Abstract
Background and aims: Specialised intestinal metaplasia and its dysplastic transformation, which precedes cancer in Barrett’s oesophagus cannot be differentiated in standard gastroscopy. The aim of this study was to investigate whether laser induced protoporphyrin IX fluorescence permits the detection of specialised intestinal metaplasia and dysplasia during endoscopy and to take biopsy specimens in a guided rather than random manner.
Methods: In 53 patients with Barrett’s oesophagus 5-aminolaevulinic acid was sprayed on the mucosa. Approximately 60 to 120 minutes later, biopsy specimens were taken based on point-like measurements of delayed fluorescence intensity ratios of protoporphyrin IX in vivo. Two independent pathologists examined the 596 biopsy specimens taken, 168 of which were selected to be investigated by a third pathologist. Among these specimens only those (n=141) with a consensus diagnosis by at least two pathologists and p53 expression as additional marker were included in the analysis.
Results: The median of normalised fluorescence intensity (ratio of delayed PpIX fluorescence intensity to immediate autofluorescence intensity) in non-dysplastic specialised intestinal metaplasia (0.51, 68% CI 0.09 to 1.92) and low grade dysplasia (1.89, 68% CI 0.55 to 3.92) differed significantly (p<0.005). Dysplasia was detected at a rate 2.8-fold higher compared with screening endoscopy despite taking fewer specimens. In addition, three early cancers were detected for the first time. Moreover, this method permitted differentiation of specialised intestinal metaplasia from junctional or gastric-fundic type epithelium (p<0.013).
Conclusions: For the first time it was possible to differentiate low grade dysplasia from non-dysplastic Barrett’s mucosa during endoscopy based on delayed laser induced fluorescence endoscopy of PpIX. Furthermore, the method helps to detect specialised intestinal metaplasia in short Barrett’s oesophagus.
Keywords: fluorescence spectroscopy, Barrett’s oesophagus
A t present the incidence of adenocarcinoma of the oesophagus and of the gastro-oesophageal junction shows the highest increase of all gastrointestinal neoplasms.1,2 As these carcinomas evolve through a series of progressively severe dysplastic changes in Barrett’s oesophagus with specialised intestinal metaplasia (SIM), endoscopic visualisation of dysplasia and short segment Barrett’s would provide the opportunity for early treatment thus improving the prognosis of this condition.3–6 For this purpose, additional endoscopic methods such as dye spraying, chromoendoscopy, for example, methylene blue, laser induced autofluorescence or fluorescence of contrast agents, and optical coherence tomography have been developed. It is still discussed controversially whether dyes, such as methylene blue, improve detection of short segments of Barrett’s mucosa and of dysplasia.7–11
Laser induced fluorescence spectroscopy (LIFS) and laser induced fluorescence endoscopy (LIFE) offer the potential to differentiate premalignant and malignant lesions from normal mucosa because of differences in their fluorescence properties. With the progression from normal to neoplasia, the predominant contribution of green autofluorescence decreases, whereas autofluorescence at longer wavelengths (red) increases.12 LIFS and LIFE were, therefore, used to fingerprint the dysplastic transformation. By using LIFS or LIFE, high grade dysplasia (HGD) was detected at high sensitivity but moderate specificity.12–14 However, low grade dysplasia (LGD) was incorrectly classified as normal.13 Furthermore inflammation,13,14 and even Barrett’s mucosa may produce false positive results.13
However, for endoscopic surveillance it is necessary to detect all three types of mucosal changes. The presence of SIM3,15–17 is essential for the development of cancer in Barrett’s oesophagus, and cancer may be preceded by either LGD or HGD, or both.18–23
The aim of our study, therefore, was to examine, whether it is possible to endoscopically locate LGD or HGD in Barrett’s oesophagus and to distinguish columnar lined oesophagus with SIM from columnar lined oesophagus without SIM, especially in short Barrett’s. In contrast with previous investigations13 we administered 5-aminolaevulinic acid (5-ALA)24–26 to stimulate the metabolic production of protoporphyrin IX (PpIX) particularly in neoplastic tissue. Furthermore, we used a tuneable, pulsed solid state laser system to record immediate and delayed fluorescence spectra online, simultaneously with white light endoscopy.27–29 By recording delayed fluorescence spectra we were able to suppress autofluorescence background effectively, exploiting the long fluorescence decay time of PpIX.
