambers only because they are filtered in the pulmonary capillary bed and do not appear at the left side of the heart. The distinction between intrapulmonary or intracardiac shunt is made by the time of appearance of the microbubbles in the left heart chambers: in intracardiac shunt the microbubbles appear generally within three heartbeats after appearance in the right heart chambers and in intrapulmonary shunt they appear 4–6 heartbeats after their initial appearance in the right side of the heart.
Arterial blood gas analysis
Arterial blood gas samples were obtained by percutaneous radial artery puncture with the subject in a seated position breathing room air, and were analysed with a standard blood gas analyser (BGElectrolytes, Instrumentation Lab. Inc., USA). AaDO2 was calculated using the alveolar gas equation.24
Lung function tests
FEV1 was obtained using a computerised spirometer (Autobox 6200; Sensor Medics, Yorba Linda, California, USA) according to standard procedures.25 The best two of three acceptable tracings for FEV1 did not vary by more than 5%. TLC was measured by a body plethysmograph (Autobox 6200; Sensor Medics).
Statistical analysis
Group comparisons were performed using the Mann-Whitney U test and Fisher’s exact test. The prevalence of HPS among cirrhotics and positive predictive values for a diagnosis of HPS with corresponding confidence intervals were calculated for different cut off values of PaO2 and AaDO2.
RESULTS
Patient characteristics according to findings on contrast echocardiography (table 1 ▶)
Table 1.
Clinical characteristics of cirrhotic patients with and without positive contrast echocardiograms
| Positive contrast echocardiogram (n=33) | Negative contrast echocardiogram (n=65) | p Value | |
| Age (y) | 53 [10] | 57 [11] | NS |
| Male (%) | 21 (64) | 44 (69) | NS |
| Aetiology of cirrhosis (%) | |||
| Alcohol | 18 (55) | 39 (60) | NS |
| HCV | 5 (15) | 14 (22) | NS |
| HBV | 2 (6) | 3 (5) | NS |
| Alcohol+HCV | 1 (3) | 1 (1.5) | NS |
| Alcohol+HBV | 1 (3) | 1 (1.5) | NS |
| Haemochromatosis | 1 (3) | 1 (1.5) | NS |
| PBC | 1 (3) | 2 (3) | NS |
| Autoimmune hepatitis | 2 (6) | 0 | NS |
| Cryptogenic | 2 (6) | 4 (6) | NS |
| Child-Pugh classification (%) | |||
| A | 4 (12) | 22 (34) | 0.02 |
| B | 9 (27) | 16 (25) | NS |
| C | 20 (61) | 27 (41) | NS |
| Child-Pugh score | 10.3 [2.5] | 9.04 [2.5] | 0.025 |
| Bilirubin* (mg/dl) | 7.4 [8.9] | 5.3 [7.8] | 0.04 |
| Albumin† (g/l) | 29.7 [6.6] | 33.3 [6.4] | 0.01 |
| Prothrombin time‡ (%) | 42.2 [17.4] | 58 [20.3] | <0.001 |
| Erythrocyte count (T/l) | 3.3 [0.6] | 3.7 [0.4] | 0.038 |
| Oesophageal varices (%) | 21 (64) | 36 (55) | NS |
| FEV1 % pred | 82 [18] | 87.6 [15] | NS |
| TLC % pred | 99 [17] | 107 [17] | NS |
| PaO2 (mm Hg) | 72.4 [14] | 84.7 [9] | <0.001 |
| AaDO2 (mm Hg) | 39.2 [13] | 27 [6.4] | 0.00001 |
| PaCO2 (mm Hg) | 31.8 [4.3] | 32 [6.4] | NS |
| Smoker (%) | 7 (21) | 11 (17) | NS |
| Ascites | 4 (12) | 13 (20) | NS |
| Chest radiograph: | |||
| Interstitial markings | 7 (21) | 2 (3) | 0.008 |
| Small pleural effusion | 2 (6) | 7 (11) | NS |
HCV, hepatitis C virus; HBV, hepatitis B virus; PBC, primary biliary cirrhosis; FEV1, forced expiratory volume in one second; TLC, total lung capacity; PaO2, partial pressure of arterial oxygen; PaCO2, partial pressure of arterial carbon dioxide; AaDO2, alveolar-arterial difference for the partial pressure of oxygen.
*Reference range <1 mg/dl.
†Reference range 34–48 g/l.
‡Reference range 75–120%.
Values are mean [SD] or number (%).
