Abstract
Background: Appendicectomy reduces the risk of having ulcerative colitis. However, its effect on the natural history of ulcerative colitis remains uncertain.
Aim: To determine whether appendicectomy reduces the overall severity of ulcerative colitis.
Patients and methods: Appendicectomy status and smoking habits were specified by direct interview in 638 patients seen consecutively between 1997 and 2000. Severity of ulcerative colitis was assessed by reviewing therapeutic needs from the onset of colitis. Additionally, the annual incidence of flare up was assessed prospectively between 1997 and 2000 in patients who had not been colectomised.
Results: The 10 year risk of colectomy was 16 (7)% in previously appendicectomised patients (n=49) compared with 33 (2)% in non-appendicectomised patients (n=589, p=0.05). Cox regression showed that previous appendicectomy and current smoking were independent factors protecting against colectomy (adjusted hazard ratio and 95% confidence intervals: 0.40 (0.20–0.78) and 0.60 (0.40–0.95), respectively). The respective proportions of appendicectomised and non-appendicectomised patients who required oral steroids and immunosuppressive therapy were not significantly different (67% v 70% and 27% v 19%, respectively). Between 1997 and 2000, ulcerative colitis was active for 48% of the time in appendicectomised patients (47 of 98 patient years) and for 62% of the time in non-appendicectomised patients (631 of 1024 patient years; p<0.01).
Conclusion: Previous appendicectomy is associated with a less severe course of ulcerative colitis. The beneficial effect of appendicectomy on the risk of colectomy is additive to that of current smoking.
Keywords: ulcerative colitis, appendicectomy, smoking
Two common environmental factors, cigarette smoking and appendicectomy, have been found to play a role in ulcerative colitis. Current smoking decreases the risk of developing ulcerative colitis1,2 and exerts a beneficial effect on the course of the disease.3–5 Conversely, smoking cessation increases the risk of ulcerative colitis6 and after acquired ulcerative colitis worsens its clinical course.7 Appendicectomy is protective. Compared with a matched control population, a smaller proportion of patients with ulcerative colitis were found to be appendicectomised.8–12 Moreover, several case reports have suggested that appendicectomy after disease onset could be a promising therapeutic option13,14 and a recent study has reported a lower percentage of ulcerative colitis relapse in 21 appendicectomised compared with non-appendicectomised patients.15
The aim of the present study was to ascertain whether previous appendicectomy influences the course of ulcerative colitis. The interrelation between smoking habits and appendicectomy was also analysed.
METHODS
Patient population
From January 1997 to December 2000, all consecutive patients with ulcerative colitis who attended our unit were included prospectively in the study. The diagnostic criteria for ulcerative colitis16 were based on the presence of at least three or four criteria after previous exclusion of infectious and neoplastic disease. The criteria were: typical case history with diarrhoea and/or blood and/or pus in the stools for more than a week in repeated episodes; typical findings on sigmoidoscopy with granulated friable mucosa with or without ulcerations of the mucosa; histological and/or cytological signs of inflammation of the mucosa; and radiological or colonoscopic signs of inflammation with a spiculated granulated inner surface of the colon proximal to the rectum and/or frank ulcerations.
Appendicectomy status and smoking habits
Appendicectomy and smoking status were specified during direct interview of the patient. Patients were classified as previously appendicectomised if appendicectomy had been performed prior to the diagnosis of colitis. They were classified as current smokers if they were smoking more than seven cigarettes per week for at least six months when ulcerative colitis was diagnosed.
Characteristics of colitis
The characteristics of colitis were completed according to retrospective analysis of medical charts. The onset of colitis, designated as the time of diagnosis, was defined as the date of first detection of unequivocal inflammatory abnormalities of the rectum, as assessed by endoscopic observations. The initial extent of colitis was determined by colonoscopy in most patients seen after 1975 whereas a few patients had only barium enema initially. After diagnosis, patients were followed clinically with 3–4 visits per year, and only investigated again in case of flare up or development of new symptoms. Morphological investigations included proctosigmoidoscopy, colonoscopy, and barium enema. The cumulative extent of colitis was established from the results of the latest colonoscopy performed from 1997. Isolated periappendicular inflammatory changes were not considered as a marker of extensive disease.
