Abstract
Background and aim: Interleukin (IL) 17 is a cytokine which exerts strong proinflammatory activities. In this study we evaluated changes in IL-17 expression in the inflamed mucosa and in the serum of patients with inflammatory bowel disease (IBD).
Methods: Tissue samples were obtained endoscopically or surgically from patients with ulcerative colitis (UC) (n=20), Crohn’s disease (CD) (n=20), infectious colitis (n=5), ischaemic colitis (n=8), and normal colorectal tissues (n=15). IL-17 expression was evaluated by a standard immunohistochemical procedure. Serum IL-17 levels were determined by ELISA. IL-17 mRNA expression was analysed by reverse transcriptase-polymerase chain reaction.
Results: IL-17 expression was not detected in samples from normal colonic mucosa, infectious colitis, or ischaemic colitis. In the inflamed mucosa of active UC and CD patients, IL-17 expression was clearly detectable in CD3+ T cells or CD68+ monocytes/macrophages. The average number of IL-17+ cells was significantly increased in active UC and CD patients compared with inactive patients. IL-17 mRNA expression was not detected in normal mucosa but was detectable in the mucosa from active UC and CD patients. IL-17 was not detected in the sera from normal individuals, infectious colitis, or ischaemic colitis patients but IL-17 levels were significantly elevated in IBD patients.
Conclusions: IL-17 expression in the mucosa and serum was increased in IBD patients. It is likely that IL-17 expression in IBD may be associated with altered immune and inflammatory responses in the intestinal mucosa.
Keywords: inflammatory bowel disease, cytokines, T cell, monocytes/macrophages, interleukin 17
Inflammatory bowel diseases (IBD) such as ulcerative colitis (UC) and Crohn’s disease (CD) are characterised by recurrent flare of inflammation and a history of chronic enterocolitis. Activation of T cells and monocytes/macrophages is regarded as an important factor in the pathogenesis of IBD.1–4
Human interleukin 17 (IL-17) is a ∼20 kDa glycoprotein of 155 amino acids, with close sequence homology to both murine IL-17, originally identified as cytotoxic T lymphocyte associated antigen 8, and viral IL-17, the open reading frame 13 of T lymphotropic Herpesvirus saimiri.5,6 IL-17 secretion has been reported to be limited in T lymphocytes, predominantly in memory CD45RO+ cells.7–9 Recently, it has been reported that IL-17F, a member of the IL-17 family, is secreted by monocyte/macrophage lineages.10 In contrast, the IL-17 receptor is widely distributed on various cell types.11,12 There is increasing evidence that IL-17 is a potent mediator of inflammatory responses in various tissues. For example, IL-17 induces several genes associated with inflammation, including IL-6, granulocyte/macrophage-colony stimulating factor, leukaemia inhibitory factor, and intercellular adhesion molecule 1.13–18 In addition, IL-17 enhances the proinflammatory responses induced by IL-1β and tumour necrosis factor α (TNF-α).19–22
IL-17 has been implicated in several inflammatory disorders, such as rheumatoid arthritis, multiple sclerosis, systemic sclerosis, systemic lupus erythematosus, psoriasis, Helicobacter pylori associated gastritis, bronchial asthma, and renal allograft rejection.21–29 However, the pathophysiological role of IL-17 in IBD remains unclear. In this study we investigated IL-17 expression in IBD patients. Our results provide evidence that IL-17 is over expressed in the inflamed mucosa of IBD and may therefore contribute to the pathophysiology of IBD.
MATERIALS AND METHODS
Tissue samples
The diagnosis of UC and CD was based on conventional clinical, endoscopic, and histopathological criteria. Surgical or biopsy specimens derived from involved areas of the colon with UC (n=20) and CD (n=20) were obtained with informed consent. Disease activity in UC patients was evaluated according to the UC disease activity index,30 and that in CD patients was determined by the CD activity index.31 All IBD patients were treated with salicylates but none was treated with immunosuppressive agents (for example, azathioprine). Biopsy samples derived from infectious colitis (n=5) and ischaemic colitis (n=8) were obtained by colonoscopy. Normal colorectal tissues were also obtained (n=15).