Thirty three of 98 patients (34%) had intrapulmonary vasodilation, as evidenced by contrast echocardiography. Demographic data of the subjects with and without a positive contrast echocardiography are shown in table 1 ▶. Patients with a positive contrast echocardiogram had more severe cirrhosis, as assessed by a significantly higher Child-Pugh score,26 compared with patients with a negative contrast echocardiography. Mean total bilirubin was significantly higher, and mean values for serum albumin, prothrombin time, and erythrocyte count were significantly lower in cirrhotics with a positive contrast echocardiography. Oesophageal varices were found more often in the group with a positive contrast echocardiography but the difference did not reach statistical significance. Lung function values (FEV1 and TLC) were slightly lower in cirrhotics with a positive contrast echocardiography compared with those with negative contrast echocardiograms. The parameters of arterial oxygenation, PaO2 and AaDO2, were highly significantly different, with lower values in the group with a positive contrast echocardiography. PaCO2 was reduced due to hyperventilation, with no difference between the two groups. The frequency of smokers was similar in both groups: seven patients (21%) in the group with a positive contrast echocardiography and 11 patients (17%) in the group with a negative contrast echocardiography. Ascites was present in four (12%) and 13 (20%) patients with and without a positive contrast echocardiography, respectively (NS). Chest radiograph showed interstitial markings, predominately in the lower lung fields, in seven (21%) and two (3%) patients with and without a positive contrast echocardiography, respectively (p<0.01). Small pleural effusions were seen in two (6%) contrast echo positive patients and in seven (11%) contrast echo negative patients (NS).
Prevalence of HPS (table 2 ▶)
Table 2.
Frequency of different cut off values for arterial oxygenation in cirrhotics with and without a positive contrast echocardiogram, the resulting prevalence of the hepatopulmonary syndrome (HPS)*, positive and negative predictive values, and overall accuracy for diagnosis of this syndrome
| Positive contrast echocardiogram (n=33) | Negative contrast echocardiogram (n=65) | Prevalence of HPS* (%) (95% CI) | Positive predictive value for diagnosis of HPS* (%) (95% CI) | Negative predictive value† for exclusion of HPS* (%) (95% CI) | Overall accuracy (95% CI) | |
| PaO2 <80 mm Hg14 | 19 (58%) | 24 (37%) | 19 (12–29) | 44 (29–60) | 75 (61–85) | 61 (51–71) |
| PaO2 <70 mm Hg5,7,8,13 | 14 (42%) | 1 (1%) | 15 (9–24) | 93 (68–100) | 77 (67–86) | 80 (70–87) |
| PaO2 <age related threshold value mm Hg | 15 (45%) | 1 (1%) | 15 (9–24) | 94 (70–100) | 78 (68–86) | 81 (71–88) |
| PaO2 <65 mm Hg | 13 (39%) | 0 | 13 (7–22) | 100 (75–100) | 76 (66–85) | 80 (70–87) |
| PaO2 <60 mm Hg | 9 (27%) | 0 | 12 (6–20) | 100 (66–100) | 73 (63–82) | 76 (66–84) |
| AaDO2 >15 mm Hg10,15 | 31 (94%) | 59 (91%) | 32 (23–42) | 34 (25–45) | 75 (34–97) | 38 (28–48) |
| AaDO2 >20 mm Hg16 | 30 (91%) | 50 (77%) | 31 (22–41) | 37 (27–49) | 83 (59–96) | 46 (36–56) |
| AaDO2 >age related threshold value mm Hg6,11 | 27 (81%) | 24 (37%) | 28 (19–37) | 53 (38–67) | 87 (74–95) | 69 (59–78) |
*Defined as: chronic liver disease and arterial hypoxaemia and pulmonary vasodilation (measured by contrast echocardiography).
†For negative predictive values the oxygenation cut off values are: PaO2 ≥80 mm Hg, ≥70 mm Hg, ≥age related threshold value, ≥65 mm Hg, ≥60 mm Hg; AaDO2 ≤15 mm Hg, ≤20 mm Hg, ≤age related threshold value.