METHODS
Experimental set up
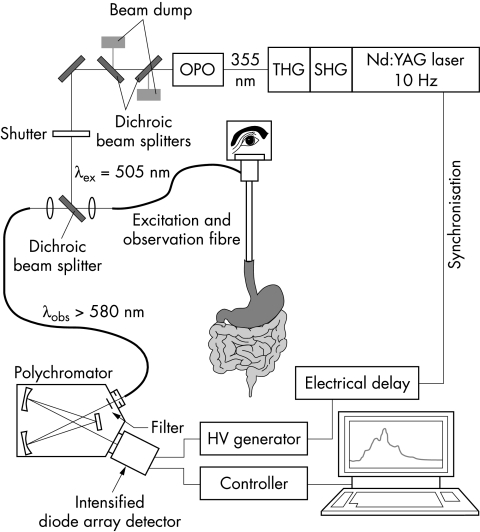
Figure 1 ▶ shows the experimental set up used to excite and detect fluorescence spectra. An optical parametric oscillator (OPO, model 355-A, GWU-Lasertechnik), pumped by the third harmonic (λ=355 nm, Epulse = 50–60 mJ, repetition rate 10 Hz) of a Q-switched Nd:YAG laser (Quanta-Ray GCR-11; Spectra-Physics), provided laser radiation tuneable between 410 nm and 2.2 μm. Energy and pulse width of the output pulses amounted typically to 2 mJ and 3 ns. The output energy of the tuneable laser beam was reduced to 150 μJ and coupled into a 600 μm hard clad silica fibre inserted into the endoscope. The laser induced fluorescence of the tissue was collected by the same fibre and guided to the entrance slit of an optical multichannel analyser, consisting of a polychromator (McPherson) and a cooled, intensified diode array detector (Princeton Instruments). A dichroic beam splitter served to decouple excitation and observation optical paths, and to separate laser induced fluorescence from back scattered laser light. In addition, a long wave pass filter (λ50% = 550 nm) allowed the residual back scattered excitation light to be suppressed. To avoid interference with the white light used for endoscopy, the intensifier of the diode array detector was opened for 20 ns only, gated by an electrical pulse of −180 V. To this end a high voltage (HV) pulse generator was triggered by a pulse provided by the power supply of the Nd:YAG laser and appropriately delayed by means of a digital delay generator.
Figure 1.
Experimental set up. THG, third harmonic generation; SHG, second harmonic generation; OPO, optical parametric oscillator.
All fluorescence spectra were corrected for the spectral transmittance of the polychromator and for the spectral sensitivity of the photocathode of the intensified diode array detector, but not for the transmittance of the long pass filter (λ50% = 550 nm) and the dichroic mirror used. In addition, the electronic background of the detector was subtracted. As fluorescence spectra were corrected for the number of laser pulses applied and for their energy, immediate and delayed spectra could be compared quantitatively.
Fluorophore
The synthesis of 5-ALA is the first step in haembiosynthesis. This pathway is tightly regulated by end point inhibition. Administration of 5-ALA leads to accumulation of PpIX, the immediate precursor of haeme in malignant and premalignant tissue,30 presumably because of an imbalance between porphobilinogen deaminase (PBGD) and ferrochelatase.24,31 The excitation spectrum of PpIX in methanol consists of the Soret band at 401 nm and four smaller Q-bands at 505, 532, 580, and 630 nm. We used the Q-band at λ=505 nm for excitation. The emission spectrum of PpIX in methanol shows bands at 635 nm and 699 nm.
Fluorescence spectra and data analysis
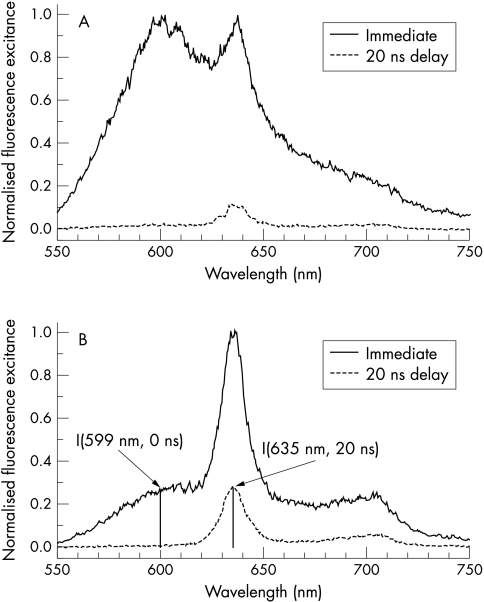
As a working hypothesis we assumed that fluorescence spectra of dysplastic lesions are dominated by PpIX fluorescence, as PpIX preferably accumulates in malignant and premalignant tissue. It was observed that immediate fluorescence spectra of dysplastic lesions exhibited PpIX fluorescence bands at λ=635 nm and λ=699 nm on top of a broad autofluorescence background as shown in fig 2B ▶. In contrast, we expected a broad autofluorescence spectrum in non-dysplastic Barrett’s mucosa without defined spectral signatures (fig 2A ▶).
Figure 2.
Fluorescence spectra of non-dysplastic and dysplastic Barrett’s mucosa. Note: the immediate spectra are similar to a certain extent, whereas the delayed spectra of dysplasia and normal mucosa differ conspicuously, in particular by about a factor of 20 in their relative PpIX fluorescence. (A) Typical fluorescence spectra of Barrett’s mucosa. Immediate spectrum: the surrounding mucosa without dysplasia exhibits a broad fluorescence spectrum without any clear spectral signature, and, therefore, the PpIX bands cannot be discriminated from the autofluorescence background. Delayed fluorescence spectrum (20 ns time delay of detection): in the delayed spectrum, a weak PpIX band at 635 nm can be discriminated. (B) Typical fluorescence spectra of dysplasia. Immediate spectrum (0 ns time delay of detection): in the spectrum, well defined fluorescence bands at λ=635 nm and at λ=699 nm appear on top of a broad background, caused by tissue autofluorescence. Delayed fluorescence spectrum (20 ns time delay of detection): because of the difference in the fluorescence decay time of PpIX (τ=16 ns) and of autofluorescence (τ about 2–4 ns), the fluorescence spectrum is virtually free of autofluorescence background and exhibits PpIX fluorescence bands only. For comparison, the prompt spectrum was normalised to the maximum intensity and the corresponding delayed spectrum was corrected for different sampling time and excitation energy.