Treatment policy
Therapy was based on the principles of maintenance treatment with sulphasalazine or mesalamine, and treatment of flare up episodes with mesalamine or prednisolone enemas, increased oral mesalamine or sulphasalazine, or systemic glucocorticoids starting at 1 mg/kg/day prednisolone for 3–4 weeks, tapering off over a period of 1–2 months, and then withheld for a few weeks. When the flare up was severe or could not be controlled by this regimen, patients received intensive intravenous treatment according to Truelove and Jewell.17 Indications for colectomy were failure of intensive intravenous treatment, protracted dependence on oral steroids, and cancer or dysplasia detected during colonoscopic surveillance. Immunosuppressive therapy was rarely prescribed before 1995. Since then, cyclosporin has been used in some cases after failure of intensive intravenous treatment and azathioprine has been proposed in some patients who had a severe attack which responded to cyclosporin and in those who were steroid dependent.
Severity of ulcerative colitis
Overall severity of disease was assessed in several ways. Firstly, the colectomy rate, the time from diagnosis to colectomy, need for systemic steroids, and need for immunosuppressive therapy were assessed retrospectively. Secondly, patients who had not been colectomised prior to inclusion were followed up prospectively from the date of inclusion to December 2000, and the activity of the disease was assessed prospectively by analysing the occurrence of flare up each year. Each patient year was considered as active if a flare up occurred during the year, and inactive in the other cases.
Statistics
Data for appendicectomised and non-appendicectomised patients were compared using the Student’s t test or χ2 test as appropriate. When analysing the effect of previous appendicectomy alone, patients were classified according to their appendicectomy status at diagnosis. Patients who were appendicectomised after diagnosis were included in the non-appendicectomised group until the time when they underwent appendicectomy. When analysing the respective effects of appendicectomy and current smoking, current smokers at diagnosis were included in the smokers group until the time when they stopped smoking, and non-smokers or former smokers were included in the non-smokers group until the time when they started or resumed smoking. For actuarial analysis, the Kaplan-Meier model was used, with the date of diagnosis as the starting point. The curves were compared using the log rank test. Multivariate analyses were performed with Cox proportional hazards regression to adjust for confounding. All baseline variables suspected as possible predictors of colectomy (young age (<20 years), old age (≥40 years), sex, ethnicity (Caucasian or not), diagnosis after 1995, familial history, extraintestinal manifestations, initial extension of colitis, smoking status, and previous appendicectomy) were entered into the model. Diagnosis after 1995 was retained as a variable because the use of immunosuppressive therapy became widespread only after this date. Results of analysis are presented as hazard ratios with 95% confidence intervals (CIs). Calculations were performed using GB-stat statistical software.
RESULTS
Effect of previous appendicectomy
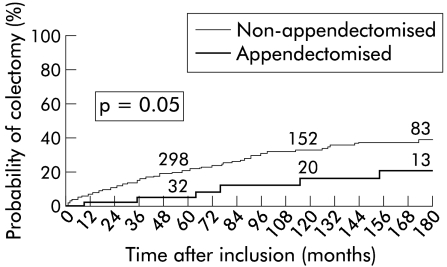
Among 638 patients with ulcerative colitis, 49 patients (8%) had undergone appendicectomy before disease onset. Appendicectomy had been performed one month to 66 years (median 17 years) before diagnosis of ulcerative colitis, at a median age of 11 years (range 6–43). Thirty five patients had undergone appendicectomy before the age of 20 years. As shown in table 1 ▶, compared with non-appendicectomised patients, appendicectomised patients were more often females and had a disease of longer duration. There were minor differences in initial extent of colitis which tended to have a more proximal location in appendicectomised patients (table 2 ▶). Cumulative location was similar in the two groups. Extent of colitis in patients with initial limited disease was observed with a similar frequency in the two groups (table 3 ▶). No patient from the appendicectomised group developed a colonic carcinoma compared with 11 non-appendicectomised patients (NS). Figure 1 ▶ shows the cumulative proportions of patients who had colectomy during the 15 years following diagnosis.
Table 1.
Main characteristics of ulcerative colitis in non-appendicectomised and appendicectomised patients
| Non-appendicectomised | Appendicectomised | p Value | |
| No of patients | 589 | 49 | |
| Female gender | 304 (52%) | 36 (73%) | 0.003 |
| Age at diagnosis (y) | 32.9 (14.4) | 35.7 (15.6) | NS |
| Duration of disease (y)* | 7.2 (8.3) | 10.1 (8.1) | 0.02 |
| Disease onset after 1995 | 155 (26%) | 12 (24%) | NS |
| Familial history | 62 (10%) | 4 (8%) | NS |
| Caucasian ethnicity | 433 (74%) | 40 (82%) | NS |
| Cigarette smoking | |||
| Never | 332 (56%) | 29 (59%) | NS |
| Latter | 8 (1%) | 1 (2%) | NS |
| Previous | 145 (25%) | 13 (27%) | NS |
| Current | 104 (18%) | 6 (12%) | NS |
| Oral contraceptive use | 75 (38%) | 5 (22%) | NS |
| Extraintestinal manifestations | 121 (21%) | 10 (20%) | NS |
*At last visit or when censored.