Immunohistochemistry
Samples were fixed in 10% buffered formalin, dehydrated in ethanol, and embedded in paraffin. Sections (4 μm) were pretreated by boiling in citrate buffer (pH 6.1) in a microwave (700 W, one minute). After cooling, non-specific binding was blocked with Dako blocking reagent (#X0909; Dako Japan, Kyoto, Japan) followed by incubation with goat polyclonal antihuman IL-17 antibody (diluted 1:100 in phosphate buffered saline (PBS) containing 5% skim milk; Santa Cruz Biotechnology, Santa Cruz, California, USA) for 48 hours at 4°C in a humidified chamber. As a negative control, sections were incubated with normal goat IgG (Santa Cruz Biotechnology). After incubation with the primary antibody, sections were reacted with 0.1% H2O2 in 0.1 M PBS and treated with biotin conjugated rabbit antigoat IgG (diluted 1:2 in PBS containing 1% skim milk; Vector, Burlingame, California, USA) for 60 minutes at room temperature and followed by avidin-biotin-peroxidase complexes (Vector). Peroxidase activity was visualised using diaminobenzidine.
For double staining with anti-IL-17 plus anti-CD3 and/or anti-IL-17 plus anti-CD68, pretreatment of the deparaffinised slides was performed as described above. The anti-IL-17 antibody (diluted 1:100) was applied first and incubated for 48 hours at 4°C in a humidified chamber. Subsequently, monoclonal mouse antihuman CD3 or CD68 (diluted 1:100 in PBS containing 5% skim milk; Novocastra, Dossenheim, Germany) was applied overnight at 4°C. Texas red labelled antigoat IgG (diluted 1:500 in PBS containing 1% skim milk and 0.1% Triton X100; Rockland, Gilbertsville, Pennsylvania, USA) and fluorescein isothiocyanate (FITC) labelled horse antimouse IgG (diluted 1:500; Vector) were applied for 120 minutes at room temperature. Images were obtained by the digital confocal laser scanning system MRC-600 (Bio Rad, Hercules, California, USA). Normal goat or mouse IgG was used as a negative control.
Enzyme linked immunosorbent assay (ELISA) for human IL-17
Serum IL-17 levels were determined by enzyme linked immunosorbent assay (ELISA) kit for human IL-17 purchased from BioSource International (Camarillo, California, USA). The lower detection limit of ELISA was 9 pg/ml for IL-17. Serum samples from 29 patients with UC (inactive 15, active 14) and 32 patients with CD (inactive 15, active 17) were evaluated. The control group consisted of 20 healthy individuals with no family history of IBD and non-immune mediated disorders, or 12 patients with non-IBD diarrhoeal illness. Whole venous blood was obtained, and serum was separated after clotting by centrifugation. Aliquots were then taken and stored at −80°C until tests were performed. Samples were diluted with PBS containing 0.1% Tween 20, and the procedure was performed according to the manual provided by BioSource.
Isolation of peripheral T cells and monocytes/macrophages
Human monocytes, positive for anti-CD68, and T cells, positive for anti-CD3, were isolated from peripheral blood by FACS vantage (Becton Dickinson, Mountain View, California, USA). The purity of each population was 95–98%.
T cells and monocytes were stimulated with 50 ng/ml of IL-2 ( R&D Systems, Minneapolis, Minnesota, USA) or 100 ng/ml of lipopolysaccharide (Sigma-Aldrich Japan, Tokyo, Japan), respectively.