As decay times of autofluorescence (τ=2–4 ns) are shorter than the fluorescence lifetime of PpIX (τ=16 ns), autofluorescence background can be suppressed by delayed observation.28,29 To this end we have delayed the electrical pulse used to open the detector by 20 ns with respect to the laser pulse. At λ=635 nm—that is, at the peak of the main fluorescence band of PpIX—high intensity of delayed fluorescence was considered as a specific marker for dysplasia (fig 2B ▶). On the other hand low intensity of delayed fluorescence was associated with non-dysplastic mucosa (fig 2A ▶). It should be noted, however, that the fluorescence intensity recorded is strongly affected by geometrical factors, for example, by the distance between probe and mucosa, and by the optical properties of the tissue—that is, by its absorption and scattering properties. For quantification we normalised the delayed PpIX fluorescence intensity I (635 nm, 20 ns) by the intensity of autofluorescence I (599 nm, 0 ns) taken from the immediate fluorescence spectrum at λ=599 nm. The ratio R=I (635 nm, 20 ns)/I(599 nm, 0 ns) strongly reduces the influence of geometrical factors and optical properties of the tissue.
Patients and procedures
Fifty three patients with Barrett’s oesophagus (34 short, 19 long, 38 men, 15 women, median age: 56 years; range 27–87) were included in our study. The diagnosis of short Barrett’s oesophagus was accepted only if SIM was diagnosed by at least two of the three pathologists. For all patients screening gastroscopies were performed (video gastroscopy, GIF Q 140, Olympus Company) with four quadrant biopsies every cm (Wilson Cook: maxum reusable biopsy forceps; Boston Scientific: megabite forceps, multibite forceps). A follow up endoscopy was performed eight weeks after screening endoscopy. An aqueous solution of 0.5 g of 5-ALA in 50 ml of sodium bicarbonate 8.4% and 50 ml of sodium chloride 0.9% was sprayed on the mucosa during gastroscopy (PW-5L-1, Olympus Company). Approximately 60 to 120 minutes later, fluorescence guided gastroscopy was performed. The optical fibre was inserted through one of the instrument channels of the endoscope and put into contact with the oesophageal tissue in a circumferential pattern. The optical fibre allowed fluorescence to be collected from a small area (1 mm2). After excitation with a laser pulse a delayed fluorescence spectrum was recorded, all spectra obtained within one second were added to improve SNR and displayed on a monitor. From such spectra fluorescence intensities of PpIX were derived and biopsies (Wilson Cook: maxum reusable biopsy forceps; Boston Scientific: megabite forceps, multibite forceps) were taken in Barrett’s segments at locations exhibiting high and, for comparison, medium and low PpIX fluorescence intensities. In addition biopsy specimens were taken from the corpus of the stomach, the distal oesophagus, and from hernias if present. Generally, a double channel endoscope (GIF 2 T 20, Olympus Company) was used to biopsy in the presence of the optical fibre, to mark the focal area of dysplastic tissue with high fluorescence intensity. The location of each biopsy specimen was determined by measuring the distance from the dental margin and by the clock position. Immediate and delayed fluorescence spectra were recorded from all biopsies ex vivo to check for sampling errors during endoscopy. In this way we found 27% sampling errors to occur during endoscopy. Therefore, we have compared only the ex vivo fluorescence data with histological examinations and p53.
Histological diagnosis
A total of 596 biopsy specimens were taken and blinded histological evaluation was performed independently by two pathologists from two different institutions. All histological specimens with dysplasia (n=69) diagnosed by at least one of the pathologists together with a random selection of normal samples (n=99) were further examined by a third pathologist from a third institution. In the final analyses only biopsy samples (n=141) were included that were evaluated by all three pathologists—with at least two of the pathologists agreeing on the diagnosis (consensus diagnosis). Pathologists had no information about the endoscopic picture, the location of the biopsy, the diagnosis of the other pathologists, and of the fluorescence intensity values. All biopsy samples were fixed in 4% neutral formaldehyde and embedded in paraffin wax. After staining with haematoxylin and eosin (H&E) and periodic acid Schiff (PAS), serial sections (4 μm) were examined. Dysplasia was defined according to the WHO classification, and SIM was diagnosed if goblet cells were present in columnar cell metaplasia.32,33 Normalised ex vivo PpIX fluorescence intensities (see above) were correlated to the consensus diagnosis. Furthermore, agreements between pathologists, p53 analyses and normalised fluorescence intensities were calculated (table 1 ▶).
Table 1.