Table 2.
Disease location in non-appendicectomised and appendicectomised patients at diagnosis and throughout the course of the disease
| Non-appendicectomised | Appendicectomised | |||
| Initial location* | Cumulative location | Initial location | Cumulative location | |
| No of patients | 580 | 589 | 49 | 49 |
| Proctitis | 182 (31) | 70 (12) | 10 (20) | 5 (10) |
| Proctosigmoiditis | 172 (30) | 112 (19) | 12 (24) | 12 (24) |
| Left sided colitis | 122 (21) | 168 (29) | 17 (35) | 13 (27) |
| Pancolitis | 104 (18) | 239 (41) | 10 (20) | 19 (39) |
* Initial data were lacking in nine patients.
Values in parentheses are percentages.
Table 3.
Maximal extent of colitis in patients with limited colitis at entry and followed up for more than six months
| Non-appendicectomised | Appendicectomised | |||||||
| Proctitis | Protosigmoiditis | Left sided | Pancolitis | Proctitis | Protosigmoiditis | Left sided | Pancolitis | |
| Proctitis | 61 (35) | 39 (23) | 38 (22) | 35 (20) | 4 (44) | 4 (44) | 0 (0) | 1 (11) |
| Proctosigmoiditis | — | 64 (39) | 48 (29) | 52 (32) | — | 8 (67) | 3 (25) | 1 (8) |
| Left sided colitis | — | — | 64 (61) | 41 (39) | — | — | 10 (59) | 7 (41) |
Values in parentheses are percentages.
Figure 1.
Cumulative percentage of patients requiring colectomy according to previous appendicectomy. Numbers above the curve indicate the number of patients at risk at the various intervals.
At 10 years, the proportions of appendicectomised and non-appendicectomised patients who required surgery were 16 (7)% and 33 (2)%, respectively (p=0.05). According to Cox proportional hazards regression, previous appendicectomy was inversely related to the risk of colectomy (adjusted hazard ratio 0.40; 95% CI 0.20–0.78; p=0.007). The respective proportions of appendicectomised and non-appendicectomised patients who required oral steroids and immunosuppressive therapy were not significantly different (67% v 70% and 27% v 19%, respectively).
Previous appendicectomy and smoking
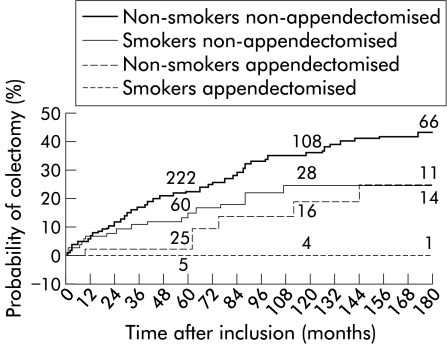
The proportion of smokers was small in the whole group (17%). Accordingly, there were only six patients (1%) who were both smokers and appendicectomised. According to actuarial analysis, the effects of appendicectomy and current smoking on the risk of colectomy were additive (fig 2 ▶). At 10 years, the proportions of patients who were non-smokers non-appendicectomised, smokers non-appendicectomised, and non-smokers appendicectomised who required surgery were 35 (3)%, 25 (5)%, and 19 (8)%, respectively. No patient who belonged to the appendicectomised smoker group underwent surgery. The Cox proportional hazards regression model selected four factors predictive of colectomy: appendicectomy, current smoking, initial left sided lesions, and initial sigmoid lesions (table 4 ▶). Both appendicectomy and current smoking therefore were independent factors protecting against colectomy. Table 5 ▶ shows that medical therapeutic requirements were significantly reduced in current smokers whereas previous appendicectomy alone had no significant effect.
Figure 2.
Cumulative percentage of patients requiring colectomy, according to current smoking and previous appendicectomy. Numbers above the curve indicate the number of patients at risk at the various intervals.
Table 4.