Reverse transcription-polymerase chain reaction (RT-PCR) for human IL-17
To confirm IL-17 expression in human peripheral monocytes and T cells, we performed RT-PCR analysis. IL-17 mRNA expression in the mucosa was also evaluated by RT-PCR. Total cellular RNA was isolated by the acid guanidium thiocyanate-phenol-chloroform (AGPC) method.32 For each sample, the first strand cDNA was synthesised using 0.5 μg of total cellular RNA with oligo(dT) primer and Superscript II reverse transcriptase (Gibco BRL, Rockville, Maryland, USA). The cDNA sample (1 μl) was amplified in 25 μl of a reaction mixture containing 10×Taq buffer (Perkin Elmer Cetus Corp., Norwalk, Connecticut, USA), 1.5 mM MgCl2, 0.1 μM of each 5` and 3` primers, and 1 U of TaqGold polymerase (Perkin-Elmer Cetus). PCR was performed in a thermal cycler (GeneAmp Model 2400; Perkin-Elmer Cetus) for 35 cycles (94°C for 30 seconds, 55°C for 30 seconds, and 72°C for 40 seconds), followed by an eight minute extension at 72°C. The PCR products (5 μl) were subjected to electrophoresis on 1.5% agarose gels and stained with 0.5 μg/ml ethidium bromide. A 100 bp DNA ladder (Gibco BRL) was used as the marker. Primers specific for the human IL-17 gene were constructed according to published sequence data26: 5`-AGA GAT ATC CCT CTG TG ATC-3`, 5`-TAC CCC AAA GTT ATC TCA GG-3`. The PCR products were ligated into the TA cloning vector (Promega, Madison, Wisconsin, USA) and sequenced by the dideoxynucleotide chain termination method.33
Statistical analysis
Evaluation of immunoreactivity for IL-17 was performed on sections by two blinded evaluators according to the method described by Middle and colleagues.34 In active CD and UC samples, inflamed regions were evaluated. The corresponding areas of the sections were marked and high power fields were counted at 400× magnification. The mean count from a total of five high power fields per slide was used.
Statistical significance of the differences was determined by the Mann-Whitney U test (Statview Version 4.5). Differences resulting in p values less than 0.05 were considered statistically significant.
RESULTS
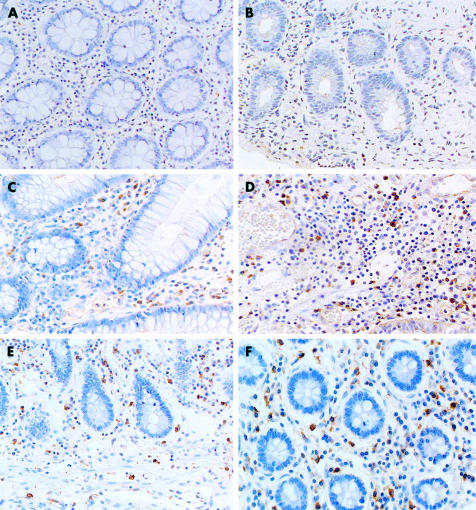
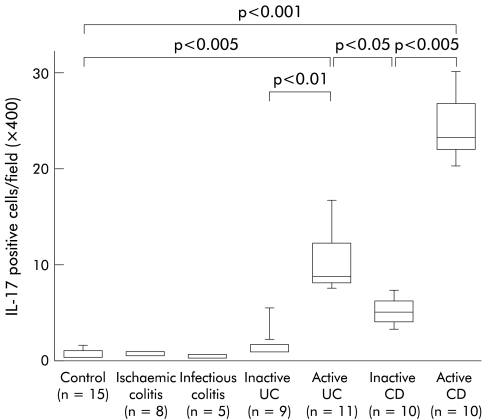
To evaluate expression of IL-17 protein in the mucosa, biopsy specimens and/or surgically resected samples were stained with anti-IL-17 antibodies. As shown in fig 1 ▶, there were no IL-17+ cells in normal colonic mucosa or in samples from ischaemic colitis. Similarly, in infectious colitis there was no immunostaining for IL-17 (data not shown). In contrast, a marked increase in IL-17+ cells was seen in the inflamed region of active UC and CD patients. In particular, IL-17+ cells markedly increased in samples from active CD patients. In active UC patients, IL-17+ cells were located mainly within the lamina propria but in active CD patients these cells were scattered throughout the submucosa and muscularis propria. As shown in fig 2 ▶, the number of IL-17+ cells was significantly increased in active IBD patients. In particular, the number of IL-17+ cells was significantly increased in active CD patients compared with active UC patients. The number of IL-17+ cells was decreased in samples from inactive IBD patients compared with active IBD patients.
Figure 1.
Immunohistochemical analysis of interleukin 17 (IL-17) protein expression in the colon. (A) Control normal mucosa (×200); (B) ischaemic colitis (×200); (C) and (D) ulcerative colitis (×200); (E) and (F) Crohn’s disease (×100 and ×200).
Figure 2.
