Abstract
Aims and methods: To examine the association between smoking and histological liver lesions in chronic hepatitis C, we studied 244 consecutive patients (152 men, 92 women; mean age 45.9 (12.6) years) with histologically proven chronic hepatitis C. Daily tobacco consumption during the six months preceding liver biopsy was recorded as the number of cigarettes smoked daily. Total lifetime tobacco consumption was recorded as the number of cigarette packs smoked per year (packs-years). Liver biopsy specimens were graded for histological activity and fibrosis according to the METAVIR scoring system.
Results: The proportion of patients with moderate (A2) or marked (A3) activity increased gradually from 62.0% in non-smokers to 81.7% in patients who smoked more than 15 cigarettes per day (p<0.009). A similar relationship was observed with total lifetime tobacco consumption: 59.0% of patients who had never smoked had grade A2 or A3 disease activity compared with 84.6% of patients who smoked more than 20 packs per year (p<0.002). Multivariate analysis showed that age over 50 years (odds ratio (OR) 5.4), alcohol intake exceeding 20 g/day (OR 2.75), and tobacco consumption of more than 15 cigarettes/day (OR 3.6) were independently related to the histological activity score. No relationship was found between the severity of fibrosis and either daily tobacco consumption or total lifetime tobacco consumption. Multivariate analysis showed that only age over 50 years (OR 8.8), daily alcohol intake exceeding 30 g/day (OR 3.4), and histological activity score (OR 7.9) were independently related to the fibrosis score.
Conclusion: This study suggests that smoking, independent of alcohol, could aggravate the histological activity of chronic hepatitis C and that patients with chronic hepatitis C virus infection should be advised to reduce or stop smoking.
Keywords: smoking, hepatitis C virus, hepatitis C infection, liver lesions
In patients with chronic hepatitis C virus (HCV) infection, both the severity and progression of liver disease markedly differ from one subject to another.1–6 This variability is not clearly explained although the role of several host, viral, and environmental factors has been suggested.5,7,8 Among environmental factors, the impact of alcohol has been largely documented in worsening both histological activity and fibrosis.5,9–13 Although experimental studies in rats have shown that nicotine can have deleterious effects on the liver, notably by aggravating the hepatotoxic effects of carbon tetrachloride (Ccl4),14 the effects of smoking on the liver in patients with HCV infection are poorly documented.15 Thus the aim of this study was to examine the association between smoking and histological liver lesions in a large cohort of consecutive patients with chronic hepatitis C.
PATIENTS AND METHODS
A total of 244 consecutive patients with histologically proven chronic hepatitis C seen in our department between February 1998 and September 1999 were included in the study. At the time of the study, none had received antiviral therapy. Baseline characteristics of the patients are shown in table 1 ▶. There were 152 men and 92 women, with a mean age of 45.9 (12.6) years. The route of HCV transmission was blood transfusion in 76 cases (31.2%), intravenous drug use in 53 cases (21.7%), and not clearly identified in 115 cases (47.1%). All had both serum anti-HCV antibodies (Ortho HCV 3.0 ELISA Test System; Ortho-clinical Diagnostics, Raritan, New Jersey, USA) and serum HCV RNA (Amplicor HCV 2.0 PCR test system; Roche Molecular Systems, Pleasanton, California, USA). None of the patients had serum hepatitis B surface antigen or antihuman immunodeficiency virus antibodies. All liver biopsy specimens were fixed in formalin, embedded in paraffin, and routinely processed for histological analysis. The histological grade of disease activity and the histological stage of fibrosis were assessed using the reproducible METAVIR scoring system as follows: grade A1 to A3 for the degree of necroinflammatory activity (A1=mild to A3=marked), and stage F0 to F4 for the degree of fibrosis (F0=no fibrosis to F4=cirrhosis).16,17 The histological index of Knodell was used to analyse separately the different components of histological activity.18
Table 1.
Baseline characteristics of the 244 patients with chronic hepatitis C virus infection
| Age (y) | 45.9 (12.6) |
| Sex | |
| Men (n (%)) | 152 (62.3%) |
| Women (n (%)) | 92 (37.7%) |
| Route of transmission | |
| Blood transfusion (n (%)) | 76 (31.2%) |
| Intravenous drug use (n (%)) | 53 (21.7%) |
| Not identified (n (%)) | 115 (47.1%) |
| Recent smoking history (cig/day)* | 7.9 (11.1) |
| Total lifetime smoking history (packs-years) | 10.2 (13.7) |
| Alcohol intake (g/day)* | 11.6 (16.8) |
| Histological activity (grade) | |
| A1 (n (%)) | 79 (32.4%) |
| A2 (n (%)) | 155 (63.5%) |
| A3 (n (%)) | 10 (4.1%) |
| Fibrosis (stage) | |
| F1 (n (%)) | 134 (55.0%) |
| F2 (n (%)) | 44 (18.0%) |
| F3 (n (%)) | 41 (16.8%) |
| F4 (n (%)) | 25 (10.2%) |
Values are mean (SD) unless otherwise indicated.