Predictors of colectomy according to multivariate Cox analysis
| Adjusted relative risk | 95% CI | p Value | |
| Current smoking | 0.60 | 0.40–0.95 | 0.03 |
| Previous appendicectomy | 0.40 | 0.20–0.78 | 0.01 |
| Left colon lesions | 2.41 | 1.69–3.43 | <0.001 |
| Sigmoid lesions | 1.69 | 1.05–2.70 | 0.03 |
Table 5.
Effect of previous appendicectomy and current smoking on medical therapeutic needs during the course of ulcerative colitis
| Non-appendicectomised | Appendicectomised | |||
| Non-smokers | Smokers | Non-smokers | Smokers | |
| n | 485 | 104 | 43 | 6 |
| Duration of disease (y) | 6.8 (8.1) | 7.7 (7.7) | 9.7 (7.0) | 8.2 (5.5) |
| No requiring oral glucocorticoids | 345 | 65 | 30 | 1 |
| Per cent | 71 | 62 | 70 | 17 |
| Relative risk | 0.88 | 0.98 | 0.23 | |
| 95% CI | 0.75–1.03 | 0.80–1.20 | 0.04–1.40 | |
| No requiring intensive intravenous treatment | 211 | 30 | 20 | 1 |
| Per cent | 44 | 29* | 47 | 17 |
| Relative risk | 0.66 | 1.07 | 0.38 | |
| 95% CI | 0.48–0.91 | 0.76–1.50 | 0.06–2.30 | |
| No requiring immunosuppressive therapy | 101 | 9 | 11 | 0 |
| Per cent | 21 | 12* | 26 | 0 |
| Relative risk | 0.42 | 1.23 | — | |
| 95% CI | 0.22–0.79 | 0.72–2.10 | — | |
*Significantly different (p<0.05) from non-appendicectomised non-smoker group.
Prospective study
The prospective study included 41 appendicectomised and 466 non-appendicectomised patients. The rate of years with active disease was 48% in appendicectomised patients (47 of 98 patient years) compared with 62% in non-appendicectomised patients (631 of 1024 patient years; p<0.01).
DISCUSSION
The present results show that previous appendicectomy has a beneficial effect on the course of ulcerative colitis. Appendicectomised patients had a less marked year by year disease activity and a decreased risk of colectomy. This effect was independent of confounding factors and additive to that of current smoking.
We believe that retrospective collection of data did not alter the results of our study. Selection bias was minimal as we did not use a postal questionnaire but included prospectively all consecutive patients seen over a four year period. The criteria used to assess the severity of disease were objective and based on therapeutic needs. In particular, the main outcome measure was colectomy, which is an unequivocal marker of the severity of ulcerative colitis, even retrospectively. The recent introduction of immunosuppressive therapy may not have influenced the results because it was used with a similar frequency in appendicectomised and non-appendicectomised patients. Likewise, the decreased colectomy rate we observed in appendicectomised patients was not a result of differences in cumulative extension of colitis which did not differ significantly in the two groups, a finding similar to that of Reif and colleagues.10 Finally, the proportion of our patients who had undergone appendicectomy previously to ulcerative colitis onset was relatively high compared with other series8–12 but it must be kept in mind that, for unknown reasons, the frequency of appendicectomy in childhood is especially high in France.18 In a recent case controlled study, Uzan and colleagues19 reported an appendicectomy rate of 8% in ulcerative colitis patients, similar to that of the present series and significantly less than 31% in age and sex matched controls.
The beneficial effect of previous appendicectomy on the course of ulcerative colitis was somewhat expected. The protective effect of appendicectomy on the development of ulcerative colitis is well documented8–12,19 and the appendix is recognised as a possible important site for priming of the cells involved in the development of ulcerative colitis. Appendiceal inflammation is observed in half of colectomy specimens from patients with ulcerative colitis20 and the appendiceal epithelium in ulcerative colitis, but not in acute appendicitis, shows intense upregulation of HLA class II and activation of macrophages.21 In the T cell receptor α chain knockout mice model, the development of colonic inflammation was suppressed in those animals that had undergone appendicectomies.22,23 In this model, appendix lymphoid follicles proliferate and produce autoantibodies against the cytoskeletal protein tropomyosin.23 In humans, tropomyosin may act as an autoantigen.24 It has been shown that in ulcerative colitis patients a large number of lamina propria B cells produce IgG against tropomyosin25 and that clinical activity correlated well with antibody titre.26 Taken together, these studies suggest that the appendix is an important site of activation of autoreactive T cells involved in colonic inflammation. One can assume that once ulcerative colitis had developed, the absence of the source activating B lymphocytes may lead to a lesser frequency of flare ups and therefore lesser disease severity. In support of this hypothesis, Naganuma and colleagues14 observed a lower recurrence rate after a first episode of ulcerative colitis in appendicectomised compared with non-appendicectomised patients, and in the prospective part of our study there was a lower annual activity rate in appendicectomised compared with non-appendicectomised patients.
