Abstract
Background and aim: Macrophage inflammatory protein 3α (MIP-3α) is a recently described lymphocyte directed C-C chemokine expressed predominately at extralymphoid sites, including the intestine. The aim of this study was to determine whether colonic epithelial cells produce MIP-3α and whether its expression is upregulated in inflammatory bowel disease.
Methods and results: We found that interleukin 1β and tumour necrosis factor α dose dependently stimulated MIP-3α production in Caco-2 and HT-29 intestinal epithelial cells. In cytokine treated Caco-2 and HT-29 cells, a significant increase in MIP-3α protein production was observed after three hours and continued for at least 24 hours. Analysis of colonic tissues by quantitative real time polymerase chain reaction and ELISA revealed significantly elevated MIP-3α mRNA levels (7.9-fold; p<0.05) and protein levels (8.9-fold; p<0.05) in Crohn’s disease compared with controls or ulcerative colitis. MIP-3α immunoreactivity in normal colon and inflammatory bowel disease was principally associated with crypt and surface epithelial cells. Moreover, MIP-3α protein levels were elevated in primary epithelial cells isolated from patients with inflammatory bowel disease.
Conclusions: These findings indicate that increased enterocyte MIP-3α production may play an important role in lymphocyte activation and recruitment to the colonic epithelium in Crohn’s disease and ulcerative colitis.
Keywords: chemokine, inflammation, enterocyte, intestine
The chemokine superfamily of chemoattractant cytokines is comprised of small (8–10 kDa), inducible, proinflammatory proteins that specialise in mobilising leucocytes to areas of immune challenge.1–4 Interaction of these molecules with their respective leucocyte receptors induces a characteristic set of responses that are necessary for leucocytes to leave the circulation and infiltrate tissues. These include formation of lamellipodia, elevation of intracellular calcium levels, modulation of adhesion molecule expression, and migration of leucocytes along a chemotactic gradient. Thus increased chemokine production and release is an important mechanism regulating leucocyte activation and recruitment in response to injury or infection.
To date, over 40 members of the chemokine family have been identified. These can be classified into one of four subfamilies according to the number and arrangement of conserved cysteine residues (C, C-C, C-X-C, or C-X3-C).2,5–7 Members of the C-C chemokine family (for example, RANTES, monocyte chemotactic protein (MCP)-1, 2, 3, and 4, macrophage inflammatory protein (MIP)-1α and β, MIP-3α and β), the C chemokine family (for example, lymphotactin), and the C-X3-C chemokine family (for example, neurotactin/fractalkine) primarily activate and recruit mononuclear cells such as monocytes/macrophages and lymphocytes. In contrast, most C-X-C chemokines (for example, interleukin (IL)-8, ENA-78, GRO-α) activate neutrophils.
MIP-3α (also known as liver and activation regulated chemokine) is a recently described C-C chemokine identified by screening the GenBank database of expressed sequence tags for novel chemokine molecules.8,9 An alternative splice variant of MIP-3α (exodus-1) which lacks an amino terminal alanine residue (Ala-27) has also been reported.10 Analysis of MIP-3α mRNA by northern blotting shows expression in both human small intestine and human colon (in addition to liver, lung, skin, prostate, and thymus). MIP-3α mRNA is evident in activated monocytes and dendritic cells,8 as well as cytokine stimulated primary keratinocytes, dermal fibroblasts, and dermal microvascular endothelial cells.11 Studies by Dieu and colleagues12,13 have also shown expression of MIP-3α mRNA and protein in crypt epithelial cells from inflamed human tonsils. Furthermore, Tanaka et al recently localised MIP-3α mRNA expression by in situ hybridisation to epithelial cells in human appendix.14 In monocytic cells, MIP-3α mRNA was rapidly upregulated by PMA and downregulated by the anti-inflammatory cytokine IL-10.8,9 These previous studies suggest that MIP-3α is secreted predominantly at extralymphoid sites in response to proinflammatory stimuli.
Baba et al have demonstrated that CCR6 (formally the orphan receptors GPR-CY4, DRY6, CKR-L3, and STRL22) is a functional receptor for MIP-3α.15 While MIP-3α is the only known chemokine ligand for CCR6, recent studies have shown that this protein can also act as a functional receptor for the antimicrobial peptides β-defensin 1 and 2.16 In contrast with expression of MIP-3α mRNA, expression of CCR6 mRNA is mainly observed in lymphoid tissues, including the spleen, lymph node, and appendix.15 CCR6 mRNA has also been detected in CD4+ and CD8+ T lymphocytes, B lymphocytes, immature dendritic cells, and activated neutrophils.15,17–19 Interestingly, functional studies have shown that binding of MIP-3α to CCR6 induces migratory responses in immature dendritic cells and lymphocytes within the memory subset.12,19–21 In keeping with these findings, CCR6 has been found to be essential for memory T cell adhesion to tumour necrosis factor α (TNF-α) activated dermal microvascular endothelial cells under physiological flow conditions.22 Moreover, a study by Campbell et al has shown that MIP-3α can mediate lymphocyte adhesion to mucosal addressin cell adhesion molecule 1 (MadCAM-1)20 which is a gastrointestinal specific counter receptor for α4β7 integrin.
Evidence from human studies and from transgenic/knockout animal models indicate that activated memory CD4+ T lymphocytes are likely to play a central role in the pathogenesis of intestinal inflammation and injury in inflammatory bowel disease (IBD). Chronic inflammatory cells in IBD, particularly Crohn’s disease, are composed of large numbers of memory CD4+ T lymphocytes.23 Moreover, CD4+ T lymphocyte mediated intestinal injury has also been reported in a number of animal models of IBD (for example, IL-2 and IL-10 knockout mice, HLA-B27/β2m transgenic rats, F1 bone marrow transplanted CD3ɛ26 transgenic mice, and the CD45RBHIGH reconstituted SCID mouse).24 The aim of this study was to determine whether colonic epithelial cells produce MIP-3α and whether its expression is upregulated in IBD. Our findings demonstrate that human enterocytes are a major source of MIP-3α and that epithelial expression of this chemokine is elevated in Crohn’s disease and ulcerative colitis. Thus increased production of MIP-3α by colonic epithelial cells may play an important role in α4β7/MAdCAM-1 mediated memory CD4+ T lymphocyte recruitment during IBD.