Number of interleukin 17 (IL-17) positive cells in the mucosa. Results are expressed as number of positive cells per field (×400). The lower and upper margins of the box represent the 25th and 75th percentiles, with the extended arms representing the 10th and 90th percentiles, respectively. The median is shown as a horizontal line within the box. UC, ulcerative colitis; CD, Crohn’s disease.
To confirm the cellular origin of IL-17 in IBD patients, samples from active CD and UC patients were double stained with fluorescence labelled anti-CD3 (specific for T cells), anti-CD68 (specific for monocytes/macrophages), and anti-IL-17 antibodies (fig 3 ▶). In samples from CD patients (fig 3A–F ▶), there were many cells double positive (yellow) for CD68/IL-17 (fig 3F ▶) as well as for CD3/IL-17 (fig 3C ▶). This suggests that monocytes/macrophages and T cells are a source of IL-17 in the inflamed mucosa of CD patients. A similar staining pattern was also observed in samples from UC patients (fig 3G–L ▶). There were double positive cells for CD3/IL-17 (fig 3I ▶) and CD68/ IL-17 (fig 3L ▶) in the inflamed mucosa of UC patients.
Figure 3.
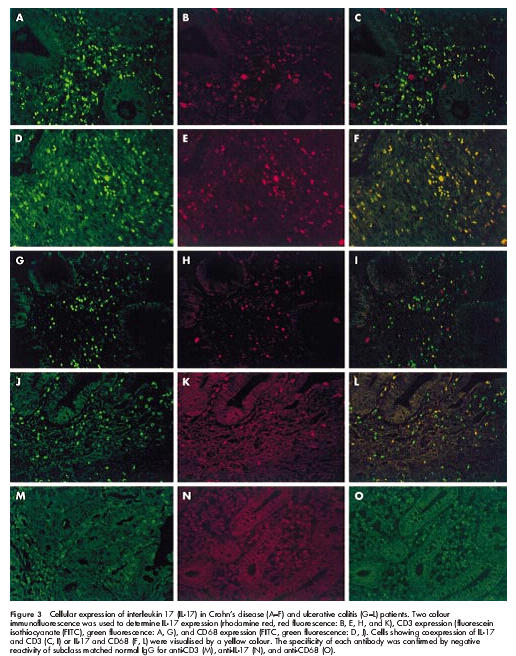
Cellular expression of interleukin 17 (IL-17) in Crohn’s disease (A–F) and ulcerative colitis (G–L) patients. Two colour immunofluorescence was used to determine IL-17 expression (rhodamine red, red fluorescence: B, E, H, and K), CD3 expression (fluorescein isothiocyanate (FITC), green fluorescence: A, G), and CD68 expression (FITC, green fluorescence: D, J). Cells showing coexpression of IL-17 and CD3 (C, I) or IL-17 and CD68 (F, L) were visualised by a yellow colour. The specificity of each antibody was confirmed by negative reactivity of subclass matched normal IgG for anti-CD3 (M), anti-IL-17 (N), and anti-CD68 (O).
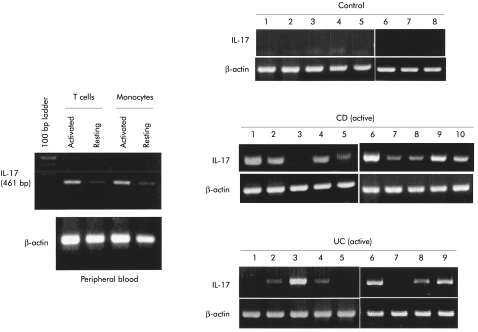
Next, we investigated IL-17 mRNA expression in the mucosa of normal individuals and active CD patients. The RT-PCR system used in this study detected IL-17 mRNA expression in T cells and monocytes/macrophages separated from peripheral blood of normal individuals (fig 4 ▶ left). In all samples from normal individuals, IL-17 mRNA expression was not detected (fig 4 ▶ right). In the inflamed mucosa of active CD patients, IL-17 mRNA expression was detected in nine of 10 samples. Similarly, in the inflamed mucosa of UC patients, it was detected in six of nine samples (fig 4 ▶ right).
Figure 4.