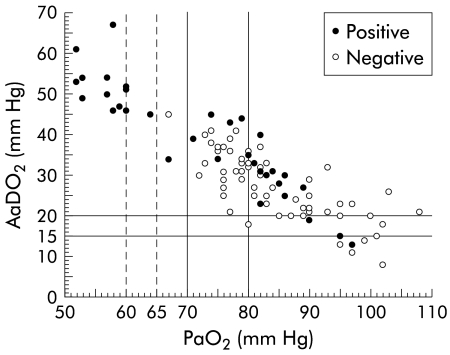
PaO2 and AaDO2 values of patients are plotted in fig 1 ▶. Vertical lines were added to show the previously published cut off values for PaO2 and AaDO2, and also PaO2 values of 60 and 65 mm Hg. The different cut off values of PaO2 and AaDO2 which, together with pulmonary vasodilation defined HPS previously, led to a wide range of prevalence rates for this syndrome (15–32%; see table 2 ▶). The prevalence of HPS was lowest when PaO2 <70 mm Hg was used as the cut off value (15%) and increased more than twofold when AaDO2 >15 mm Hg (32%) and >20 mm Hg (31%) were used. Overall, prevalence was higher when AaDO2 cut off values were used (28–32%) compared with PaO2 cut off values (15–19%). Introduction of an age related threshold value for PaO2 led to a prevalence of 15%. As expected, PaO2 <65 mm Hg and <60 mm Hg, threshold values for more severe hypoxaemia which were not used previously in definitions of HPS, resulted in the lowest prevalence rates (13% and 12%, respectively).
Figure 1.
Cirrhotic patients with positive or negative transthoracic contrast echocardiography according to their PaO2 and AaDO2 values. Previously published cut off values for a definition of hepatopulmonary syndrome were added as vertical lines in addition to those marking PaO2 values of 60 and 65 mm Hg. PaO2, arterial partial pressure of oxygen; AaDO2, alveolar-arterial difference for PaO2.
Positive predictive value for HPS (table 2 ▶)
Positive predictive values for different cut off values of arterial oxygenation for a diagnosis of HPS are presented in table 2 ▶. The positive predictive value for a diagnosis of HPS was highest (100%) with PaO2 <65 mm Hg and <60 mm Hg, and slightly lower (93% and 94%) with PaO2 <70 mm Hg and <age related threshold value, respectively. Using other oxygenation cut off values (PaO2 <80 mm Hg, AaDO2 >15 mm Hg, AaDO2 >20 mm Hg, and >age related threshold value) resulted in very low positive predictive values for a diagnosis of HPS.
Negative predictive value for HPS and overall accuracy (table 2 ▶)
Negative predictive values for exclusion of HPS ranged from 73% to 78% when PaO2 was used as a cut off, and increased to 83% for AaDO2 >20 mm Hg and to 87% for AaDO2 >age related threshold value. Overall accuracy (relative part of correctly classified patients) was highest for PaO2 <age related threshold value (81%), followed by PaO2 <70 mm Hg (80%), and PaO2 <65 mm Hg (80%).
Frequency of a positive contrast echocardiography according to different grades of gas exchange abnormalities
In the patient group with moderate to severe gas exchange abnormalities, the frequency of a positive contrast echocardiography was high: 14/15 (93%) patients with PaO2 <70 mm Hg, 15/16 (94%) patients with PaO2 <age related threshold value, 13/13 (100%) patients with PaO2 <65 mm Hg, and 9/9 (100%) patients with PaO2 <60 mm Hg had a positive contrast echocardiography.
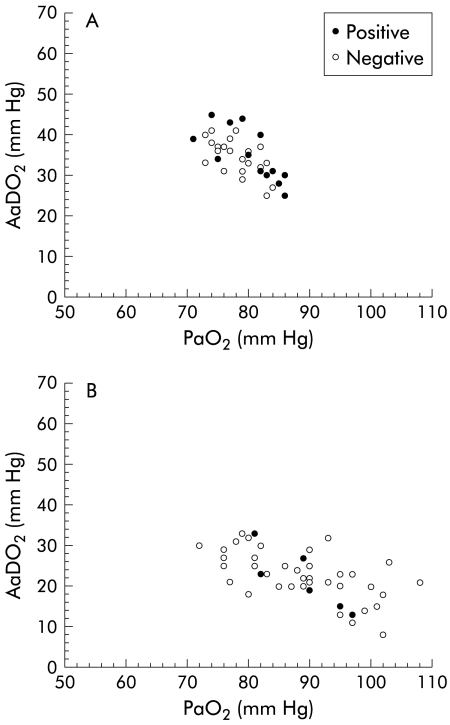
In contrast, in the normoxaemic group (PaO2 >70 mm Hg) with an elevated AaDO2 (>age related threshold value), 13/36 (36%) patients had a positive contrast echocardiography (fig 2A ▶).
Figure 2.
(A) Subgroup of patients with only mild oxygenation abnormalities (PaO2 >70 mm Hg and AaDO2 >age related threshold value), plotted according to their PaO2 and AaDO2 values, with positive or negative transthoracic contrast echocardiography. (B) Subgroup of patients with normal gas exchange (AaDO2 <age related threshold value), plotted according to their PaO2 and AaDO2 values, with positive or negative transthoracic contrast echocardiography. PaO2, arterial partial pressure of oxygen, AaDO2, alveolar-arterial difference for PaO2.