MATERIALS AND METHODS
Cell culture
Caco-2 cells (American Type Culture Collection) were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum, 100 units/ml penicillin G sodium, 100 μg/ml streptomycin sulphate, and non-essential amino acids (Sigma, St Louis, Missouri, USA). HT-29 cells (American Type Culture Collection) were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum, 100 units/ml penicillin G sodium, and 100 μg/ml streptomycin sulphate. Both cell lines were incubated at 37°C in an atmosphere of 5% CO2 and 95% air. All cell culture experiments were carried out using 12 or 96 well tissue culture plates (Corning Costar, Acton, Massachusetts, USA). For cytokine stimulation experiments, confluent monolayers of Caco-2 or HT-29 cells were incubated with complete tissue culture media containing 0.01–100 ng/ml recombinant IL-1β or TNF-α (R&D Systems, Minneapolis, Minnesota, USA).
Human colonic tissue specimens
For studies of MIP-3α mRNA and protein levels, full thickness cryopreserved human colonic tissue samples were obtained from the tissue bank of the Massachusetts General Hospital, Center for the Study of Inflammatory Bowel Disease. Tissue samples were studied from patients who had undergone colectomy for ulcerative colitis or Crohn’s colitis. Areas of uninvolved colon were also studied from patients undergoing colectomy for colon cancer to serve as non-inflamed controls. For immunohistochemical studies of MIP-3α production, cryopreserved human biopsy specimens from normal colon, ulcerative colitis, and Crohn’s colitis were obtained from the tissue bank of the Massachusetts General Hospital, Center for the Study of Inflammatory Bowel Disease. All cryopreserved tissue samples were stored at −80°C.
For MIP-3α ELISA, the full thickness colonic tissue specimens were homogenised in phosphate buffered saline (PBS) containing a cocktail of protease inhibitors (Mini-Complete; Roche Molecular Biochemicals, Indianapolis, Indiana, USA) supplemented with 1 mM PMSF. After centrifugation at 20 000 g for 10 minutes at 4°C, the total protein level of the supernatants was determined by the BCA protein assay (Pierce, Rockford, Illinois, USA). For analysis of MIP-3α mRNA levels by real time quantitative polymerase chain reaction (Taqman assay), full thickness colonic tissue samples were homogenised in denaturing solution according to the manufacturer’s instructions (Stratagene, La Jolla, California, USA).
Preparation of isolated colonic epithelial cells
Isolated colonic epithelial cells were obtained from human colonic tissue by dispase treatment and Percoll density centrifugation, as described previously.25 Intestinal tissues for epithelial cell isolation were obtained from patients who had undergone colectomy for ulcerative colitis or Crohn’s colitis. The disease activity (active/inactive) of each tissue was assessed according to standard histological criteria.26,27 Areas of uninvolved colon were also studied from patients undergoing colectomy for colon cancer to serve as non-inflamed controls. Briefly, tissues were washed with RPMI plus antibiotics, incubated with 1/mM dithiothreitol (Life Technologies, Grand Island, New York, USA) to remove mucus, and then incubated with 2.4 U/ml dispase II (Roche) at 37°C for two hours. Liberated cells were centrifuged through a discontinuous Percoll density gradient (Sigma). Cells were used only if viability was >95%, as assessed by trypan blue exclusion. The degree of contaminating mononuclear cells was <5%, as assessed by morphology and immunostaining.
For MIP-3α ELISA, isolated epithelial cell preparations were washed three times by centrifugation at 2000 g for two minutes at 4°C in PBS containing a cocktail of protease inhibitors (Mini-Complete; Roche) supplemented with 1 mM PMSF. After washing, cells were resuspended in 0.5 ml PBS containing protease inhibitors, sonicated, and centrifuged at 20 000 g for 10 minutes at 4°C. The total protein level of the supernatants was then determined by the BCA protein assay (Pierce).
MIP-3α ELISA
To measure MIP-3α protein levels, a sandwich ELISA was developed. The wells of a Maxisorp 96 well microtitre plate (NUNC) were coated with 2.5 μg/ml goat-antihuman MIP-3α antibody (R&D Systems) in 50 μl of carbonate coating buffer (pH 9.6) overnight at 4°C. The goat antihuman MIP-3α antibody shows less than 10% cross reactivity with recombinant murine TECK and no cross reactivity with other chemokines, including human MCP-1, 2, 3, RANTES, MIP-1α, or MIP-1β. After washing with PBS containing 0.05% Tween 20 (PBS-T), wells were blocked with 4% bovine serum albumin (BSA) in PBS-T for one hour at room temperature. After further washing, duplicate 50 μl aliquots of sample or recombinant MIP-3α were added for one hour at room temperature. To detect bound MIP-3α, the goat antihuman MIP-3α antibody was biotinylated using NHS-LC biotin (Pierce) and incubated at 1 μg/ml in blocking buffer for one hour at room temperature. Following additional washing, streptavidin-biotin horseradish peroxidase complex (Amersham, Biosciences Piscataway, New Jersey, USA), diluted 1:1000 in PBS-T, was added and the plate was incubated at room temperature for 30 minutes. After washing, 50 μl of tetramethylbenzidine substrate solution (Kirkegaard and Perry Labs, Gaithersburg, Maryland, USA) was added to each well and, following colour development, the reaction was stopped with 50 μl of 1 M o-phosphoric acid. The optical density at 450 nm was read using an automated microtitre plate photometer (Dynatech, Chantilly, Virginia, USA). The sample concentration of MIP-3α was determined by comparison with a standard curve generated using recombinant MIP-3α. The MIP-3α ELISA had a sensitivity of 15–30 pg/ml, an intra-assay variation of 2.6% (n=4), and an inter-assay variation of 4.6% (n=4).
Analysis of steady state MIP-3α mRNA levels by reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated from Caco-2 cells or cryopreserved human colonic tissue resection specimens using guanidine isothiocyanate-phenol-chloroform extraction.28 RNA (1 μg) was reversed transcribed using random hexamer primers and Moloney murine leukaemia virus reverse transcriptase, as previously described,29 and the resulting cDNA was stored at −20°C. Polymerase chain reaction (PCR) amplification of MIP-3α and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNAs was performed as previously described with minor modifications.29 Briefly, cDNA samples (2 μl) were amplified by initial denaturation at 94°C for five minutes followed by an initial annealing step at 55°C (for MIP-3α) or 60°C (for GAPDH) for five minutes. Samples were then subjected to 24 cycles (for GAPDH) or 40 cycles (for MIP-3α) of extension at 72°C for 1.5 minutes, denaturation at 94°C for one minute, and annealing at 55°C (for MIP-3α) or 60°C (for GAPDH) for one minute, followed by a final extension at 72°C for 10 minutes. The primers used were as follows: MIP-3α sense, 5′ GAG-TTT-GCT-CCT-GGC-TGC-TTT 3′; MIP-3α antisense, 5′ TTT-ACT-GAG-GAG-ACG-CAC-AAT 3′; GAPDH sense, 5′ CCA-CCC-ATG-GCA-AAT-TCC-ATG-GCA 3′; and GAPDH antisense, 5′ TCT-AGA-CGG-CAG-GTC-AGG-TCC-ACC 3′. PCR products were analysed by electrophoresis through 1% agarose gels containing 500 ng/ml ethidium bromide and visualised using an ultraviolet transilluminator at 312 nm (Fisher Biotech, Pittsburgh, Pennsylvania, USA). Expected sizes for MIP-3α and GAPDH PCR products were 259 and 600 base pairs, respectively.