Interobserver agreement: histology, p53 protein expression, and fluorescence spectroscopy
| By biopsy sample | By patient | |||
| Method | κ | agreement (%) | κ | agreement (%) |
| Pathologist A v B | 0.18 | 52 | 0.15 | 47 |
| Pathologist B v C | 0.39 | 67 | 0.13 | 43 |
| Pathologist A v C | 0.67 | 87 | 0.46 | 76 |
| P 53v consensus diagnosis | 0.38 | 66 | 0.55 | 80 |
| Fluorescence v consensus | 0.40 | 76 | 0.67 | 86 |
| P 53 v fluorescence | 0.17 | 50 | 0.42 | 71 |
| P 53 v pathologist A | 0.31 | 68 | 0.72 | 88 |
| P 53 v pathologist B | 0.30 | 66 | 0.36 | 66 |
| P 53 v pathologist C | 0.35 | 67 | 0.30 | 64 |
| Fluorescence v pathologist A | 0.35 | 65 | 0.67 | 83 |
| Fluorescence v pathologist B | 0.12 | 36 | 0.35 | 66 |
| Fluorescence v pathologist C | 0.23 | 62 | 0.56 | 82 |
p53 protein expression
All 168 biopsy samples examined by the third pathologist were subsequently examined for p53 protein expression as an additional biological marker.34 The investigator had no prior information on the results of endoscopy, histological examination, and fluorescence spectroscopy. For p53 protein expression immunohistochemistry was performed on 4 μm sections of fixed and paraffin wax embedded tissue. The sections were dewaxed in xylene and rehydrated through graded alcohol. Activity of endogenous peroxidase was blocked incubating the sections for 10 minutes in 3% hydrogen peroxide. Retrieval of antigen in fixed tissues was accomplished by microwave treatment of slides in citrate buffer for three times at 700 W. After preincubating the slides in normal horse serum, the sections were incubated for one hour with the DO-1 monoclonal mouse antibody (Oncogene Science, Cambridge, USA) at a dilution of 1:100. The DO-1 antibody that was used recognised an epitope of the wild type p53 protein as well as of the p53 mutant. Bound DO-1 antibodies were detected by application of the Vectastain ABC kit (Vector, Burlingame, USA). Finally, the slides were counterstained with haematoxylin. A positive control specimen was prepared from tissue known to express p53 protein, and a negative control slide was prepared from the original paraffin blocks using a non-immune serum supernatant instead of the primary antibody.
Ethical guidelines
The protocol followed in the trials was approved by the ethics committee of the Medical Faculty (Charité) of the Humboldt University. Written informed consent was obtained from each patient.
Analysis of results and statistics
As the central 68% mass is characteristic for the lognormal distribution, results are expressed as median with the 68% confidence interval (CI) in parentheses, unless otherwise stated.35 Statistical significance of the differences for non-normally distributed data was tested by the the Mann-Whitney U test.36 Statistical analyses were performed using the SPSS software (SPSS 10.07-G).
To improve the comparability of normalised fluorescence intensity (R) of groups with different numbers of samples (for example, LGD, non-dysplastic) we used the normalised cumulative frequency as a way to illustrate the distribution of the parameter R. The normalised cumulative frequency of biopsy samples diagnosed to be of a specific type by pathology is the sum of biopsy samples—ordered for increasing R values—normalised to the total number of biopsy samples.37,38
RESULTS
Fifty five fluorescence guided endoscopies were performed in 53 patients with Barrett’s oesophagus. From the 596 biopsy specimens taken only 141 samples with a consensus diagnosis by at least two pathologist and additional p53 expression measurement were included in the final analysis. Administration of 5-ALA and endoscopies were well tolerated by all patients.
Comparison between screening endoscopy and fluorescence guided endoscopy
LIFS led to a considerable increase in the number of dysplasia detected. Based on the consensus diagnoses of two pathologists dysplasia was observed by LIFS in 14 of 53 patients (4 patients HGD, 10 patients LGD), whereas in only five of these patients dysplasia had been found by previous routine screening procedures. Among the 14 patients with dysplasia detected by LIFS eight patients carried dysplasia in long, six patients in short Barrett’s segments. In contrast, dysplasia was detected by routine screening endoscopy only in long segments. Interestingly, detection of dysplasia was improved by LIFS despite fewer biopsy specimens taken (median LIFS: 7; median screening endoscopy: 28). Furthermore, early cancers were detected in three patients by LIFS. In one of these patients HGD was suspected by screening endoscopy.
Fluorescence data of dysplastic and non-dysplastic mucosa
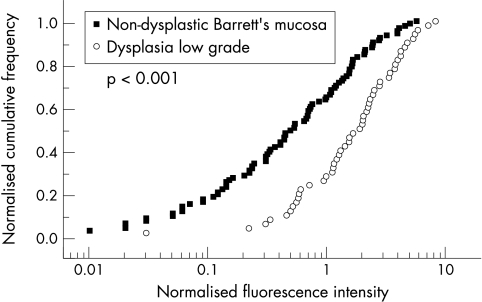
Typical fluorescence spectra of non-dysplastic mucosa and dysplastic mucosa are shown in figure 2A ▶ and figure 2B ▶, respectively. We have plotted (see fig 3 ▶) normalised cumulative frequencies37,38 versus fluorescence intensities R (see section fluorescence spectra and data analysis) for biopsy specimens classified as dysplasia or non-dysplastic Barrett’s mucosa in long and short Barrett’s according to the consensus diagnosis. The median of normalised fluorescence intensity R of the 50 biopsy specimens with LGD was Rmedian = 1.89 (CI: 0.55 to 3.92), whereas it was Rmedian = 0.51 (CI: 0.09 to 1.92) in the 91 specimens with non-dysplastic Barrett’s mucosa. The significantly higher median of normalised fluorescence intensity of dysplastic tissue compared with non-dysplastic Barrett’s mucosa with SIM (p<0.001) clearly confirms our working hypothesis that PpIX accumulates preferably in malignant and premalignant tissue and hence leads to higher normalised PpIX fluorescence intensities. The normalised fluorescence intensity in the nine samples with HGD was Rmedian =0.95 (CI 0.4 to 2.30) was within the range of R-values of LGD. The Rmean (SD) of the early cancer specimens was 1.73 (1.3). Based on these results, a value of Rdiscr =0.8 of the normalised fluorescence intensities of biopsies was chosen to discriminate between dysplasia (R≥0.8) and non-dysplastic (R<0.8) tissue. Following this approach, 38 of the 50 biopsy specimens with dysplasia according to the consensus diagnosis were correctly classified as malign and 58 out of 91 non-dysplastic Barrett’s mucosa as benign. In this way, a sensitivity of 76%, a specificity of 63%, a positive predictive value (PPV) of 52%, and a negative predictive value (NPV) of 82% were achieved. At a discriminator value Rdiscr >0.8, the number of false positives will be reduced, at the same time increasing the number of false negatives for low grade dysplasia. More importantly the number of false negatives of HGD would be unacceptable large. The value of Rdiscr =0.8 leads to about the same specificity for LGD and HGD.