*Within the six months before liver biopsy.
Tobacco consumption was estimated in a standardised evaluation of smoking history19: each patient was asked to complete a questionnaire (table 2 ▶) on the day of liver biopsy. The questionnaire included the following items: age at the beginning of regular smoking, smoking duration, and daily amount of tobacco smoked in the six months preceding liver biopsy. Tobacco consumption was expressed as the number of cigarettes smoked daily (cig/day) and as packs-years—that is, the product of the average number of packs smoked per day by the number of years as a smoker.20 Patients were classified into three or four groups according to tobacco consumption. A total of 137 patients (56.1%) were non-smokers at the time of liver biopsy, 47 patients (19.3%) smoked 1–15 cig/day, and 60 patients (24.6%) smoked more than 15 cig/day. Information on total lifetime smoking consumption was known for 234 patients. One hundred patients (42.7%) had never smoked, 46 patients (19.7%) had smoked 1–10 packs-years, 49 patients (20.9%) had smoked 11–20 packs-years, and 39 patients (16.7%) has smoked more than 20 packs-years. Thirty seven patients (15.2%) had stopped smoking at least six months before liver biopsy. Alcohol intake was also estimated at the time of liver biopsy as the number of units (equivalent to 10 g of alcohol) per day during the previous six months. Patients were classified into four groups according to alcohol intake as follows: mild (1–20 g/day; 79 patients, 32.4%), moderate (21–40 g/day; 49 patients, 20.1%), heavy (>40 g/day; 39 patients, 16.0%), or none (77 patients, (31.5%). We checked that smoking consumption and alcohol intake had been stable during the six months preceding liver biopsy.
Table 2.
Smoking history (according to the Textbook of pulmonary disease, 5th Edn, 199419)
| Age when the patient started to smoke |
| How old was the patient when he/she became a regular smoker? |
| Duration of smoking |
| Were there periods when the patient stopped smoking? |
| Is the patient currently smoking? |
| At what age did the patient stop smoking? |
| Amount smoked |
| Average lifetime number of cigarettes smoked per day. |
| Average lifetime number of cigars smoked per day. |
| Average lifetime amount of pipe tobacco smoked per day. |
| Packs-years of cigarettes smoked (average packs/day×number of years as a smoker). |
Statistical analysis
Results are expressed as means (SD) and percentages. Univariate analysis was performed using the overall χ2 test and the χ2 test for a linear trend for categorical data to identify factors associated with the histological activity score or the histological fibrosis score. Thereafter, stepwise logistic regression analysis was used to explore the independence of the factors found to be significant in the univariate analysis. All factors with a p≤0.10 in univariate analysis were tested in the model. Odds ratios (OR) were estimated from the model and are presented with their 95% confidence intervals (CI). p values <0.05 were considered significant.
RESULTS
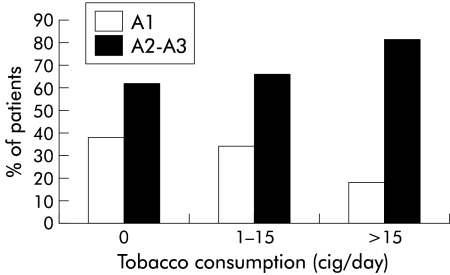
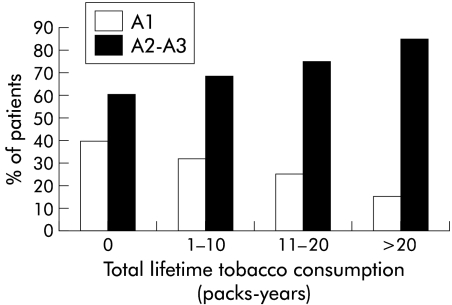
The relationship between tobacco consumption (cig/day and packs-years) and histological activity is presented in figs 1 and 2 ▶ ▶. As shown in fig 1 ▶, the proportion of patients with moderate or marked histological activity (A2–A3) increased gradually with daily tobacco consumption during the six months before liver biopsy: grade A2 or A3 was found in 85 (62.0%) of 137 patients who did not smoke, in 31 (66.0%) of 47 patients who smoked 1–15 cig/day, and in 49 (81.7%) of 60 patients who smoked more than 15 cig/day (p<0.009, test for a linear trend). Likewise, the proportion of patients with mild histological activity (A1) fell as tobacco consumption increased. A similar relationship was observed with total lifetime smoking (fig 2 ▶). The proportion of patients with moderate and marked activity (A2–A3) increased gradually from 59.0% in the 100 patients who had never smoked to 67.4% in the 46 light smokers (1–10 packs-years), 75.5% in the 49 moderate smokers (11–20 packs-years), and 84.6% in the 39 heavy smokers (more than 20 packs-years) (p<0.002, test for a linear trend). Similarly, the proportion of patients with mild histological activity (A1) fell with the number of packs-years.