Reverse transcription-polymerase chain reaction (RT-PCR) analysis of interleukin 17 (IL-17) mRNA expression. Purified T cells were stimulated by interleukin 2 for three hours, and purified monocytes/macrophages were stimulated by lipopolysaccharide for three hours. Total cellular RNA was extracted by the acid guanidium thiocyanate-phenol-chloroform (AGPC) method and IL-17 mRNA expression was evaluated by RT-PCR analysis. Similarly, IL-17 mRNA expression was investigated in mucosal samples from normal individuals and in active Crohn’s disease (CD) and ulcerative colitis (UC) patients.
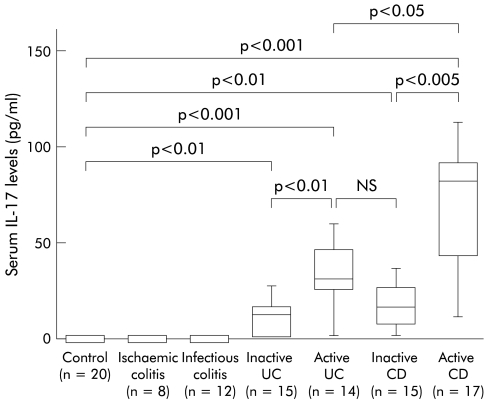
We evaluated serum IL-17 levels in IBD patients. As shown in fig 5 ▶, IL-17 was not detected in the sera of normal individuals, or in infectious colitis or ischaemic colitis. Serum IL-17 was significantly elevated in patients with active UC and CD patients compared with healthy individuals. In particular, IL-17 levels were markedly elevated in active CD patients.
Figure 5.
Serum interleukin 17 (IL-17) levels in patients with inflammatory bowel disease. IL-17 level was determined by ELISA for human IL-17 purchased from Bio Source International (Camarillo, California, USA). The lower limit of ELISA was 9 pg/ml of IL-17. The lower and upper margins of the box represent the 25th and 75th percentiles, with the extended arms representing the 10th and 90th percentiles, respectively. The median is shown as a horizontal line within the box. UC, ulcerative colitis; CD, Crohn’s disease.
DISCUSSION
Altered immune responses have been implicated in the pathogenesis of IBD. Many studies addressing the pathogenesis of IBD have focused on impaired mucosal cytokine secretion. For example, a growing number of studies have reported an increase in mucosal concentrations of proinflammatory cytokines and chemokines, including IL-1β, TNF-α, IL-6, IL-8, IL-12, IL-16, IL-18, interferon γ, and others.34–41
IL-17 is mainly derived from activated T cells.7–9 Recent reports have shown that IL-17F, a member of the IL-17 family, is secreted by monocytes/macrophages.10 IL-17 stimulates expression and production of proinflammatory cytokines in human cells,12–17 and the proinflammatory nature of IL-17 depends on activation of the transcription factor nuclear factor κB.18,21 Infiltration of large numbers of T cells and monocytes/macrophages into the inflamed mucosa has been described in IBD patients1–4 but the role of IL-17 secretion by inflammatory cells has not been investigated. Therefore, the aim of this study was to evaluate the possibility that IL-17 activity is elevated in patients with IBD. IL-17 secretion was initially investigated in the inflamed mucosa of IBD patients by immunohistochemical techniques. The number of IL-17+ cells was significantly elevated in the inflamed mucosa of IBD. In particular, the number of IL-17+ cells was increased in active lesions of CD patients. Double staining using either anti-CD3 or anti-CD68 with anti-IL-17 antibodies revealed that T cells and monocytes/macrophages are the local source of IL-17 in the inflamed mucosa of IBD patients. In accordance with the biological properties of IL-17, our results suggest that IL-17 derived from activated T cells and monocytes/macrophages could be involved in induction and persistence of mucosal inflammatory responses in IBD.
Previous studies have shown that several proinflammatory cytokines are elevated in the sera of IBD patients. For example, in IBD patients elevation in serum levels of IL-6, TNF-α, CC-chemokine eotaxin, and macrophage inhibitory factor has been reported.42–46 Elevation of proinflammatory cytokines is considered to be associated with the severity of gut inflammation. In this study, we showed significant elevation of serum IL-17 levels in active IBD patients. Notably, serum IL-17 levels were significantly higher in active CD patients than in active UC patients. These results are compatible with immunohistochemical observations indicating that the number of IL-17+ cells was greater in the inflamed mucosa of active CD patients compared with UC patients. With respect to the study of the relationship between serum IL-17 levels and disease activity, previous reports23,25,29 found that serum IL-17 levels were altered according to disease activity. The usefulness of serum IL-17 in estimating disease activity of IBD patients remains unclear. Further studies involving a greater number of patients will clarify its usefulness in IBD patients.