Interestingly, the beneficial effect of previous appendicectomy appeared additive to that of current smoking. Of note, the very few ulcerative colitis patients who were both smokers and appendicectomised experienced a particularly benign course (none of six required surgery). This suggests that appendicectomy and smoking are acting through different mechanisms. Indeed, the beneficial effect of smoking is believed to originate from changes in mucus permeability27 and/or inhibition of cytokine production28 and does not interfere with the B lymphocyte response.
In a recent study,11 Andersson et al showed that appendicectomy for an inflammatory condition but not for non-specific abdominal pain was protective against the risk of developing ulcerative colitis. The pathology reports of the removed appendix were not available in our series. Therefore, it was not possible to distinguish between patients operated on for an inflammatory condition and those operated on for other diagnoses. In France during the 1980s, the appendicectomy rate was more than four times the rate observed in the UK or USA.18 This rate has decreased in recent years29 but the proportion of removed appendices with no or minimal inflammatory changes is still more than 25%.30 It seems plausible therefore that a significant proportion of our patients were appendicectomised while not having an inflammatory condition, suggesting that it is the removal of the appendix which is beneficial, rather than inflammation of the appendix.
In conclusion, ours is the first study to demonstrate a negative association between appendicectomy and the risk of colectomy for ulcerative colitis. Patients genetically at high risk of developing ulcerative colitis may be considered as candidates for appendicectomy with the objectives of preventing the development of ulcerative colitis and also decreasing its severity. The effects of appendicectomy after disease onset need further study.
REFERENCES
- 1.Calkins BM. A meta-analysis of the role of smoking in inflammatory bowel disease. Dig Dis Sci 1989;34:1841–54. [DOI] [PubMed] [Google Scholar]
- 2.Lindberg E, Tysk C, Andersson K, et al. Smoking and inflammatory bowel disease, a case control study. Gut 1988;29:352–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyko EJ, Perera DR, Koepsell TD, et al. Effects of cigarette smoking on the clinical course of ulcerative colitis. Scand J Gastroenterol 1988;23:1147–52. [DOI] [PubMed] [Google Scholar]
- 4.Fraga XF, Vergara M, Medina C, et al. Effects of smoking on the presentation and clinical course of inflammatory bowel disease. Eur J Gastroenterol Hepatol 1997;9:683–7. [DOI] [PubMed] [Google Scholar]
- 5.Mokbel M, Carbonnel F, Beaugerie L, et al. Effect of smoking on the long-term course of ulcerative colitis. Gastroenterol Clin Biol 1998;22:858–62. [PubMed] [Google Scholar]
- 6.Motley RJ, Rhodes J, Ford GA, et al. Time relationships between cessation of smoking and onset of ulcerative colitis. Digestion 1987;37:125–7. [DOI] [PubMed] [Google Scholar]
- 7.Beaugerie L, Massot N, Carbonnel F, et al. Impact of cessation of smoking on the course of ulcerative colitis. Am J Gastroenterol 2001;96:2113–16. [DOI] [PubMed] [Google Scholar]
- 8.Smithson JE, Radford-Smith G, Jewell GP. Appendectomy and tonsillectomy in patients with inflammatory bowel disease. J Clin Gastroenterol 1995;21:283–6. [DOI] [PubMed] [Google Scholar]
- 9.Rutgeerts P, D’Haens G, Hiele M, et al. Appendectomy protects against ulcerative colitis. Gastroenterology 1994;106:1251–3. [DOI] [PubMed] [Google Scholar]
- 10.Reif S, Lavy A, Keter D, et al. Appendectomy is more frequent but not a risk factor in Crohn’s disease while being protective in ulcerative colitis: a comparison of surgical procedures in inflammatory bowel disease. Am J Gastroenterol 2001;96:829–32. [DOI] [PubMed] [Google Scholar]
- 11.Andersson RE, Olaison G, Tysk C, et al. Appendectomy and protection against ulcerative colitis. N Engl J Med 2001;344:808–14. [DOI] [PubMed] [Google Scholar]