In the group with normal gas exchange, defined by AaDO2 <age related threshold value, 6/47 (13%) patients had a positive contrast echocardiography (fig 2B ▶).
Clinical features of patients with “clinically significant” and “subclinical” HPS (table 3 ▶)
Table 3.
Clinical features of cirrhotics with “clinically significant” hepatopulmonary syndrome (HPS), “subclinical” HPS, and no HPS
| “Clinically significant” HPS* (n=14) and positive contrast echo | “Subclinical” HPS† (n=13) and positive contrast echo | No HPS (n=71) | p Value | |
| Dyspnoea | 8 (57%) | 1 (8%) | 4 (6%) | <0.001 |
| Spider naevi | 11 (79%) | 7 (54%) | 28 (39%) | <0.05 |
| Palmar erythema | 8 (57%) | 4 (31%) | 35 (49%) | NS |
| Child-Score | 11.6 (1.9) | 10.5 (3.1) | 9 (2.5) | <0.05 |
| OLT | 2 (14%) | 5 (38%) | 12 (17%) | |
| Complications | 0 | Embolic brain infarct 2; wound infection 1; prolonged weaning 1 | 0 | |
| Outcome | Both died, 2 and 10 months after OLT | 1 died, 6.5 months after OLT | 2 died, 7.5 and 9 months after OLT |
*Defined as hypoxaemia (PaO2 <70 mm Hg).
†Defined as normoxaemia (PaO2 ≥70 mm Hg and AaDO2 >age related threshold).
For clinical purposes, we subdivided the patient group with intrapulmonary vasodilation (assessed by a positive contrast echocardiography) and abnormal gas exchange into two groups: “clinically significant” HPS, characterised by hypoxaemia (PaO2 <70 mm Hg) and “subclinical” HPS, characterised by normoxaemia (PaO2 ≥70 mm Hg but AaDO2 >age related threshold). Fourteen patients with “clinically significant” HPS had a higher frequency of dyspnoea at rest (57%) compared with 13 “subclinical” HPS patients (8%) and the patient group without HPS (6%; p<0.001). Spider naevi were found more often in the “clinically significant” HPS group (79%) compared with the “subclinical” HPS group (54%) and the group without HPS (39%; p<0.05). The frequency of palmar erythema was not significantly different between the three groups. The Child-Pugh score was highest in “clinically significant” HPS patients (11.6 (1.9)), followed by the “subclinical” HPS patients (10.5 (3.1)); it was lowest in the patient group without HPS (9 (2.5); p<0.05).
Nineteen patients (19%) underwent orthotopic liver transplantation (OLT): two from the “clinically significant” HPS group, five from the “subclinical” HPS group, and 12 from the group without HPS. Two patients with “subclinical” HPS experienced cerebral embolic events. The first patient had two episodes of paresis of the right arm four years after transplantation; no cardiac or arterial source for cerebral embolism could be detected (no arrhythmias, normal transoesophageal echo, no patent foramen ovale, normal sonography of the carotid and vertebral arteries). The second patient had embolic brainstem infarct three years after OLT and no source for the emboli could be detected. Wound infection (abdominal wall abscess) occurred in one patient with “subclinical” HPS. None of the transplanted patients had respiratory problems in the postoperative period except for one “subclinical” HPS patient who needed prolonged weaning from the ventilator. Mortality was 37% (3/8) in the HPS group compared with 17% (2/12) in the group without HPS. Both transplanted patients with “clinically significant” HPS died two and 10 months after OLT because of progression of their underlying disease (OLT was performed because of hepatocellular carcinoma; they developed multiple metastasis formation after several weeks). One patient with “subclinical” HPS died 6.5 months after OLT due to an ileus, and two transplanted patients without HPS died 7.5 and 9 months after OLT (relapse of hepatocellular carcinoma; unknown reason).
DISCUSSION
Our study shows that defining hypoxaemia in HPS by different, previously used, cut off values for arterial oxygenation leads to wide variation in the prevalence of HPS in the same sample of cirrhotics. This clearly demonstrates that previously published data on the prevalence of HPS using different oxygenation cut off values cannot be compared directly.