Figure 3.
Consensus histological diagnosis compared with normalised PpIX fluorescence intensity: dysplasia compared with non-dysplastic Barrett’s mucosa (SIM).
Speculating that for further therapeutic decision making it might be sufficient to suspect dysplasia in a patient by recording at least one normalised fluorescence intensity value above 0.8 a sensitivity of 100%, a specificity of 67%, a PPV of 89%, and a NPV of 100% would have been obtained.
Fluorescence data of short Barrett’s oesophagus
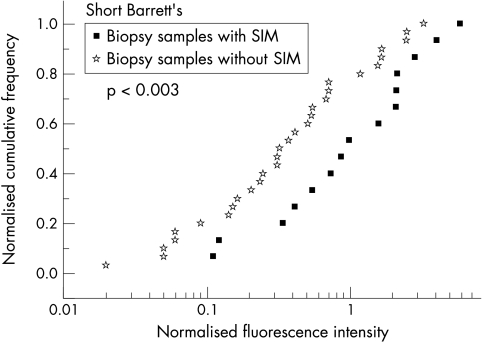
In this section we correlate normalised fluorescence intensities R with pathology of biopsy specimens of endoscopically short Barrett’s only (fig 4 ▶). The analyses were performed based on the consensus diagnosis. The median of normalised fluorescence intensities of columnar epithelium with SIM was Rmedian =0.91 (CI: 0.2 to 2.50), whereas Rmedian =0.31 (CI: 0.07 to 1.55) was obtained for gastric fundic type or junctional type epithelium (p<0.02). We have chosen a value of Rdiscr =0.7 to distinguish SIM (R≥0.7) from junctional or gastric fundic type (R<0.7) by normalised fluorescence intensity, resulting in a sensitivity of 67%, a specificity of 70%, a PPV of 53%, and a NPV of 81%.
Figure 4.
Consensus histological diagnosis compared with normalised PpIX fluorescence intensity: short Barrett’s—junctional or gastric-fundic type epithelium compared with SIM.
p53 protein expression and fluorescence data
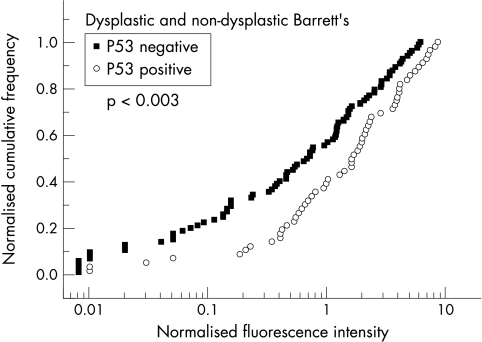
In figure 5 ▶ normalised cumulative frequencies of p53 negative (Rmedian =0.64, CI: 0.05 to 2.9) and positive biopsies (Rmedian =1.55, CI: 0.4 to 4.5) are plotted versus normalised fluorescence intensities R. With a value of Rdiscr =0.7 chosen for discrimination a sensitivity of 68%, a specificity of 52%, a PPV of 49%, and a NPV of 71% was deduced.
Figure 5.
P53 expression versus normalised PpIX fluorescence intensity.
DISCUSSION
The efficacy of regular screening endoscopy in Barrett’s oesophagus is still under debate, although it is known that cancer is preceded by dysplastic transformation of Barrett’s mucosa. On the one hand, dysplasia and cancer may be missed even when four quadrant biopsy specimens are taken, because dysplasia and cancer may occur in mucosa that appears normal in white light endoscopy.3 On the other hand, the cost effectiveness of surveillance is questionable because of the low overall cancer incidence. As dysplasia and adenocarcinoma in Barrett’s oesophagus are primarily associated with SIM,39–43 the cost per cancer diagnosed would be lower if only patients with SIM were screened.44 Apart from SIM, dysplasia, and cancer, the different types of columnar epithelium cannot be differentiated by their appearance during white light endoscopy and can, hence, be overlooked.45,46 Several new methods (for example, dye staining, LIFS, LIFE, optical coherence tomography) have, therefore, been introduced with the intention of making SIM, dysplasia and small carcinomas visible during endoscopy.
Panjehpour et al13 obtained promising results on Barrett’s oesophagus by using laser induced autofluorescence spectroscopy. This technique permitted HGD to be differentiated from non-dysplastic mucosa with high sensitivity and moderate specificity. However, HGD could be detected only when large areas were involved. Furthermore, mixed HGD and LGD were correctly classified as premalignant in only 28% of the biopsy specimens and LGD were classified as benign. Moreover, the authors concluded from preliminary data that active inflammation might be misclassified as premalignant.