In conclusion, we have demonstrated that mucosal IL-17 expression and serum IL-17 levels are elevated in active IBD patients. These results suggest that IL-17 may play an important role in the pathophysiology of IBD patients. Further studies in larger numbers of IBD patients are necessary to determine the mechanisms involved in elevated mucosal IL-17 secretion and the immunological role of IL-17 in IBD.
Acknowledgments
The authors thank Dr T Tsujikawa and Dr M Sasaki for technical assistance. The study was supported in part by Grant-in-Aid for Scientific Research (B) from the Ministry of Education, Culture, Sports, Science and Technology, Japan (12470121 and 13470119).
Abbreviations
IBD, inflammatory bowel disease
UC, ulcerative colitis
CD Crohn’s disease
IL, interleukin
TNF, tumour necrosis factor
FITC, fluorescein isothiocyanate
ELISA, enzyme linked immunosorbent assay
RT-PCR, reverse transcription-polymerase chain reaction
AGPC, acid guanidium thiocyanate-phenol-chloroform
PBS, phosphate buffered saline
REFERENCES
- 1.Schreiber S. Monocytes or T cells in Crohn’s disease: does IL-16 allow both to play at that game? Gut 2001;49:746–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zareie M, Singh PK, Irvine EJ, et al. Monocytes/macrophage activation by normal bacteria and bacterial products: implications for altered epithelial function in Crohn’s disease. Am J Pathol 2001:158;1101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boirivant, M, Marini, M, DiFelice, et al. Lamina propria T cells in Crohn’s disease and other gastrointestinal inflammation show defective CD2 pathway-induced apoptosis. Gastroenterology 1999;116:557–65. [DOI] [PubMed] [Google Scholar]
- 4.Saubermann LJ, Probert CSJ, Christ AD, et al. Evidence of T cell receptor β-chain patterns in inflammatory and noninflammatory bowel disease states Am J Physiol 1999;276:G613–21. [DOI] [PubMed] [Google Scholar]
- 5.Fossiez F, Banchereau J, Murry R, et al. Interleukin-17. Intern Rev Immunol 1998;16:541–51. [DOI] [PubMed] [Google Scholar]
- 6.Yao Z, Painter SL, Fanslow WC, et al. Human IL-17: a novel cytokine derived from T cells. J Immunol 1995;155:5483–6. [PubMed] [Google Scholar]
- 7.Kennedy J, Rossi DL, Zurawski SM, et al. Mouse IL-17: a cytokine preferentially expressed by αβ TCR+ CD4-CD8- T cells. J Interferon Cytokine Res 1996;16:611–17. [DOI] [PubMed] [Google Scholar]
- 8.Albanesi C, Scarponi C, Cavani A, et al. Interleukin-17 is produced by both Th1 and Th2 lymphocytes, and modulates interferon-γ- and interleukin-4-induced activation of human keratinocytes. J Invest Dermatol 2000;115:81–7. [DOI] [PubMed] [Google Scholar]
- 9.Shin HC, Benbernou N, Esnault S, et al. Expression of IL-17 in human memory CD45RO+ T lymphocytes and its regulation by protein kinase A pathway. Cytokine 1999;11:257–66. [DOI] [PubMed] [Google Scholar]
- 10.Starnes T, Robertson MJ, Sledge G, et al. IL-17F, a novel cytokine selectively expressed in activated T cells and monocytes, regulates angiogenesis and endothelial cell cytokine production. J Immunol 2001;167:4137–40. [DOI] [PubMed] [Google Scholar]
- 11.Yao Z, Spriggs MK, Derry JM, et al. Molecular characterization of the human interleukin (IL)-17 receptor. Cytokine 1997;9:794–800. [DOI] [PubMed] [Google Scholar]
- 12.Yao Z, Fanslow WC, Seldin MF, et al. Herpesvirus saimiri encodes a new cytokine, IL-17, which bind to a novel cytokine receptor. Immunity 1995;3:811–21. [DOI] [PubMed] [Google Scholar]
- 13.Fossiez F, Djossou O, Chomarat P, et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med 1996;183:2593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chabaud M, Fossiez F, Taupin JL, et al. Enhancing effects of IL-17 on IL-1-induced IL-6 and leukemia inhibitory factor production by rheumatoid arthritis synoviocytes and its regulation by Th2 cytokines. J Immunol 1998;161:409–14. [PubMed] [Google Scholar]