Available data of previous publications on the frequency of positive contrast echocardiograms and the prevalence of HPS are provided in table 4 ▶ and grouped according to their preferred cut off value defining arterial hypoxaemia. The prevalence of HPS in our patients using PaO2 <70 mm Hg as the cut off value (15%) was within the reported range of 5–17.5%.5,7,8,11 Using PaO2 <80 mm Hg, the prevalence in our study (19%) was higher compared with the study of Vedrinne and colleagues (8%).10 This can be explained by the higher rate of hypoxaemic patients in our study population (43% of all patients had PaO2 values <80 mm Hg) compared with those in Vedrinne et al’s study10 (24% of all patients had PaO2 values <80 mm Hg). With AaDO2 >15 mm Hg as the cut off, the prevalence in our patients (31%) was also higher compared with the studies of Rolla and colleagues15 (20%) and Martinez-Palli and colleagues12 (19%). In the study of Rolla et al, fewer patients had a positive contrast echocardiography (27%) and patients were less hypoxaemic (mean PaO2 85.1 mm Hg v 80.5 mm Hg in our patients; mean AaDO2 17.8 mm Hg v 31.2 mm Hg in our patients). In the abstract by Martinez-Palli et al, no detailed data are provided on the frequency of positive contrast echocardiograms. In addition, AaDO2 >age related threshold was associated with a higher prevalence in our study (28%) compared with the studies of Stoller and colleagues13 (4%) and Aller and colleagues6 (16%). In Stoller et al’s study, HPS was diagnosed in four of 98 patients but the frequency of intrapulmonary vasodilation and arterial blood gas analysis in the remaining 94 patients were not provided. In the study of Aller and colleagues6 a different formula for calculation of AaDO2 may have been the reason for the different prevalence values. Also, the contrast agent used may have contributed to the different prevalence data. Krowka and colleagues7 and Stoller and colleagues13 in part used indocyanine green dye solution which provides microbubbles with diameters of up to 90 μm, while we and others (Hopkins and colleagues,8 Abrams and colleagues,5 Rolla and colleagues,15 and Aller and colleagues6) used saline solution which creates microbubbles of 15–180 μm. Vedrinne and colleagues10 used a modified fluid gelatine solution which creates microbubbles of 10±2 μm. The frequency of intrapulmonary vasodilation in our patients (34%), measured by transthoracic contrast echocardiography, was within the range reported in the literature (5–47 %, see table 3 ▶).
Table 4.
Frequency of a positive contrast echocardiography and prevalence of hepatopulmonary syndrome (HPS) among cirrhotics, reported in previous publications
| Cut off value for arterial oxygenation | n | % positive echo | % HPS | Reference |
| PaO2 <70 mm Hg | 38 | 13 | 5 | Krowka7 |
| 53 | 47 | 15 | Hopkins8 | |
| 47 | 23 | 13 | Jensen11 | |
| 40 | 38 | 17.5 | Abrams5 | |
| PaO2 <80 mm Hg | 37 | 32 | 8 | Vedrinne10 |
| 37 | 51* | 13* | Vedrinne10 | |
| AaDO2 >15 mm Hg | 45 | 27 | 20 | Rolla15 |
| 36 | NR | 19 | Martinez-Palli12 | |
| AaDO2 >age related threshold | 98 | NR | 4 | Stoller13 |
| 88 | 28 | 16 | Aller6 | |
| 88 | 42* | 22* | Aller6 |
*Transoesophageal echocardiography.
Figure 1 ▶ clearly illustrates that most of the study patients, those with as well as those without a positive contrast echocardiography, had AaDO2 values >15 mm Hg (90/98; 92%) and >20 mm Hg (80/98; 82%); this frequency was reduced with AaDO2 >age related threshold (51/98; 52%). In contrast, the frequency of abnormal PaO2 values was lower: 43% of all study patients had a PaO2 <80 mm Hg, 16% had a PaO2 <70 mm Hg or <age related threshold value, and 12% had a PaO2 <60 mm Hg. This higher frequency of patients with abnormal values of AaDO2 compared with PaO2 may be explained by the higher sensitivity of AaDO2 in describing the oxygenation abnormality because it includes the PaCO2 determination (which is low as a result of hyperventilation). Thus PaO2 may be normal yet as a result of hypocapnia there is an increased AaDO2.1,2
The resulting positive predictive values of all three AaDO2 cut off values for a diagnosis of HPS were low (34%, 37%, and 53%, respectively). In contrast, PaO2 cut off values generally showed a higher positive predictive value. Using PaO2 <80 mm Hg as the cut off, the positive predictive value was still low (44%) but increased with PaO2 <70 mm Hg (93%) and <age related threshold value (94%). No patient without a positive contrast echocardiography had PaO2 values below 65 mm Hg; thus cut off levels for more severe hypoxaemia (PaO2 <65 mm Hg and <60 mm Hg) led to a positive predictive value of 100%. Consequently, cirrhotics with PaO2 <70 mm Hg or <age related threshold value had a higher probability of HPS. Moreover, when PaO2 was below 65 mm Hg, a diagnosis of HPS was definitely established.