In this study we provide evidence that, with the aid of time delayed PpIX fluorescence spectroscopy, LGD can be discriminated from non-dysplastic Barrett’s mucosa with a sensitivity of 76% and a specificity of 63%. Furthermore, a distinction can be made between SIM and other types of columnar epithelium in short Barrett’s oesophagus.
A possible explanation for the improvement in the detection of dysplasia and SIM is the larger penetration depth of the laser light associated with the longer excitation wavelength (λ=505 nm), compared with the penetration depth at the wavelength (λ=410 nm) used previously.13 Furthermore, bleaching of the fluorophore may be reduced by the use of green rather than blue light13,14 resulting in an increased sensitivity of dysplastic lesions. In addition, pretreatment of these patients with precursors (5-ALA) of fluorophores, which accumulate preferentially in malignant tissue, might increase the sensitivity of detection of dysplasia compared with endogenous fluorophores (for example, collagen, elastin, NADH, flavins) detected by autofluorescence spectroscopy.13 In our case we exploited the long fluorescence decay time (τ about 16 ns) of PpIX fluorescence compared with decay times (τ about 2–4 ns) of autofluorescence27–29 to suppress autofluorescence background by delayed observation of laser induced fluorescence. Furthermore, pulsed excitation and observation within 20 ns permitted fluorescence spectra to be recorded under normal endoscopic conditions—that is, in the presence of permanent white light illumination.
The interobserver disagreement of our pathologists is high, and mainly caused by distinguishing LGD from non-dysplastic mucosa. However, this is consistent with published literature, there a high degree (around 40%) of interobserver variability is reported.47–51 To handle this problem all samples included in our final analyses had been diagnosed by three different pathologists, and in addition the expression of p53 was determined. For comparison of normalised fluorescence intensities and p53 expression with histological examination we used consensus diagnosis,51 as a consensus diagnosis of LGD among gastrointestinal pathologists suggests an increased risk of progression from LGD to HGD or carcinoma.52
Although normalised fluorescence intensities correlate with p53 expression to a certain extent (see table 1 ▶), the latter parameter cannot replace the consensus diagnosis. Despite all these reservations, time gated fluorescence spectroscopy seems to be a reliable method for detecting low grade dysplasia in patients with Barrett’s mucosa. This conclusion is supported by the nearly tripled detection rate of dysplasia compared with screening endoscopy. In addition, delayed observation of PpIX fluorescence aids detection of specialised metaplasia in short Barrett’s.
The price of our fluorescence guided endoscopy system is rather high, but a dedicated system could be built more cheaply. Nevertheless, the reduced number of histological biopsy specimens required in the case of fluorescence spectroscopy (median 7 compared with median 28 biopsy samples per patient in conventional endoscopy) would lower the expenses for the screening procedure in the present investigation by the same amount. Moreover, our equipment can also be used for screening of other diseases, such as ulcerative colitis, early stomach cancers, flat adenomas, and polyps.
In conclusion, our data show that time gated detection of protoporphyrin IX fluorescence improves fingerprinting of short Barrett’s (SIM) and dysplastic lesions during endoscopy for target biopsies. Furthermore, LGD can be localised by this technique. Our present set up is a research instrument, which is not intended to be used in routine endoscopy. However, a commercial instrument based on the same principle could be designed for this purpose.
Acknowledgments
This study was supported by a grant from Charité University and Ludwig Demling Prize 1997.
Abbreviations
SIM, specialised intestinal metaplasia
LIFS, laser induced fluorescence spectroscopy
LIFE, laser induced fluorescence endoscopy
HGD, high grade dysplasia
LGD, low grade dysplasia
5-ALA, 5-aminolaevulinic acid
PpIX, protoporphyrin IX
OPO, optical parametric oscillator
HV, high voltage
PBGD, porphobilinogen deaminase
R, normalised fluorescence intensity
SNR, signal to noise ratio
REFERENCES
- 1.Pera M, Cameron AJ, Trastek VF, et al. Increasing incidence of adenocarcinoma of the oesophagus and esophagogastric junction. Gastroenterology 1993;104:510–13. [DOI] [PubMed] [Google Scholar]
- 2.Blot WJ, Devesa SS, Fraumeni JF. Continuing climb in rates of oesophageal adenocarcinoma: an Update. JAMA 1993;270:1320. [PubMed] [Google Scholar]
- 3.Reid BJ, Weinstein WM, Lewin KJ, et al. Endoscopic biopsy can detect high-grade dysplasia or early adenocarcinoma in Barrett’s oesophagus without grossly recognisable neoplastic lesions. Gastroenterology 1987;93:1–11. [DOI] [PubMed] [Google Scholar]