Figure 1.
Relationship between histological activity (METAVIR scoring system; A1=mild to A3=marked) and smoking (cig/day within the six months before biopsy) in patients with chronic hepatitis C virus infection (p<0.009, test for a linear trend).
Figure 2.
Relationship between histological activity (METAVIR scoring system; A1=mild to A3=marked) and smoking (packs-years) in patients with chronic hepatitis C virus infection (p<0.002, test for a linear trend).
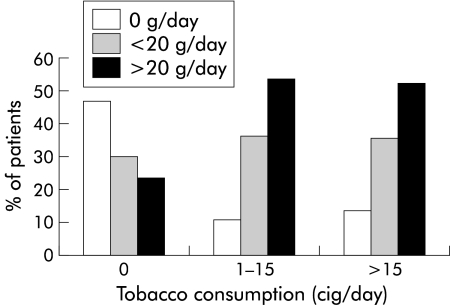
A significant relationship between smoking and alcohol intake was found: 46.7% of patients who did not smoke did not drink alcohol and 51.7% of patients who smoked more than 15 cig/day drank more than 20 g/day (p<0.001) (fig 3 ▶). In the univariate analysis, A2–A3 activity was also associated with an older age (p=0.003) and alcohol intake exceeding 20 g/day (p=0.003). As several factors were associated together, a multivariate analysis was performed to explore factors independently associated with activity. Apart from alcohol intake and smoking (cig/day and packs-years), this multivariate analysis included age at biopsy and sex. Age over 50 years (OR 5.4 (95% CI 2.4–12.4)), alcohol intake exceeding 20 g/day (OR 2.75 (95% CI 1.2–6.2)), and smoking more than 15 cig/day (OR 3.6 (95% CI 1.5–8.8)) were independently associated with histological activity (table 3 ▶). The component of the Knodell index activity which was significantly affected by tobacco consumption was periportal necrosis: the proportion of patients with moderate or marked periportal necrosis (Knodell index 3 or 4) was significantly higher in patients who smoked more than 15 cig/day than in patients who did not smoke or smoked less than 15 cig/day (62.7% v 78.9%, respectively; p=0.03).
Figure 3.
Relationship between alcohol intake (g/day) and tobacco consumption (cig/day within the six months before biopsy) in patients with chronic hepatitis C virus infection (p<0.001).
Table 3.
Logistic regression analysis for activity
| Variable | Patients with A2–A3 activity | Odds ratio | 95% CI | p Value |
| Age (y) | ||||
| ≤40 | 59% | 1 | — | |
| 41–50 | 63% | 1.6 | 0.8–3.4 | NS |
| >50 | 82% | 5.4 | 2.4–12.4 | 0.0001 |
| Alcohol intake (g/day) | ||||
| 0 | 55% | 1 | — | |
| ≤20 | 67% | 1.5 | 0.7–3.1 | NS |
| >20 | 80% | 2.75 | 1.2–6.2 | 0.04 |
| Tobacco consumption (cig/day) | ||||
| 0 | 62% | 1 | — | |
| ≤15 | 66% | 1.2 | 0.5–2.8 | NS |
| >15 | 82% | 3.6 | 1.5–8.8 | 0.007 |
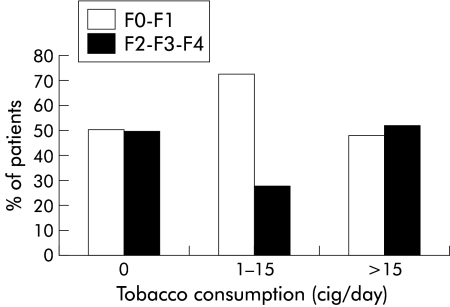
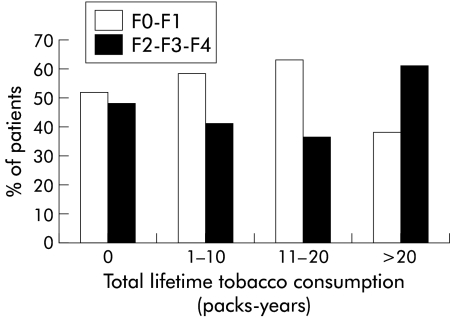
In contrast, no significant relationship was found between histological fibrosis and either daily or lifetime smoking history (figs 4, 5 ▶ ▶). As in the univariate analysis, F2–F3–F4 fibrosis was associated with older age (p<0;001), alcohol intake more than 30 g/day, moderate or marked activity (A2–A3) (p<0.001), and, to a less extent, duration of infection (p=0.07). A multivariate analysis was performed. The three parameters which were independently associated with stage of fibrosis in the multivariate analysis were age over 50 years (OR 8.8 (95% CI 3.9–20.0)), alcohol intake exceeding 30 g/day (OR 3.4 (95% CI 1.3–9.0)), and histological activity (OR 7.9 (95% CI 3.5–18.0)) (table 4 ▶).