- 15.Cai XY, Gommol-CP J, Justice L, et al. Regulation of granulocyte colony-stimulating factor gene expression by interleukin-17. Immunol Lett 1998;62:51–8. [DOI] [PubMed] [Google Scholar]
- 16.Jovanovic DV, Di Battista JA, Martel PJ, et al. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-1β and TNF-α, by human macrophages. J Immunol 1998;60:3513–21. [PubMed] [Google Scholar]
- 17.Albanesi C, Cavani A, Girolomoni G. IL-17 is produced by nickel-specific T lymphocytes and regulates ICAM-1 expression and chemokine production in human keratinocytes: Synergistic or antagonist effects with Ischaem-γ and TNF-α. J Immunol 1999;162:494–502. [PubMed] [Google Scholar]
- 18.Awane M, Andres PG, Li DJ, et al. NF-κB-inducing kinase is a common mediator of IL-17-, TNF-α-, and IL-1β-induced chemokine promoter activation in intestinal epithelial cells. J Immunol 1999;162:5337–44. [PubMed] [Google Scholar]
- 19.Katz Y, Nadiv O, Beer Y. Interleukin-17 enhances tumor necrosis factor alpha-induced synthesis of interleukins 1, 6, and 8 in skin and synovial fibroblasts: a possible role as a “fine-tuning cytokine” in inflammation processes. Arthritis Rheum 2001;44:2176–84. [DOI] [PubMed] [Google Scholar]
- 20.LeGrand A, Fermor B, Fink C, et al. Interleukin-1, tumor necrosis factor alpha, and interleukin-17 synergistically up-regulate nitric oxide and prostaglandin E2 production in explants of human osteoarthritic knee menisci. Arthritis Rheum 2001;44:2078–83. [DOI] [PubMed] [Google Scholar]
- 21.Shimada M, Andoh A, Hata K, et al. IL-6 secretion by human pancreatic periacinar myofibroblasts in response to inflammatory mediators. J Immunol 2002;168: 861–8. [DOI] [PubMed] [Google Scholar]
- 22.Chabaud M, Lubberts E, Joosten L, et al. IL-17 derived from juxta-articular bone and synovium contributes to joint degradation in rheumatoid arthritis. Arthritis Res 2001;3:168–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matusevicius D, Kivisakk P, He B, et al. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult Scler 1999;5:101–4. [DOI] [PubMed] [Google Scholar]
- 24.Kurasawa K, Hirose K, Sano H, et al. Increased interleukin-17 production in patients with systemic sclerosis. Arthritis Rheum 2000;43:2455–63. [DOI] [PubMed] [Google Scholar]
- 25.Wong CK, Ho CY, Li EK, et al. Elevation of proinflammatory cytokine (IL-18, IL-17, IL-12) and Th2 cytokine (IL-4) concentrations in patients with systemic lupus erythematosus. Lupus 2000;9:589–93. [DOI] [PubMed] [Google Scholar]
- 26.Albanesi C, Scarponi C, Cavani A, et al. Interleukin-17 is produced by both Th1 and Th2 lymphocytes, and modulates interferon-γ- and interleukin-4-induced activation of human keratinocytes. J Invest Dermatol 2000;115:81–7. [DOI] [PubMed] [Google Scholar]
- 27.Luzza F, Parrello T, Monteleone G, et al. Up-regulation of IL-17 is associated with bioactive IL-8 expression in Helicobacter pylori-infected human gastric mucosa. Up-regulation of IL-17 is associated with bioactive IL-8 expression in Helicobacter pylori-infected human gastric mucosa. J Immunol 2000; 165:5332–7. [DOI] [PubMed] [Google Scholar]
- 28.Linden A. Role of interleukin-17 and the neutrophil in asthma. Int Arch Allergy Immunol 2001;126:179–84. [DOI] [PubMed] [Google Scholar]
- 29.Hsieh HG, Loong CC, Lui WY, et al. IL-17 expression as a possible predictive parameter for subclinical renal allograft rejection. Transpl Int 2001;14:289–98. [DOI] [PubMed] [Google Scholar]
- 30.Singleton JW. Clinical features, course, and laboratory findings in ulcerative colitis. In: Kirsner JB, Shorter RG, eds. Inflammatory bowel disease, 4th edn. Baltimore: Williams and Wilkins, 1995:335–43.