In the patient group with a normal PaO2 (>70 mm Hg) but elevated AaDO2, the frequency of positive contrast echocardiography was relatively low (36%). This frequency may be increased when using transoesophageal contrast echocardiography which has been shown previously to be superior to the transthoracic approach in the detection of intrapulmonary vasodilation in cirrhotics.6,10 In the study of Vedrinne and colleagues,10 the frequency of positive contrast echocardiography and the prevalence for HPS in cirrhotics increased by 5% and 19%, respectively, when the transoesophageal approach was used compared with the transthoracic procedure (see table 4 ▶). In concordance, using the transoesophageal technique, Aller et al reported an increase of 14% and 6% for detection of intrapulmonary vasodilation and prevalence for HPS (see table 4 ▶).6 Therefore, it seems reasonable that some of our patients with normoxaemia but elevated AaDO2 and a negative transthoracic contrast echocardiography would have had a positive transoesophageal contrast echocardiography. However, most studies investigating HPS have used transthoracic contrast echocardiography for the detection of intrapulmonary vasodilation,5,7,8,11–13,15 and all reviews on HPS describe transthoracic contrast echocardiography as the method of assessment of intrapulmonary vasodilation,1–4,16,27–29 apart from lung perfusion scan. Moreover, for our screening study with a relatively high patient number (127 patients) the usefulness of the transoesophageal technique was limited due to the imposed risk as an invasive procedure in patients who had a history of variceal bleeding. However, the correlation between intrapulmonary vasodilation and mild gas exchange abnormalities seems to be weak and other reasons for an elevated AaDO2 should be considered in these patients. In fact, of our 23 patients with normal PaO2 but elevated AaDO2 and negative contrast echocardiography, six had mild ascites, two had small pleural effusions, and two had mild pulmonary function test abnormalities (FEV1 and/or TLC between 66% and 85% of predicted), which might contribute to the elevated AaDO2.
Interstitial markings were observed significantly more often in contrast echo positive patients. They are typical of HPS,29–33 are predominately localised in the lower lung fields, and may reflect pulmonary vascular dilatations.
Patients with normal gas exchange may also show a positive contrast echocardiography (6/47; 13% in our study); this is in agreement with previous studies.5–8,10,11,15
As expected, a high percentage of patients with “clinically significant” HPS felt dyspnoeic (57%) whereas dyspnoea was seldom noted in the patient groups with “subclinical” HPS (8%) and without HPS (6%). It has been noted previously that patients with cutaneous spider naevi have more profound gas exchange abnormalities and more intrapulmonary vasodilation,34–36 suggesting that spider naevi might be a cutaneous marker of intrapulmonary vascular dilatations.3 Our study supports these previous reports with small patient numbers, and showed that there was a significant correlation between cutaneous spider naevi and the severity of HPS. Conflicting data exist in the literature regarding the correlation between HPS and the severity of liver disease. Whereas a study by Abrams and colleagues17 showed significantly lower PaO2 values, higher AaDO2 values, and greater shunt fractions in Child-Pugh A cirrhosis compared with Child-Pugh B and C classes, another study by Vachiéry and colleagues37 showed that hypoxaemic cirrhotics had a significantly higher Child-Pugh score. Our study clearly showed a significant correlation between the severity of HPS and Child-Pugh score. Patients with “subclinical” HPS bear the risk of developing “clinically significant” HPS during the course of their disease but the risk is unknown and should be determined in a prospective follow up study. Deterioration of gas exchange is not the only risk in these patients; they may develop other complications of intrapulmonary vasodilation, such as venous emboli passing through dilated intrapulmonary vessels into the systemic circulation. Two of our 13 “subclinical” HPS patients had embolic brain infarcts after OLT with no cardiac or arterial source detected for the cerebral embolism. Cerebral embolism due to venous emboli has been described previously in a few patients with HPS after OLT.38–40 For the development of wound infections after OLT, as observed in one of our patients, HPS may also play an important pathogenetic role.40
In conclusion, the use of various cut off values defining arterial hypoxaemia in HPS described in previously published studies leads to a wide range of prevalence rates for HPS in patients with cirrhosis. PaO2 values below 70 mm Hg or below the age related threshold value predict the presence of HPS with high probability in the absence of intrinsic cardiopulmonary diseases, whereas a PaO2 value <65 mm Hg definitely predicts a diagnosis of HPS. The severity of HPS is clearly correlated with the degree of liver disease. Whereas dyspnoea is often noted in patients with “clinically significant” HPS, patients with “subclinical” HPS may have complications not directly associated with impaired gas exchange but caused by dilated intrapulmonary vessels, such as venous emboli passing through the pulmonary circulation to the brain.