Figure 4.
Relationship between histological fibrosis (METAVIR scoring system; F0=no fibrosis to F4=cirrhosis) and smoking (cig/day within the six months before biopsy) in patients with chronic hepatitis C virus infection (p<0.51, test for a linear trend).
Figure 5.
Relationship between histological fibrosis (METAVIR scoring system; F0=no fibrosis to F4=cirrhosis) and smoking (packs-years) in patients with chronic hepatitis C virus infection (p=0.11).
Table 4.
Logistic regression analysis for fibrosis
| Variable | Patients with F2–F3–F4 fibrosis | Odds ratio | 95% CI | p Value |
| Age (y) | ||||
| ≤40 | 26% | 1 | — | |
| 41–50 | 35% | 1.5 | 0.7–3.3 | NS |
| >50 | 53% | 8.8 | 3.9–20.0 | 0.0001 |
| Alcohol intake (g/day) | ||||
| 0 | 44% | 1 | — | |
| ≤30 | 32% | 0.4 | 0.2–0.9 | 0.04 |
| >30 | 74% | 3.4 | 1.3–9.0 | 0.03 |
| Activity | ||||
| A1 | 15% | 1 | — | |
| A2–A3 | 59% | 7.9 | 3.5–18.0 | 0.0001 |
DISCUSSION
This study shows a clear relationship between daily tobacco consumption and the severity of histological activity in patients with chronic hepatitis C. Indeed, the proportion of patients with moderate and marked activity (A2–A3) increased gradually from 62.0% in patients who did not smoke to 81.7% in patients who smoked more than 15 cig/day (p<0.009). As confounding factors, namely alcohol intake, could explain this relationship, a multivariate analysis was performed. It showed that, in addition to two previously identified factors (that is, age over 50 years and alcohol intake exceeding 20 g/day), tobacco consumption higher than 15 cig/day within the six months before biopsy was associated with the severity of histological activity. Thus independent of alcohol intake, tobacco consumption higher than 15 cig/day was associated with aggravation of histological activity in patients with chronic hepatitis C. In our study, both univariate and multivariate analyses showed the relationship between tobacco consumption and histological activity. This result is somewhat different from a recent report which found a significant relationship in age adjusted and multivariate analyses but not in univariate analysis.15 Interestingly, the total lifetime smoking history was not independently associated with histological activity, suggesting that smoking may have a rapid and reversible hepatotoxic effect.
The mechanism by which smoking may increase histological activity in chronic hepatitis C has to be determined. Nicotine, a major component of tobacco smoke, is rapidly absorbed through the lungs and released into the circulation. It is mainly metabolised in the liver. The effects of nicotine on the liver have been studied in rats, both alone and combined with Ccl4.14 Rats receiving nicotine alone developed significant liver lesions characterised by steatosis and focal or confluent necrosis. CCl4 induced liver lesions were also aggravated by nicotine. Tobacco induced liver lesions have been ascribed in vitro and in vivo to oxidative stress associated with lipid peroxidation.21–24 In patients with chronic hepatitis C, the reduction in the concentrations of hepatic, plasmatic, and lymphocytic glutathione could favour the hepatotoxic effect of smoking.25 Up to now, toxicity of nicotine on the liver has only been studied in animals, not permitting extrapolation to humans. Thus the mechanism of the relationship between tobacco and histological activity that we found in our HCV infected patients remains to be clarified.