Analysis of MIP-3α mRNA levels using real time quantitative RT-PCR (Taqman assay)
MIP-3α mRNA levels and GAPDH mRNA levels in full thickness colonic tissue samples were quantified using a fluorogenic 5′-nuclease PCR assay30 with a GeneAmp 5700 sequence detection system (ABI/Perkin-Elmer). For each standard or sample, duplicate reactions containing 2.5 μl of cDNA were incubated for two minutes at 50°C, denatured for 10 minutes at 95°C, and subjected to 40 cycles of annealing at 55°C for 20 seconds, extension at 60°C for one minute followed by denaturation at 95°C for 15 seconds. The gene specific primers used were: MIP-3α sense primer, 5′ CTG-GCT-GCT-TTG-ATG-TCA-GTG 3′; MIP-3α antisense primer, 5′ GCA-GTC-AAA-GTT-GCT-TGC-TGC 3′; GAPDH sense primer, 5′ GAC-CAC-AGT-CCA-TGC-CAT-CA 3′; and GAPDH antisense primer, 5′ CAT-CAC-GCC-ACA-GTT-TCC-C 3′. To detect amplicons generated using the gene specific primers dual labelled fluorogenic (Taqman) probes containing FAM (at the 5′ end) and TAMRA (at the 3′ end) were synthesised (Sigma-Genosys, The Woodlands, Texas, USA). The Taqman probes used were: MIP-3α, 5′ TGC-TAC-TCC-ACC-TCT-GCG-GCG-AA 3′; and GAPDH, 5′ ACC-CAG-AAG-ACT-GTG-GAT-GGC-CCC 3′. The number of amplicons in each sample reaction was determined by comparison with a standard curve generated using five 10-fold dilutions of plasmids (10 pg to 1 fg) containing MIP-3α or GAPDH cDNA. MIP-3α mRNA levels are presented as the number of amplicons per 104 GAPDH amplicons.
Immunohistochemical studies of MIP-3α expression in human colon
Cyropreserved biopsy tissues were sectioned (6 μm thickness), fixed in 80% acetone for one minute, and air dried prior to storage over desiccant at −20°C. For immunostaining, sections were brought to room temperature, post fixed in 80% acetone for two minutes, air dried, and rehydrated in Tris buffered saline (TBS). Sections were then incubated in methanol containing 0.3% hydrogen peroxide to block endogenous peroxidase activity, washed (3×5 minutes in TBS), and incubated with unbound avidin and biotin. After washing, sections were incubated for one hour in TBS containing 1% BSA, 0.5% cold water fish gelatin, and 1.5% normal goat serum. The blocking solution was then removed by washing and sections were incubated overnight at 4°C with 5 μg/ml murine antihuman MIP-3α mAb (R&D Systems) or an IgG1 isotype control monoclonal antibody (Pharmingen, San Diego, California, USA). The following day sections were washed and then incubated with a biotin conjugated goat antimouse IgG (1:200; Caltag Laboratories, Burlingame, California, USA) for one hour at room temperature. Following washing, sections were treated with peroxidase conjugated streptavidin (Vector Laboratories, Burlingame, California, USA) for one hour at room temperature, washed again, and incubated with 3,3′-diaminobenzidine (1 mg/ml) and H2O2 for five minutes at room temperature. Staining present in each section was then intensified by incubation in 0.5% CuSO4 for five minutes at room temperature. Sections were then counterstained with methyl green, dehydrated in ascending ethanol solutions, cleared with xylene, and mounted with Permount.
Statistical analyses
Statistical analyses were performed using SigmaStat for Windows version 2.0 (SPSS Science, Chicago, Illinois, USA). Analysis of variance followed by t tests were used for intergroup comparisons, except where otherwise stated.
RESULTS
Human gastrointestinal epithelial cell lines produce MIP-3α in response to cytokine stimulation
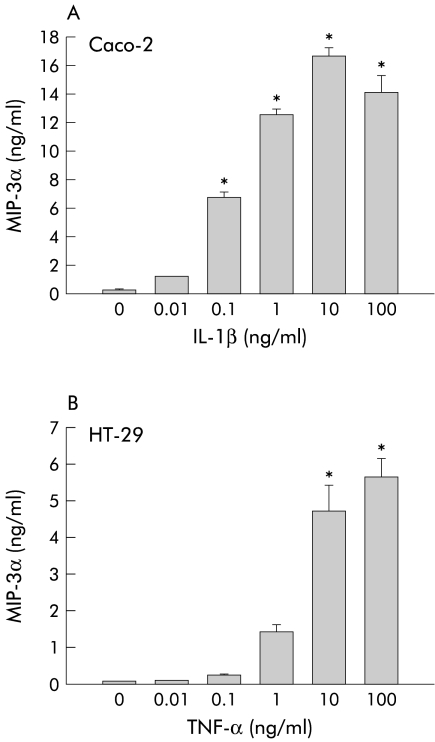
Studies from our laboratory and others indicate that human intestinal epithelial cells produce both C-C and C-X-C chemokines when stimulated by IL-1β, TNF-α, pathogenic bacteria, and viruses—for example, rotavirus.31–36 To determine whether enterocytes also produce MIP-3α, conditioned media from a variety of cytokine stimulated human gastrointestinal epithelial cell lines were tested for MIP-3α protein levels by ELISA. As shown in fig 1A ▶, non-stimulated Caco-2 monolayers secreted small amounts of MIP-3α. Exposure to IL-1β dose dependently increased levels of MIP-3α in conditioned media after 24 hours. Significantly increased levels of MIP-3α were observed at a concentration of 0.1 ng/ml IL-1β with maximal secretion occurring at 10 ng/ml IL-1β. A similar pattern was observed with HT-29 monolayers stimulated by TNF-α (fig 1B ▶). Again, conditioned media from non-stimulated HT-29 monolayers contained little MIP-3α. Stimulation of HT-29 monolayers with TNF-α also dose dependently increased MIP-3α secretion. Statistically significant increases were observed at concentrations of 10–100 ng/ml.
Figure 1.