- 31.Best WR, Becktel JM, Singleton JW, et al. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology 1976;70:439–44. [PubMed] [Google Scholar]
- 32.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987;162:156–9. [DOI] [PubMed] [Google Scholar]
- 33.Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 1977;74:5463–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Middel P, Reich K, Polzien F, et al. Interleukin 16 expression and phenotype of interleukin 16 producing cells in Crohn’s disease. Gut 2001;49:795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahida YR, Wu K, Jewell DP. Enhanced production of interleukin-1β by mononuclear cells isolated from mucosa with active ulcerative colitis of Crohn’s disease. Gut 1989;30:835–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lampinen M, Carlson M, Sangfelt P, et al. IL-5 and TNF-α participate in recruitment of eosinophils to intestinal mucosa in ulcerative colitis. Dig Dis Sci 2001;46:2004–9. [DOI] [PubMed] [Google Scholar]
- 37.Atreya R, Mudter J, Finotto S, et al. Blockade of interleukin-6 trans signaling suppress T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in Crohn disease and experimental colitis in vivo. Nat Med 2000;6:583–8. [DOI] [PubMed] [Google Scholar]
- 38.Papadakis KA, Targan SR. The role of chemokines and chemokine receptors in mucosal inflammation. Inflamm Bowel Dis 2000;6:303–13. [DOI] [PubMed] [Google Scholar]
- 39.Omata F, Birkenbach M, Matsuzaki S, et al. The expression of IL-12 p40 and its homologue, Epstein-Barr virus-induced gene 3, in inflammatory bowel disease. Inflamm Bowel Dis 2001;7:215–20. [DOI] [PubMed] [Google Scholar]
- 40.Bisping G, Lugering N, Lutke-Brintrup S, et al. Patients with inflammatory bowel disease (IBD) reveal increased induction capacity of intracellular interferon-gamma (Ischaem-γ) in peripheral CD8+ lymphocytes co-cultured with intestinal epithelial cells. Clin Exp Immunol 2001;123:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garside P. A role for IL-18 in intestinal inflammation? Gut 2001;48:6–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holub MC, Mako E, Devay T, et al. Increased interleukin-6 levels, interleukin-6 receptor and gp130 expression in peripheral lymphocytes of patients with inflammatory bowel disease. Scand J Gastroenterol 1998;228(suppl):47–50. [PubMed] [Google Scholar]
- 43.Louis E, Belaiche J, van Kemseke C, et al. A high serum concentration of interleukin-6 is predictive of relapse in quiescent Crohn’s disease. Eur J Gastroenterol 1997;9:939–44. [DOI] [PubMed] [Google Scholar]
- 44.Komatsu M, Kobayashi D, Saito K, et al. Tumor necrosis factor-α in serum of patients with inflammatory bowel disease as measured by ba highly sensitive immuno-PCR. Clin Chem 2001;47:1297–301. [PubMed] [Google Scholar]
- 45.Chen W, Paulus B, Shu D, et al. Increased serum levels of eotaxin in patients with inflammatory bowel disease. Scand J Gastroenterol 2001;36:515–20. [DOI] [PubMed] [Google Scholar]
- 46.Murakami H, Akbar SM, Matsui H, et al. Macrophage migration inhibitory factor in the sera and at the colonic mucosa in patients with ulcerative colitis: clinical implications and pathogenic significance. Eur J Clin Invest 2001;31:337–43. [DOI] [PubMed] [Google Scholar]