Acknowledgments
The authors thank the nursing staff of the intensive care unit, Department of Internal Medicine IV, University of Vienna, Austria, for help in performing the study.
Abbreviations
HPS, hepatopulmonary syndrome
PaO2, partial pressure of arterial oxygen
AaDO2, alveolar-arterial difference for the partial pressure of oxygen
PaCO2, partial pressure of arterial carbon dioxide
FEV1, forced expiratory volume in one second
TLC, total lung capacity
OLT, orthotopic liver transplantation
REFERENCES
- 1.Krowka MJ, Cortese DA. Hepatopulmonary syndrome. Current concepts in diagnostic and therapeutic considerations. Chest 1994;105:1528–37. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez-Roisin R, Agusti AG, Roca J. The hepatopulmonary syndrome: new name, old complexities. Thorax 1992;47:897–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lange PA, Stoller JK. The hepatopulmonary syndrome. Ann Intern Med 1995;122:521–9. [DOI] [PubMed] [Google Scholar]
- 4.Krowka MJ. Hepatopulmonary syndrome. In: Plevak D, Seeff L, eds. Critical care issues in liver transplantation: The American Association for the Study of the Liver and the International Liver Transplantation Society; 1999:58–65.
- 5.Abrams GA, Jaffe CC, Hoffer PB, et al. Diagnostic utility of contrast echocardiography and lung perfusion scan in patients with hepatopulmonary syndrome. Gastroenterology 1995;109:1283–8. [DOI] [PubMed] [Google Scholar]
- 6.Aller R, Moya JL, Moreira V, et al. Diagnosis of hepatopulmonary syndrome with contrast transesophageal echocardiography: advantages over contrast transthoracic echocardiography. Dig Dis Sci 1999;44:1243–8. [DOI] [PubMed] [Google Scholar]
- 7.Krowka MJ, Tajik AJ, Dickson ER, et al. Intrapulmonary vascular dilatations (IPVD) in liver transplant candidates. Screening by two-dimensional contrast-enhanced echocardiography. Chest 1990;97:1165–70. [DOI] [PubMed] [Google Scholar]
- 8.Hopkins WE, Waggoner AD, Barzilai B. Frequency and significance of intrapulmonary right-to-left shunting in end-stage hepatic disease. Am J Cardiol 1992;70:516–19. [DOI] [PubMed] [Google Scholar]
- 9.Park SC, Beerman LB, Gartner JC, et al. Echocardiographic findings before and after liver transplantation. Am J Cardiol 1985;55:1373–8. [DOI] [PubMed] [Google Scholar]
- 10.Vedrinne JM, Duperret S, Bizollon T, et al. Comparison of transesophageal and transthoracic contrast echocardiography for detection of an intrapulmonary shunt in liver disease. Chest 1997;111:1236–40. [DOI] [PubMed] [Google Scholar]
- 11.Jensen DM, Pothamsetty S, Ganger D, et al. Clinical manifestations of cirrhotic patients with intrapulmonary shunts. Gastroenterology 1994;106:A912. [Google Scholar]
- 12.Martinez-Palli G, Barberà J, Visa J, et al. Hepatopulmonary syndrome: Prevalence and clinical markers. Eur Respir J 1996;9:179. [Google Scholar]
- 13.Stoller JK, Lange PA, Westveer MK, et al. Prevalence and reversibility of the hepatopulmonary syndrome after liver transplantation. The Cleveland Clinic experience. West J Med 1995;163:133–8. [PMC free article] [PubMed] [Google Scholar]
- 14.Sherlock S. Disorders of the liver and the biliary system, 8th edn. Oxford: Blackwell, 1989.
- 15.Rolla G, Brussino L, Colagrande P, et al. Exhaled nitric oxide and oxygenation abnormalities in hepatic cirrhosis. Hepatology 1997;26:842–7. [DOI] [PubMed] [Google Scholar]
- 16.Castro M, Krowka MJ. Hepatopulmonary syndrome. A pulmonary vascular complication of liver disease. Clin Chest Med 1996;17:35–48. [DOI] [PubMed] [Google Scholar]
- 17.Abrams GA, Nanda NC, Dubovsky EV, et al. Use of macroaggregated albumin lung perfusion scan to diagnose hepatopulmonary syndrome: a new approach. Gastroenterology 1998;114:305–10. [DOI] [PubMed] [Google Scholar]
- 18.Murray JF, Nadel JA. Textbook of respiratory medicine. 2nd edn. Philadelphia: Saunders, 1994.