Human gastrointestinal epithelial cell lines produce macrophage inflammatory protein (MIP)-3α in response to cytokine stimulation. (A) Confluent Caco-2 cell monolayers were exposed to recombinant interleukin (IL)-1β (0.01–100 ng/ml) for 24 hours. MIP-3α protein levels in conditioned media were then measured by ELISA. (B) Confluent HT-29 cell monolayers were exposed to recombinant tumour necrosis factor α (TNF-α 0.01–100 ng/ml) for 24 hours and MIP-3α protein levels in conditioned media were then measured by ELISA. Data are expressed as mean (SEM) (n=4). *p<0.05 versus non-stimulated cells.
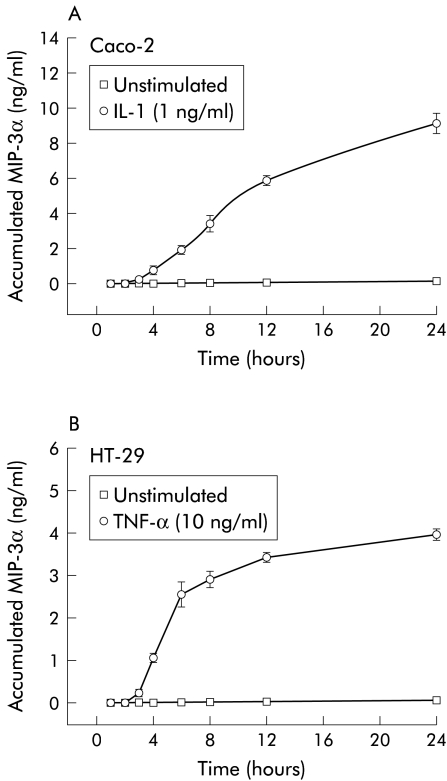
Previous studies indicate that some chemokines (for example, IL-8 and MCP-1) are produced with rapid kinetics for short periods whereas others (for example, ENA-78 and RANTES) are produced with slower kinetics and are secreted over longer time courses. These differences in chemokine gene expression suggest that temporal chemokine gradients are likely to be functionally important for leucocyte trafficking to areas of inflammation in vivo. To determine whether production of MIP-3α by intestinal epithelial cells followed either rapid- or delayed-type kinetics, we next examined the time course of its production by ELISA in Caco-2 cells treated with IL-1β (fig 2A ▶). Control Caco-2 monolayers secreted little MIP-3α over the 24 hour period of the experiment. In contrast, a significant increase in MIP-3α protein production was detected in conditioned media from Caco-2 cells stimulated by IL-1β (1 ng/ml) for three hours. The rate of MIP-3α production by IL-1β treated Caco-2 cells was essentially linear up to 12 hours after which it began to decrease. A similar time course of MIP-3α production was also observed when HT-29 cells were treated with 10 ng/ml TNF-α (fig 2B ▶) or 1 ng/ml IL-1β (data not shown).
Figure 2.
Time course of macrophage inflammatory protein (MIP)-3α protein production by cytokine stimulated Caco-2 and HT-29 cells. (A) Confluent Caco-2 cell monolayers were incubated with or without recombinant interleukin (IL)-1β (1 ng/ml) for 0–24 hours. Accumulated MIP-3α protein levels in conditioned media from non-stimulated cells or cells treated with IL-1β were then measured by ELISA. Data are expressed as mean (SD) (n=4). (B) Confluent HT-29 cell monolayers were incubated with or without recombinant tumour necrosis factor α (TNF-α 10 ng/ml) for 0–24 h. Accumulated MIP-3α protein levels in conditioned media from non-stimulated cells or cells treated with TNF-α were then measured by ELISA. Data are expressed as mean (SD) (n=4).
MIP-3α production by human intestinal epithelial cells lines requires de novo mRNA synthesis
Previous studies have shown that the production of chemokines is largely regulated via upregulation of gene transcription.2,37 To determine whether the increased production of MIP-3α by cytokine stimulated intestinal epithelial cell lines required increased gene expression, we next examined steady state MIP-3α mRNA levels in IL-1β-treated Caco-2 cells by RT-PCR (fig 3A ▶). Steady state MIP-3α mRNA is undetectable in unstimulated Caco-2 cells. Following stimulation by IL-1β (1 ng/ml), steady state MIP-3α mRNA levels increase rapidly and are maximal after 2–8 hours after which they slowly decline. Analysis using ImageQuant software (Amersham Biosciences) indicated that at 24 hours MIP-3α mRNA levels were ∼ 65% of those observed at four hours. Similar findings were observed when HT-29 cells were stimulated by TNF-α (10 ng/ml) except that steady state MIP-3α mRNA levels begin to decline after six hours (fig 3B ▶). Image analysis indicated that MIP-3α mRNA levels at 24 hours were ∼45% of maximal levels (four hours). These findings indicate that MIP-3α protein production by cytokine stimulated intestinal epithelial cell lines appears to follow increases in steady state MIP-3α mRNA levels.
Figure 3.
Kinetics of increased steady state macrophage inflammatory protein (MIP)-3α mRNA levels in cytokine stimulated Caco-2 and HT-29 cells. (A) Confluent Caco-2 cell monolayers were exposed to recombinant interleukin (IL)-1β (1 ng/ml) for 0–24 hours. Steady state levels of MIP-3α mRNA and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA were then determined by reverse transcription-polymerase chain reaction (RT-PCR). (B) Confluent HT-29 cell monolayers were exposed to recombinant tumour necrosis factor α (TNF-α 10 ng/ml) for 0–24 hours. Steady state levels of MIP-3α mRNA and GAPDH mRNA were determined by RT-PCR.
In order to investigate whether MIP-3α protein production required de novo mRNA synthesis, we also performed experiments using actinomycin D, an inhibitor of mRNA production. Pretreatment of Caco-2 cells with actinomycin D (10 μg/ml) for 30 minutes almost completely abolished MIP-3α secretion by Caco-2 cells in response to IL-1β (94% inhibition; p<0.01). Taken together, these data suggest that cytokine stimulated MIP-3α production by intestinal epithelial cells is controlled primarily at the level of gene transcription.