- 12.Koutroubakis IE, Vlachonikolis IG. Appendectomy and the development of ulcerative colitis: results of a metaanalysis of published case-control studies. Am J Gastroenterol 2000;95:171–6. [DOI] [PubMed] [Google Scholar]
- 13.Jarnerot G, Andersson M, Franzen L. Laparoscopic appendectomy in patients with refractory ulcerative colitis. Gastroenterology 2001;120:1562–3. [DOI] [PubMed] [Google Scholar]
- 14.Naganuma M, Iizuka B, Torii A, et al. Appendectomy protects against the development of ulcerative colitis and reduces its recurrence: Results of a multicenter case-controlled study in Japan. Am J Gastroenterol 2001;96:1123–6. [DOI] [PubMed] [Google Scholar]
- 15.Okazaki K, Onodera H, Watanabe N, et al. A patient with improvement of ulcerative colitis after appendectomy. Gastroenterology 2000;119:502–6. [DOI] [PubMed] [Google Scholar]
- 16.Langholz E, Munkholm P, Davidsen M, et al. Course of ulcerative colitis: analysis of changes in disease activity over years. Gastroenterology 1994;107:3–11. [DOI] [PubMed] [Google Scholar]
- 17.Truelove SC, Jewell DP. Intensive intravenous regimen for severe attacks of ulcerative colitis. Lancet 1974;7866:1067–70. [DOI] [PubMed] [Google Scholar]
- 18.Tiret L, Rotman N, Hatton F, et al. La chirurgie digestive en France. Une étude épidémiologique nationale (1978–1982). Gastroenterol Clin Biol 1988;12:354–60. [PubMed] [Google Scholar]
- 19.Uzan A, Jolly D, Berger E, et al. Effet protecteur de l’appendicectomie contre la rectocolite hémorragique. Etude cas-témoins. Gastroenterol Clin Biol 2001;25:239–42. [PubMed] [Google Scholar]
- 20.Scott IS, Sheaff M, Coumbe A, et al. Appendiceal inflammation in ulcerative colitis. Histopathology 1998;33:168–73. [DOI] [PubMed] [Google Scholar]
- 21.Waraich T, Sarsfield P, Wright DH. The accessory cell populations in ulcerative colitis: a comparison between the colon and appendix in colitis and acute appendicitis. Hum Pathol 1997;28:297–303. [DOI] [PubMed] [Google Scholar]
- 22.Bhan AK, Mizoguchi E, Smith RN, et al. Spontaneous chronic colitis in TCR alpha-mutant mice; an experimental model of human ulcerative colitis. Int Rev Immunol 2000;19:123–38. [DOI] [PubMed] [Google Scholar]
- 23.Mizoguchi A, Mizoguchi E, Chiba C, et al. Role of appendix in the development of inflammatory bowel disease in TCR-alpha mutant mice. J Exp Med 1996;184:707–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geng X, Biancone L, Dai HH, et al. Tropomyosin isoforms in intestinal mucosa: production of autoantibodies to tropomyosin isoforms in ulcerative colitis. Gastroenterology 1998;114:912–22. [DOI] [PubMed] [Google Scholar]
- 25.Onuma EK, Amenta PS, Ramaswamy K, et al. Autoimmunity in ulcerative colitis (UC): a predominant colonic mucosal B cell response against human tropomyosin isoform 5. Clin Exp Immunol 2000;121:466–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakamaki S, Takayanagi N, Yoshizaki N, et al. Autoantibodies against the specific epitope of human tropomyosin(s) detected by a peptide-based enzyme immunoassay in sera of patients with ulcerative colitis show antibody dependent cell mediated cytotoxicity against HLA-DPw9 transfected L cells. Gut 2000;47:236–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zijlstra FJ, Srivastava ED, Rhodes M, et al. Effect of nicotine on rectal mucus and mucosal eicosanoids. Gut 1994;35:247–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madretsma GS, Donze GJ, van Dijk APM, et al. Nicotine inhibits the in vitro production of interleukin 2 and tumor necrosis factor-α by human mononuclear cells. Immunopharmacology 1996;35:47–51. [DOI] [PubMed] [Google Scholar]
- 29.Fingerhut A, Hay JM, Millat B, et al. General and gastrintestinal tract surgery in France. Arch Surg 1998;133:568–74. [DOI] [PubMed] [Google Scholar]
- 30.Barrat C, Catheline JM, Rizk N, et al. Does laparoscopy reduce the incidence of unnecessary appendicectomies? Surg Laparosc Endosc 1999;9:27–31. [PubMed] [Google Scholar]