- 4.Levine DS, Haggitt RC, Blount PL, et al. An endoscopic biopsy protocol can differentiate high-grade dysplasia from early adenocarcinoma in Barrett’s oesophagus. Gastroenterology 1993;105:40–50. [DOI] [PubMed] [Google Scholar]
- 5.Weston A P, Krmpotich P, Makdisi WF, et al. Short segment Barrett’s oesophagus: clinical and histological features, associated endoscopic findings, and association with gastric intestinal metaplasia. Am J Gastroenterol 1996;91:981–6. [PubMed] [Google Scholar]
- 6.McClave SA, Boyce HW JR, et al. Early diagnosis of columnar-lined oesophagus: a new endoscopic criterion. Gastrointest Endosc 1987;33:413–16. [DOI] [PubMed] [Google Scholar]
- 7.Canto MI, Setrakian S, Petras RE, et al. Methylene blue selectively stains intestinal metaplasia in Barrett`s oesophagus. Gastrointest Endosc 1996;44:1–7. [DOI] [PubMed] [Google Scholar]
- 8.Canto MI, Setrakian S, Willis J, et al. Methylene blue-directed biopsies improve detection of intestinal metaplasia and dysplasia in Barrett’s oesophagus. Gastrointest Endosc 2000;51:560–8. [DOI] [PubMed] [Google Scholar]
- 9.Sueoka N, Nishigaki H, Hiratsuka T, et al. Combined magnification video-endoscopy with methylene blue staining for the assessment of intestinal metaplasia in patients with Barrett’s oesophagus. Gastrointest Endosc 1999;49:AB54. [Google Scholar]
- 10.Jobson B, Goenka P, Manalo G, et al. Methylene blue staining for intestinal metaplasia in Barrett’s oesophagus—Is it as good as we think? Gastrointest Endosc 1999;49:AB52. [Google Scholar]
- 11.Ray MB, Mayfield-Stokes S, Cecil B, et al. Results of methylene blue-directed biopsy is similar to conventional biopsy for the diagnosis of intestinal metaplasia and dysplasia in Barrett`s oesophagus. Gastrointest Endosc 1999;49:AB54. [Google Scholar]
- 12.DuVall A, Wilson BC, Marcon N. Light induced fluorescence endoscopy. In: Cotton PB, Tytgat GNJ, Williams CB, et al, eds. Annual of gastrointestinal endoscopy. 10th edn. London: Rapid Science Publishers, 1997:25–30.
- 13.Panjehpour M, Overholt BF, Vo-Dinh T, et al. Endoscopic fluorescence detection of high-grade dysplasia in Barrett’s oesophagus. Gastroenterology 1996;111:93–101. [DOI] [PubMed] [Google Scholar]
- 14.Haringsma J, Tytgat GNJ. Detection of dysplasia in Barrett’s oesophagus using light-induced fluorescence endoscopy. Endoscopy 1997;29:E35. [Google Scholar]
- 15.McArdle JE, Lewin KJ, Randall G, et al. Distribution of dysplasia and early invasive carcinoma in Barrett’s oesophagus. Hum Pathol 1992;23:479–82. [DOI] [PubMed] [Google Scholar]
- 16.Gottfried MR, McClave SA, Boyce HW. Incomplete intestinal metaplasia in the diagnosis of columnar lined oesophagus (Barrett’s oesophagus). Am Clin Pathol 1989;92:741–6. [DOI] [PubMed] [Google Scholar]
- 17.Schnell TG, Sontag SJ, Chejfec G. Adenocarcinoma arising in tongues or short segments of Barrett`s oesophagus. Dig Dis Sci 1992;37:137–43. [DOI] [PubMed] [Google Scholar]
- 18.Tytgat GNJ. Does endoscopic surveillance in oesophageal columnar metaplasia (Barrett’s oesophagus) have any real value? Endoscopy 1995;27:19–26. [DOI] [PubMed] [Google Scholar]
- 19.Miros M, Kerlin P, Walker N. Only patients with dysplasia progress to adenocarcinoma in Barrett’s oesophagus. Gut 1991;32:1441–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt HG, Riddell RH, Walther B, et al. Dysplasia in Barrett’s oesophagus. J Cancer Res Clin Oncol 1985;110:145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menke-Pluymers MBE, Mulder AH, Hop WCJ, et al. Dysplasia and aneuploidy as marker of malignant degeneration in Barrett’s oesophagus. Gut 1994;35:1348–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reid BJ, Blount Pl, Rubin CE, et al. Flow-cytometric and histological progression to malignancy in Barrett’s oesophagus: prospective endoscopic surveillance of a cohort. Gastroenterology 1992;102:1212–19. [PubMed] [Google Scholar]
- 23.Hong MK, Laskin WB, Herman BE, et al. Expansion of the ki-G7 proliferative compartment correlates with degree of dysplasia in Barrett’s oesophagus. Cancer 1995;75:423–9. [DOI] [PubMed] [Google Scholar]
- 24.Kennedy JC, Pottier RH, Pross DC. Photodynamic therapy with endogenous protoporphyrin IX: basic principles and present clinical experience. J Photochem Photobiol B Biol 1990;6:143–5. [DOI] [PubMed] [Google Scholar]
- 25.Ortner M, Ebert B, Nolte D, et al. Endoscopic detection of colonic dysplasia in inflammatory bowel disease using delayed laser-induced fluorescence spectroscopy. Gastroenterology 1997;112:A632. [Google Scholar]
- 26.Ortner M, Ebert B, Nolte D, et al. Endoscopic investigation of Barrett’s oesophagus by laser induced fluorescence spectroscopy. Endoscopy 1997;29:E1. [Google Scholar]
- 27.Ebert B, Nolte D, Rinneberg H, et al. Characteristic porphyrin-like autofluorescence in primary colon tumours and lymph nodes. In: Proceedings of optical biopsies. Bellingham, Washington: SPIE, 1995:57–67.