MIP-3α mRNA and protein levels are increased in active Crohn’s disease
As our in vitro studies demonstrated that human intestinal epithelial cell lines can upregulate MIP-3α mRNA and protein production in response to proinflammatory cytokines, we next examined MIP-3α expression in normal human colon and in colonic resection tissue from patients with IBD. To quantify MIP-3α mRNA levels, a real time quantitative PCR (Taqman) assay was performed. As shown in fig 4A ▶, colonic tissue from patients with active Crohn’s disease showed significantly elevated MIP-3α mRNA levels (7.9-fold; p<0.05) compared with non-inflamed control colon. MIP-3α mRNA levels in colonic tissues from patients with active ulcerative colitis were also elevated (2.8-fold) but this increase did not reach statistical significance. Homogenates of each tissue sample were also subjected to ELISA to quantify MIP-3α protein levels. As shown in fig 4B ▶, significantly increased MIP-3α protein levels (8.9-fold; p<0.05) were observed in colonic tissue from patients with active Crohn’s disease compared with normal colon. MIP-3α protein levels in colonic tissue from patients with active ulcerative colitis were similar to those found in non-inflamed control colon. Further analysis of the data presented in fig 4 ▶ indicated a strong positive correlation between MIP-3α mRNA and protein levels in the colonic tissue specimens examined (r=0.844, p<0.001 by Pearson product moment test).
Figure 4.
Macrophage inflammatory protein (MIP)-3α mRNA and protein levels are elevated in Crohn’s disease. Colonic tissue samples were obtained from patients undergoing colectomy for ulcerative colitis or Crohn’s colitis and from patients undergoing colectomy for colon cancer to serve as non-inflamed controls. (A) Total RNA derived from each tissue was reverse transcribed and subjected to real time quantitative reverse transcription-polymerase chain reaction (RT-PCR) to evaluate MIP-3α and GAPDH mRNA levels. Results are expressed as the number of MIP-3α amplicons per 104 glyceraldehyde-3-phosphate dehydrogenase (GAPDH) amplicons. The horizontal bar represents the mean value for each group. (B) Tissue homogenates from each specimen were subjected to ELISA to quantify MIP-3α protein levels. Results are expressed as ng of MIP-3α per mg of total homogenate protein. The horizontal bar represents the mean value for each group.
Enterocytes are the primary source of MIP-3α immunoreactivity in normal colon and inflammatory bowel disease
We next performed immunohistochemical studies to examine the sites of MIP-3α production in human colonic tissue. As shown in fig 5B ▶, in normal colon MIP-3α immunoreactivity was principally associated with both crypt and surface epithelial cells. Focal staining of occasional cells in the lamina propria was also observed. Some non-specific staining of lamina propria cells was evident in normal colon sections using an isotype control monoclonal IgG1 antibody (fig 5A ▶) but little staining of enterocytes was observed.
Figure 5.
Enterocytes are the primary site of macrophage inflammatory protein (MIP)-3α production in normal colon and inflammatory bowel disease. (A, B) Normal colon. MIP-3α immunoreactivity is principally associated with colonic epithelial cells (B). Minimal staining of enterocytes was observed in normal colon using an isotype control monoclonal IgG1 antibody (A). (C, D) Ulcerative colitis. Similar to normal colon, MIP-3α immunoreactivity in ulcerative colitis was mainly associated with epithelial cells (D). Little staining of epithelial cells was seen in ulcerative colitis tissue samples stained with the isotype control monoclonal antibody (C). (E, F) Crohn’s disease. Again, in colonic tissues from patients with Crohn’s disease MIP-3α immunoreactivity was primarily observed in epithelial cells (F). In contrast, Crohn’s disease sections treated with the isotype control monoclonal antibody showed little immunostaining (E). (G, H) Antibody controls. Minimal staining of epithelial cells was seen in Crohn’s disease tissue samples when the primary antibody was omitted (G). As expected, no staining of Crohn’s disease tissues was observed on omission of both the primary and secondary antibodies (H). Magnification for each panel, ×200.
Sites of MIP-3α production in colonic tissues from patients with ulcerative colitis and Crohn’s disease were also investigated. Similar to our findings in normal colon, crypt epithelial cells were a major source of MIP-3α immunoreactivity in ulcerative colitis (fig 5D ▶) and Crohn’s disease (fig 5F ▶). In both tissues, some positive cells were also observed in the lamina propria or submucosal tissues. Again, little or no staining of enterocytes was observed when ulcerative colitis or Crohn’s disease tissue sections were stained with the isotype control monoclonal IgG1 antibody (fig 5C, 5E ▶, respectively). Interestingly, a similar pattern of staining to that seen with the isotype control monoclonal antibody was observed when the primary antibody was omitted (fig 5G ▶). This finding suggests that the staining seen in fig 5A, 5C, and 5E ▶ is likely non-specific. As expected, no staining was observed when both the primary and secondary antibodies were omitted (fig 5H ▶). Taken together, our immunohistochemical findings indicate that enterocytes, rather than lamina propria cells, are likely to be the major source of MIP-3α production in normal colon and IBD.
MIP-3α expression by primary epithelial cells is upregulated in IBD
As the findings of our immunohistochemical study clearly demonstrated strong expression of MIP-3α by colonic epithelial cells, we next sought to determine whether epithelial expression of this chemokine is elevated during IBD. As shown in fig 6 ▶, production of MIP-3α by primary epithelial cells isolated from patients with inactive ulcerative colitis was similar to that seen in normal colon (as determined by ELISA of tissue homogenates). In contrast, MIP-3α protein levels in primary epithelial cells isolated from patients with active ulcerative colitis were significantly elevated (2.6-fold; p<0.01) compared with epithelial cells isolated from non-inflamed control colon. A similar increase in epithelial cell MIP-3α protein levels was seen in patients with active Crohn’s disease (2.4-fold; p<0.05). Interestingly, a significant increase in MIP-3α protein production was observed in primary epithelial cells obtained from patients with inactive Crohn’s disease (1.7-fold; p<0.05).
Figure 6.
Macrophage inflammatory protein (MIP)-3α protein production by primary epithelial cells is increased during inflammatory bowel disease. Epithelial cells were isolated from tissue samples obtained from patients undergoing colectomy for ulcerative colitis (UC) or Crohn’s disease (CD), and from tissue samples obtained from patients undergoing colectomy for colon cancer to serve as non-inflamed controls. Homogenates of each tissue were prepared and subjected to ELISA to quantify MIP-3α protein levels. Results are expressed as ng of MIP-3α per mg of total homogenate protein. The horizontal bar represents the mean value for each group.
DISCUSSION
The aim of this study was to investigate whether human colonic epithelial cells produce the memory CD4+ T lymphocyte directed chemokine MIP-3α and to determine whether its expression was elevated in IBD. Our findings demonstrated that human intestinal epithelial cell lines can dose dependently upregulate MIP-3α production following stimulation by the proinflammatory cytokines IL-1β and TNF-α. Furthermore, in cytokine stimulated Caco-2 intestinal epithelial cells, we found that MIP-3α production requires de novo mRNA synthesis. Immunohistochemical analysis of normal human colon and colonic tissue from patients with ulcerative colitis or Crohn’s disease indicated that, in vivo, MIP-3α immunoreactivity was associated primarily with crypt epithelial cells and to a lesser extent with surface enterocytes. We also showed that MIP-3α mRNA and protein levels were significantly elevated in Crohn’s colitis compared with ulcerative colitis or normal colon. Moreover, we found that primary epithelial cells isolated from patients with IBD contained elevated levels of MIP-3α protein. These findings indicate that colonic epithelial cells are an important source of MIP-3α and that increased production of this chemokine may play a role in the recruitment of CD4+ T lymphocytes and immature dendritic cells to the epithelial layer in IBD.