- 19.Schenk P, Globits S, Koller J, et al. Accuracy of echocardiographic right ventricular parameters in patients with different end-stage lung diseases prior to lung transplantation. J Heart Lung Transplant 2000;19:145–54. [DOI] [PubMed] [Google Scholar]
- 20.Kim WR, Krowka MJ, Plevak DJ, et al. Accuracy of Doppler echocardiography in the assessment of pulmonary hypertension in liver transplant candidates. Liver Transpl 2000;6:453–8. [DOI] [PubMed] [Google Scholar]
- 21.Burghuber OC. Doppler assessment of pulmonary haemodynamics in chronic hypoxic lung disease. Thorax 1996;51:9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rich S. Nomenclature and classification of pulmonary hypertension. In: Primary Pulmonary Hypertension: Executive Summary from the World Symposium—Primary Pulmonary Hypertension, Evian, France, 1998:25–7. Co-sponsered by The World Health Organization.
- 23.Teague SM, Sharma MK. Detection of paradoxical cerebral echo contrast embolization by transcranial Doppler ultrasound. Stroke 1991;22:740–5. [DOI] [PubMed] [Google Scholar]
- 24.West JB. Pulmonary pathophysiology—the essentials, 4th edn. Baltimore: Williams and Wilkins, 1990.
- 25.American Thoracic Society Executive Committee. Recommended standardized procedures for pulmonary testing. Am Rev Respir Dis 1978;118:55–72.677558 [Google Scholar]
- 26.Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973;60:646–9. [DOI] [PubMed] [Google Scholar]
- 27.Krowka MJ. Clinical management of hepatopulmonary syndrome. Semin Liver Dis 1993;13:414–22. [DOI] [PubMed] [Google Scholar]
- 28.Herve P, Lebrec D, Brenot F, et al. Pulmonary vascular disorders in portal hypertension. Eur Respir J 1998;11:1153–66. [DOI] [PubMed] [Google Scholar]
- 29.Muller C, Schenk P. Hepatopulmonary syndrome. Wien Klin Wochenschr 1999;111:339–47. [PubMed] [Google Scholar]
- 30.Stanley NN, Woodgate DJ. Mottled chest radiograph and gas transfer defect in chronic liver disease. Thorax 1972;27:315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krowka MJ, Cortese DA. Severe hypoxemia associated with liver disease: Mayo Clinic experience and the experimental use of almitrine bismesylate. Mayo Clin Proc 1987;62:164–73. [DOI] [PubMed] [Google Scholar]
- 32.Hourani JM, Bellamy PE, Tashkin DP, et al. Pulmonary dysfunction in advanced liver disease: frequent occurrence of an abnormal diffusing capacity. Am J Med 1991;90:693–700. [PubMed] [Google Scholar]
- 33.McAdams HP, Erasmus J, Crockett R, et al. The hepatopulmonary syndrome: radiologic findings in 10 patients. AJR Am J Roentgenol 1996;166:1379–85. [DOI] [PubMed] [Google Scholar]
- 34.Robin ED, Horn B, Goris ML, et al. Detection, quantitation and pathophysiology of lung “spiders”. Trans Assoc Am Physicians 1975;88:202–16. [PubMed] [Google Scholar]
- 35.Rodriguez-Roisin R, Roca J, Agusti AG, et al. Gas exchange and pulmonary vascular reactivity in patients with liver cirrhosis. Am Rev Respir Dis 1987;135:1085–92. [DOI] [PubMed] [Google Scholar]
- 36.Andrivet P, Cadranel J, Housset B, et al. Mechanisms of impaired arterial oxygenation in patients with liver cirrhosis and severe respiratory insufficiency. Effects of indomethacin. Chest 1993;103:500–7. [DOI] [PubMed] [Google Scholar]
- 37.Vachiéry F, Moreau R, Hadengue A, et al. Hypoxemia in patients with cirrhosis: relationship with liver failure and hemodynamic alterations. J Hepatol 1997;27:492–5. [DOI] [PubMed] [Google Scholar]
- 38.Abrams GA, Rose K, Fallon MB, et al. Hepatopulmonary syndrome and venous emboli causing intracerebral hemorrhages after liver transplantation: a case report. Transplantation 1999;68:1809–11. [DOI] [PubMed] [Google Scholar]
- 39.Oh KS, Bender TM, Bowen A, et al. Plain radiographic, nuclear medicine and angiographic observations of hepatogenic pulmonary angiodysplasia. Pediatr Radiol 1983;13:111–5. [DOI] [PubMed] [Google Scholar]
- 40.Egawa H, Kasahara M, Inomata Y, et al. Long-term outcome of living related liver transplantation for patients with intrapulmonary shunting and strategy for complications. Transplantation 1999;67:712–17. [DOI] [PubMed] [Google Scholar]