- 28.Kohl M, Neukammer J, Sukowski U, et al. Delayed observation of laser-induced fluorescence for imaging of tumours. Appl Phys 1993;56:131–8. [Google Scholar]
- 29.Kohl M, Sukowski U, Ebert B, et al. Imaging of superficially growing tumours by time delayed observation of laser-induced fluorescence. In: Doughterty TJ, Katzir A, eds. Optical methods for tumour treatment and detection: Mechanisms and techniques in photodynamic therapy II. Bellingham, Washington: Proc SPIE,1993:206–21.
- 30.Schoenfeld N, Epstein O, Lahav M, et al. The haeme biosynthetic pathway in lymphocytes of patients with malignant lymphoproliferative disorders. Cancer Lett 1988;43:43–8. [DOI] [PubMed] [Google Scholar]
- 31.Hinnen P, DeRooij FWM, VanVetthuysen ML, et al. Biochemical basis of 5-aminolaevulinic acid-induced protoporphyrin IX accumulation: a study in patients with (pre)malignant lesions of the oesophagus. Br J Cancer 1998;78:679–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watanabe H, Jass JR, Sobin LH, et al. WHO histological typing of oesophageal and gastric tumours. In: Berlin: Springer-Verlag, 1990:38–9.
- 33.Lewin KJ, Appelman HD. Barrett’s oesophagus-columnar dysplasia and adenocarcinoma of the oesophagus. In: Lewin KJ, Appelman HD ed. Tumors of the esophagus and stomach. Bethesda: Armed Force Institute of Pathology, 1996:113.
- 34.Klump B, Holzmann K, Kühn A, et al. Distribution of cell populations with DNA aneuploidy and p53 protein expression in ulcerative colitis. Eur J Gastroenterol Hepatol 1997;9:789–94. [DOI] [PubMed] [Google Scholar]
- 35.Lothar Sachs: Angewandte Statistik. 8. Berlin: Springer-Verlag, 1997:175.
- 36.Mann HB, Whitney DR. On a test of whether one of the two random variables is stochastically larger than the other. Ann Math Statistics 1947;18:50–60. [Google Scholar]
- 37.Lothar Sachs: Angewandte Statistik. 8. Berlin: Springer-Verlag, 1997:107.
- 38.Richard F Mould. Introductory medical statistics. 2nd edn. Bristol: Adam Hilger, 1989:9.
- 39.Spechler SJ, Zeroogian JM, Antonioli DA, et al. Prevalence of metaplasia at the gastroesophageal junction. Lancet 1994;344:1533–6. [DOI] [PubMed] [Google Scholar]
- 40.Reid BJ, Weinstein WM. Barrett’s oesophagus and adenocarcinoma. Ann Rev Med 1987;38:477–92. [DOI] [PubMed] [Google Scholar]
- 41.Haggitt RC. Adenocarcinoma in Barrett’s oesophagus: A new epidemic? Hum Pathol 1992;23:475–6. [DOI] [PubMed] [Google Scholar]
- 42.Hassall E. Barrett’s oesophagus: new definitions and approaches in children. J Pediatr Gastroenterol Nutr 1993;16:345–64. [PubMed] [Google Scholar]
- 43.Vieth M, Stolte M. Barrett’s oesophagus, Barrett’s dysplasia, Barrett’s carcinoma. The endoscopic diagnosis with no biopsy is inadequate. Gastroenterology 1997;112:A674. [Google Scholar]
- 44.Nilson J, Skobe V, Johansson J, et al. Screening for oesophageal adenocarcinoma: an evaluation of a surveillance program for columnar metaplasia of the oesophagus. Scand J Gastroenterol 2000;35:10–16. [PubMed] [Google Scholar]
- 45.Spechler SJ, Goyal RK. Barrett’s oesophagus. N Engl J Med 1986;315:362–71. [DOI] [PubMed] [Google Scholar]
- 46.Kim SL, Waring JP, Spechler SJ, et al. Diagnostic inconsistencies in Barrett’s oesophagus. Gastroenterology 1994;107:945–9. [PubMed] [Google Scholar]
- 47.Lam St, Palcic B, McLean D, et al. Detection of early lung cancer using low dose Photofrin II. Chest 1990;97:333–7. [DOI] [PubMed] [Google Scholar]
- 48.Barr H, Shepherd NA, Dix A, et al. Eradication of high-grade dysplasia in columnar-lined (Barrett’s) oesophagus by photo dynamic therapy with endogenously generated protoporphyrin IX. Lancet 1996;348:584–5. [DOI] [PubMed] [Google Scholar]
- 49.Sagan C, Flejou JF, Diebold MD, et al. Reproducibility of histological criteria of dysplasia in Barrett’s mucosa. Gastroenterol Clin Biol 1994;18:31–4. [PubMed] [Google Scholar]
- 50.Reid BJ, Hagitt RC, Rubin CE, et al. Observer variation in the diagnosis of dysplasia in Barrett’s oesophagus. Human Pathol 1988;19:166–78. [DOI] [PubMed] [Google Scholar]
- 51.Wallace MB, Perelman LT, Backman V, et al. Endoscopic detection of dysplasia in patients with Barrett’s oesophagus using light scattering spectroscopy. Gastroenterology 2000;119:677–82. [DOI] [PubMed] [Google Scholar]
- 52.Skacel M, Petras RE, Gramlich TL, et al. The diagnosis of low-grade dysplasia in Barrett’s oesophagus and its implication for disease progression. Am J Gastroenterol 2000;95:3383–7. [DOI] [PubMed] [Google Scholar]