A number of studies have shown that human intestinal epithelial cell lines produce both C-X-C and C-C chemokines in response to proinflammatory stimuli such as IL-1β and TNF-α as well during infection by enteroinvasive bacteria (for example, Salmonella dublin) and viruses (for example, rotavirus).31–36 Interestingly, studies from our group and others demonstrated that intestinal epithelial cells can differentially regulate both C-X-C and C-C chemokine gene expression.29,33 More specifically, some chemokines (for example, IL-8 and MCP-1) are produced with rapid kinetics for short periods whereas others (for example, ENA-78 and RANTES) are produced with slower kinetics and are secreted over longer time courses. In this study we found that levels of MIP-3α mRNA and protein were rapidly upregulated in cytokine stimulated Caco-2 and HT-29 cells with kinetics similar to those seen for IL-8 and MCP-1. However, in contrast with the transient production of IL-8 and MCP-1 protein, which ceases after 6–8 hours of stimulation, secretion of MIP-3α by these cell lines continued, albeit at a reduced rate, for 12–24 hours, similar to the time course of ENA-78 production. Similar findings have recently been reported by Izadpanah and colleagues.38 Interestingly, these authors also demonstrated that, using polarised Caco-2 cells grown in Transwell cultures, MIP-3α was secreted mainly into the basal compartment following IL-1β stimulation. Taken together with previous studies these findings suggest that differential upregulation of C-C and C-X-C chemokine gene expression following cell activation is likely to be an important mechanism for “fine tuning” leucocyte recruitment during inflammatory responses in vivo.
Epithelial cell production of chemokines that regulate innate immune responses has been reported in colonic tissues from patients with IBD. In a previous study our group has shown that enterocytes are a major source of the neutrophil directed C-X-C chemokine ENA-78 in colonic tissue from patients with ulcerative colitis and in normal colon.29 Similar findings have been reported by Z’Graggen and colleagues.39 However, in contrast with these findings, we and other workers found that colonic IL-8 mRNA and protein was produced mainly by activated monocytes/macrophages and endothelial cells, and to a lesser extent by neutrophils and epithelial cells.2,31,40–42 Production of the C-C chemokine MCP-1 by enterocytes in normal colon and colonic tissue from IBD has been reported by Reinecker and colleagues.43 Moreover, these authors found that MCP-1 mRNA levels were markedly upregulated in IBD tissue compared with normal colon. In situ hybridisation studies of IBD tissues have also demonstrated MCP-1 mRNA in lamina propria macrophages, smooth muscle cells, and endothelial cells.44
More recent studies have demonstrated that human colonic epithelial cells also produce a variety of chemokines that likely regulate adaptive immune responses. Epithelial production of the CD4+ T cell directed C-X-C chemokines IP-10 and MIG has been reported in non-inflamed colon.45 Enterocyte cell expression of IP-10, MIG, and I-TAC mRNA and protein is rapidly upregulated in human intestinal xenografts stimulated by interferon γ or interferon γ plus IL-1β.45 As MIP-3α is chemotactic for memory CD4+ T cells and immature dendritic cells, this chemokine is also likely to play an important role in colonic adaptive immune responses. Tanaka et al have shown that epithelial cells, especially those lining lymphoid follicles, are a major site of MIP-3α production in murine intestinal tissues.14 These authors also found that MIP-3α production was markedly elevated following treatment of mice with lipopolysaccharide. In this study, we demonstrated that in normal colon and IBD epithelial cells, particularly in the crypt region, are a major site of MIP-3α production. Izadpanah et al have recently reported predominantly epithelial production of MIP-3α in IL-1α treated human intestinal zenografts and IBD.38 However, in contrast with our study, these authors found only minimal epithelial MIP-3α immunoreactivity in normal colonic tissue. While the reason for this difference is unclear, our observation that normal non-inflamed epithelial cells express significant amounts of MIP-3α constitutively suggests this protein may have important homeostatic functions in the colon. In support of this, Rescigno et al have shown recently that immature dendritic cells are recruited to a subepithelial position in the mouse intestine where, on upregulation of occludin, they intercalate with the epithelial layer to directly sample bacteria present in the lumen.46 Taken together with our findings, these data suggest that constitutive colonic MIP-3α production may be a mechanism for the recruitment of immature dendritic cells to the epithelium where the luminal contents can be continuously screened for pathogens.
In this study, we found that MIP-3α mRNA and protein levels were significantly elevated in colonic tissues from patients with active Crohn’s disease but not active ulcerative colitis. In contrast, we found that MIP-3α protein levels in isolated primary colonic epithelial cells were increased in both ulcerative colitis and Crohn’s disease. The reason for the discrepancy between MIP-3α production in active ulcerative colitis tissue and isolated colonic epithelial cells is unclear. One possible explanation is that the level of secreted MIP-3α is higher in Crohn’s disease tissue than ulcerative colitis tissue. Alternatively, there may be other non-epithelial sources of MIP-3α production in active Crohn’s disease.
The exact molecular mechanisms that give rise to ulcerative colitis and Crohn’s disease remain poorly understood. However, it is believed that, in genetically susceptible individuals, both disorders result from inappropriate or excessive immune responses to environmental factors such as elements of the intestinal microflora.47 Intestinal epithelial cells form the firstline of defence against pathogenic lumenal microbes and are recognised as active participants in mucosal immune and inflammatory reactions. Our finding of increased MIP-3α protein production by epithelial cells isolated from patients with Crohn’s disease and ulcerative colitis suggests that this chemokine may play an important role in the pathogenesis of human IBD. Interesting, Izadpanah et al have reported recently that MIP-3α protein production is upregulated following infection of enterocytes in vitro and in vivo by the exogenous enteric pathogens S dublin, E coli O29:NM, and S typhi.38 It should be noted however that based on the findings of this study we cannot exclude the possibility that the increased levels of epithelial MIP-3α production seen in vivo may be secondary to ongoing inflammation within intestinal tissues.
Evidence from human studies and from transgenic/knockout animal models (for example, IL-2 and IL-10 knockout mice, HLA-B27/β2m transgenic rats, F1 bone marrow transplanted CD3ɛ26 transgenic mice, and the CD45RBHIGH reconstituted SCID mouse) indicate that activated CD4+ T lymphocytes are likely to play a central role in the generation of intestinal inflammation and injury in IBD.48,49 In particular, Crohn’s disease is characterised by large numbers of infiltrating CD4+ T lymphocytes.23 The human studies presented herein are in agreement with recent experiments that demonstrate that MIP-3α mRNA levels are significantly elevated in chronically inflamed colons from IL-10 knockout mice and the CD4+CD45RBHIGH T cell transfer model of colitis.50 Moreover, inhibition of colonic inflammation in the IL-10 knockout model by treatment with anti-IL-12 monoclonal antibody resulted in downregulation of MIP-3α mRNA levels. Previous studies have shown that binding of MIP-3α to its receptor, CCR6, induces migratory responses in memory CD4+ T lymphocytes and immature dendritic cells.12,19–21 MIP-3α has also been shown to induce T lymphocyte adhesion to the gastrointestinal specific vascular addressin MadCAM-1.20 As active Crohn’s disease and the IL-10 knockout model of colitis are characterised by a prominent infiltration of the colonic mucosa by α4β7 bearing CD4+ T lymphocytes,23,51 these data suggest that MIP-3α may play an important role in the recruitment of these cells to the epithelial layer during intestinal inflammation by inducing their adhesion to MAdCAM-1, a gut specific vascular addressin.
Acknowledgments
This work was supported by National Institute of Health grants DK-54920, AI-23504, AI-24671, and AI-44236, and the Massachusetts General Hospital/Center for the Study of Inflammatory Bowel Diseases (DK-43551). ACK is the recipient of a Career Development Award from the Crohn’s and Colitis Foundation of America. The authors would also like to thank Dan Brown for his assistance with the immunohistochemical studies.
Abbreviations
MIP, macrophage inflammatory protein
MCP-1, monocyte chemotactic protein 1
MAdCAM-1, mucosal addressin cell adhesion molecule 1
IL, interleukin
TNF-α, tumour necrosis factor α
IBD, inflammatory bowel disease
PBS, phosphate buffered saline
BSA, bovine serum albumin
GAPDH, glyceraldehyde-3-phosphate dehydrogenase
RT-PCR, reverse transcription-polymerase chain reaction
TBS, Tris buffered saline
REFERENCES
- 1.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell 1994;76:301–14. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Baruch A, Michiel DF, Oppenheim JJ. Signals and receptors involved in recruitment of inflammatory cells. J Biol Chem 1996;270:11703–6. [DOI] [PubMed] [Google Scholar]
- 3.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol 1997;15:675–705. [DOI] [PubMed] [Google Scholar]
- 4.Taub DD. Chemokine-leukocyte interactions. The voodoo that they do so well. Cytokine Growth Factor Rev 1996;7:355–76. [DOI] [PubMed] [Google Scholar]
- 5.Bazan JF, Bacon KB, Hardiman G, et al A new class of membrane-bound chemokine with a CX3C motif. Nature 1997;385:640–4. [DOI] [PubMed] [Google Scholar]
- 6.Kelner GS, Kennedy J, Bacon KB, et al Lymphotactin: a cytokine that represents a new class of chemokine. Science 1994;266:1395–9. [DOI] [PubMed] [Google Scholar]
- 7.Pan Y, Lloyd C, Zhou H, et al Neurotactin, a membrane-anchored chemokine upregulated in brain inflammation. Nature 1997;387:611–17. [DOI] [PubMed] [Google Scholar]
- 8.Hieshima K, Imai T, Opdenakker G, et al. Molecular cloning of a novel human CC chemokine liver and activation-regulated chemokine (LARC) expressed in liver. Chemotactic activity for lymphocytes and gene localization on chromosome 2. J Biol Chem 1997;272:5846–53. [DOI] [PubMed] [Google Scholar]
- 9.Rossi DL, Vicari AP, Franz-Bacon K, et al. Identification through bioinformatics of two new macrophage proinflammatory human chemokines: MIP-3alpha and MIP-3beta. J Immunol 1997;158:1033–6. [PubMed] [Google Scholar]
- 10.Hromas R, Gray PW, Chantry D, et al. Cloning and characterization of exodus, a novel beta-chemokine. Blood 1997;89:3315–22. [PubMed] [Google Scholar]
- 11.Homey B, Dieu-Nosjean MC, Wiesenborn A, et al. Up-regulation of macrophage inflammatory protein-3 alpha/CCL20 and CC chemokine receptor 6 in psoriasis. J Immunol 2000;164:6621–32. [DOI] [PubMed] [Google Scholar]
- 12.Dieu MC, Vanbervliet B, Vicari A, et al. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med 1998;188:373–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dieu-Nosjean MC, Massacrier C, Homey B, et al. Macrophage inflammatory protein 3alpha is expressed at inflamed epithelial surfaces and is the most potent chemokine known in attracting Langerhans cell precursors. J Exp Med 2000;192:705–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka Y, Imai T, Baba M, et al. Selective expression of liver and activation-regulated chemokine (LARC) in intestinal epithelium in mice and humans. Eur J Immunol 1999;29:633–42. [DOI] [PubMed] [Google Scholar]
- 15.Baba M, Imai T, Nishimura M, et al. Identification of CCR6, the specific receptor for a novel lymphocyte-directed CC chemokine LARC. J Biol Chem 1997;272:14893–8. [DOI] [PubMed] [Google Scholar]
- 16.Yang D, Chertov O, Bykovskaia SN, et al. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science 1999;286:525–8. [DOI] [PubMed] [Google Scholar]
- 17.Yamashiro S, Wang JM, Yang D, et al. Expression of CCR6 and CD83 by cytokine-activated human neutrophils. Blood 2000;96:3958–63. [PubMed] [Google Scholar]
- 18.Greaves DR, Wang W, Dairaghi DJ, et al. CCR6, a CC chemokine receptor that interacts with macrophage inflammatory protein 3alpha and is highly expressed in human dendritic cells. J Exp Med 1997;186:837–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Power CA, Church DJ, Meyer A, et al. Cloning and characterization of a specific receptor for the novel CC chemokine MIP-3alpha from lung dendritic cells. J Exp Med 1997;186:825–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell JJ, Hedrick J, Zlotnik A, et al. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science 1998;279:381–4. [DOI] [PubMed] [Google Scholar]
- 21.Liao F, Rabin RL, Smith CS, et al. CC-chemokine receptor 6 is expressed on diverse memory subsets of T cells and determines responsiveness to macrophage inflammatory protein 3 alpha. J Immunol 1999;162:186–94. [PubMed] [Google Scholar]
- 22.Fitzhugh DJ, Naik S, Caughman SW, et al. Cutting edge: C-C chemokine receptor 6 is essential for arrest of a subset of memory T cells on activated dermal microvascular endothelial cells under physiologic flow conditions in vitro. J Immunol 2000;165:6677–81. [DOI] [PubMed] [Google Scholar]
- 23.James SP, Fiocchi C, Graeff AS, et al. Phenotypic analysis of lamina propria lymphocytes. Predominance of helper-inducer and cytolytic T-cell phenotypes and deficiency of suppressor-inducer phenotypes in Crohn’s disease and control patients. Gastroenterology 1986;91:1483–9. [PubMed] [Google Scholar]
- 24.Morales VM, Snapper SB, Blumberg RS. Probing the gastrointestinal immune function using transgenic and knockout technology. Curr Opin Gastroenterol 1996;12:577–83. [Google Scholar]
- 25.Panja A, Siden E, Mayer L. Synthesis and regulation of accessory/proinflammatory cytokines by intestinal epithelial cells. Clin Exp Immunol 1995;100:298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riddell RH. Histopathology of ulcerative colitis. In: Allan RN, Rhodes JM, Hanauer SB, et al, eds. Inflammatory bowel diseases, 3rd edn. New York: Churchill Livingstone, 1997:291–309.
- 27.Goldblum JR, Petras RE. Histopathology of Crohn’s disease. In: Allan RN, Rhodes JM, Hanauer SB, et al, eds. Inflammatory bowel diseases, 3rd edn. New York: Churchill Livingstone, 1997:311–16.
- 28.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate- phenol-chloroform extraction. Anal Biochem 1987;162:156–9. [DOI] [PubMed] [Google Scholar]
- 29.Keates S, Keates AC, Mizoguchi E, et al. Enterocytes are the primary source of the chemokine ENA-78 in normal colon and ulcerative colitis. Am J Physiol 1997;273:G75–82. [DOI] [PubMed] [Google Scholar]
- 30.Holland PM, Abramson RD, Watson R, et al. Detection of specific polymerase chain reaction product by utilizing the 5′—3′ exonuclease activity of Thermus aquaticus DNA polymerase. Proc Natl Acad Sci USA 1991;88:7276–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelly CP, Keates S, Siegenberg D, et al. IL-8 secretion and neutrophil activation by HT-29 colonic epithelial cells. Am J Physiol 1994;267:G991–7. [DOI] [PubMed] [Google Scholar]
- 32.Eckmann L, Jung HC, Schurer-Maly C, et al. Differential cytokine expression by human intestinal epithelial cell lines: regulated expression of interleukin 8. Gastroenterology 1993;105:1689–97. [DOI] [PubMed] [Google Scholar]
- 33.Yang SK, Eckmann L, Panja A, et al. Differential and regulated expression of C-X-C, C-C, and C- chemokines by human colon epithelial cells. Gastroenterology 1997;113:1214–23. [DOI] [PubMed] [Google Scholar]
- 34.Jung HC, Eckmann L, Yang SK, et al. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest 1995;95:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eckmann L, Kagnoff MF, Fierer J. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect Immun 1993;61:4569–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Casola A, Estes MK, Crawford SE, et al. Rotavirus infection of cultured intestinal epithelial cells induces secretion of CXC and CC chemokines. Gastroenterology 1998;114:947–55. [DOI] [PubMed] [Google Scholar]
- 37.Ye J, Young HA. Negative regulation of cytokine gene transcription. FASEB J 1997;11:825–33. [DOI] [PubMed] [Google Scholar]
- 38.Izadpanah A, Dwinell MB, Eckmann L, et al. Regulated MIP-3alpha/CCL20 production by human intestinal epithelium: mechanism for modulating mucosal immunity. Am J Physiol Gastrointest Liver Physiol 2001;280:G710–19. [DOI] [PubMed] [Google Scholar]
- 39.Z’Graggen K, Walz A, Mazzucchelli L, et al. The C-X-C chemokine ENA-78 is preferentially expressed in intestinal epithelium in inflammatory bowel disease. Gastroenterology 1997;113:808–16. [DOI] [PubMed] [Google Scholar]
- 40.Koch AE, Polverini PJ, Kunkel SL, et al. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science 1992;258:1798–801. [DOI] [PubMed] [Google Scholar]
- 41.Baggiolini M, Loetscher P, Moser B. Interleukin-8 and the chemokine family. Int J Immunopharmacol 1995;17:103–8. [DOI] [PubMed] [Google Scholar]
- 42.Baggiolini M, Walz A, Kunkel SL. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest 1989;84:1045–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reinecker HC, Loh EY, Ringler DJ, et al. Monocyte-chemoattractant protein 1 gene expression in intestinal epithelial cells and inflammatory bowel disease mucosa. Gastroenterology 1995;108:40–50. [DOI] [PubMed] [Google Scholar]
- 44.Grimm MC, Elsbury SK, Pavli P, et al. Enhanced expression and production of monocyte chemoattractant protein-1 in inflammatory bowel disease mucosa. J Leukoc Biol 1996;59:804–12. [DOI] [PubMed] [Google Scholar]
- 45.Dwinell MB, Lugering N, Eckmann L, et al. Regulated production of interferon-inducible T-cell chemoattractants by human intestinal epithelial cells. Gastroenterology 2001;120:49–59. [DOI] [PubMed] [Google Scholar]
- 46.Rescigno M, Urbano M, Valzasina B, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol 2001;2:361–7. [DOI] [PubMed] [Google Scholar]
- 47.Stenson WF. Inflammatory Bowel Disease In: Yamada T, ed. Textbook of gastroenterology, 2nd edn. Philadelphia: JB Lippincott Company, 1995:1748–806.
- 48.Elson CO, Sartor RB, Tennyson GS, et al. Experimental models of inflammatory bowel disease. Gastroenterology 1995;109:1344–67. [DOI] [PubMed] [Google Scholar]
- 49.Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology 1998;115:182–205. [DOI] [PubMed] [Google Scholar]
- 50.Scheerens H, Hessel E, Waal-Malefyt R, et al. Characterization of chemokines and chemokine receptors in two murine models of inflammatory bowel disease: IL-10−/− mice and Rag-2−/− mice reconstituted with CD4+CD45RBhigh T cells. Eur J Immunol 2001;31:1465–74. [DOI] [PubMed] [Google Scholar]
- 51.Davidson NJ, Leach MW, Fort MM, et al. T helper cell 1-type CD4+ T cells, but not B cells, mediate colitis in interleukin 10-deficient mice. J Exp Med 1996;184:241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]