In our study, the degree of fibrosis was independently related to age at liver biopsy, alcohol consumption, and histological activity, confirming previous results.5,10–12 In contrast, we observed no relationship between the degree of fibrosis and daily tobacco consumption during the six months preceding liver biopsy. This was not surprising as the action of smoking appears to be rapid and reversible. Likewise, the total lifetime smoking history was not associated with the degree of fibrosis. This was more surprising but could be explained by the fact that the duration of HCV infection was known in only half of the study population and probably did not overlap perfectly with the duration of smoking. In a recent report,15 a significant relationship was shown between tobacco consumption and histological fibrosis. This result was obtained only when the Knodell score activity was excluded from the multivariate analysis, which led to the suggestion that the fibrotic effect of smoking was the consequence of enhanced activity.
All of these data support a deleterious role of smoking in patients with chronic hepatitis C but we cannot exclude the fact that confounding, not yet identified, factors, such as social or environmental variables, could explain the relationship between tobacco and histological activity. Additional studies are required to clarify this relationship. Investigations are needed for testing such a role in other chronic liver diseases. In this respect, it has already been suggested that cigarette smoking was independently related to the risk of cirrhosis both in alcoholics and in asymptomatic chronic hepatitis B virus carriers.26,27
In conclusion, we showed that smoking could aggravate the histological activity of chronic hepatitis C. Cessation or reduction of tobacco consumption should therefore be recommended in patients with chronic hepatitis C.
Abbreviations
HCV, hepatitis C virus
OR, odds ratio
CCl4
carbon tetrachloride
REFERENCES
- 1.EASL International Consensus Conference on Hepatitis C. Consensus statement. J Hepatol 1999;30:956–61. [PubMed] [Google Scholar]
- 2.Roudot-Thoraval F, Pawlotsky JM, Deforges L, et al. Anti-HCV seroprevalence in pregnant women in France. Gut 1993;34(suppl 2):S55–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiyosawa K, Tanaka E, Sodeyama T, et al. Transmission of hepatitis C in an isolated area in Japan: community-acquired infection. Gastroenterology 1994;106:1596–602. [DOI] [PubMed] [Google Scholar]
- 4.Tong MJ, El-Farra NS, Reikes A, et al. Clinical outcomes after transfusion associated hepatitis C. N Engl J Med 1995;332:1463–6. [DOI] [PubMed] [Google Scholar]
- 5.Roudot-Thoraval F, Bastie A, Pawlotsky JM, et al. Epidemiological factors affecting the severity of hepatitis C virus related liver disease: a French survey of 6664 patients. Hepatology 1997;26:485–90. [DOI] [PubMed] [Google Scholar]
- 6.Seef LB, Miller RN, Rabkin CS, et al. 45-years follow-up of hepatitis C virus infection in healthy young adults. Ann Intern Med 2000;132:105–11. [DOI] [PubMed] [Google Scholar]
- 7.Pawlotsky JM, Tsakiris L, Roudot-Thoraval F, et al. Relationship between hepatitis C virus genotypes and sources of infection in patients with chronic hepatitis C. J Infect Dis 1995;171:1607–16. [DOI] [PubMed] [Google Scholar]
- 8.Zeuzem S, Franke A, Lee JH, et al. Phylogenic analysis of hepatitis C virus isolates and their correlation to viremia, liver function tests and histology. Hepatology 1996;24:1003–9. [DOI] [PubMed] [Google Scholar]
- 9.Corrao G, Arico S. Independent and combined action of hepatitis C virus infection and alcohol consumption on the risk of symptomatic liver cirrhosis. Hepatology 1998;27:914–19. [DOI] [PubMed] [Google Scholar]
- 10.Pessione F, Degos F, Marcellin P, et al. Effect of alcohol consumption on serum hepatitis C virus RNA and histological lesions in chronic hepatitis C. Hepatology 1998;27:1717–22. [DOI] [PubMed] [Google Scholar]
- 11.Ostapowicz G, Watson KJR, Locarnini SA, et al. Role of alcohol in the progression of liver disease caused by hepatitis C infection. Hepatology 1998,27:1730–5. [DOI] [PubMed] [Google Scholar]
- 12.Lonjon I, Hézode C, Roudot-Thoraval F, et al. Alcohol intake, even moderate, could increase histological activity and fibrosis in patients with chronic active hepatitis C. J Hepatol 1998;28(suppl):55A. [Google Scholar]
- 13.Wiley TE, McCarthy M, Breidi L, et al. Impact of alcohol on the histological and clinical progression of hepatitis C infection. Hepatology 1998;28:805–9. [DOI] [PubMed] [Google Scholar]
- 14.Yuen ST, Gogo AR, Luk ISC, et al. The effect of nicotine and its interaction with carbon tetrachloride in the rat liver. Pharmacol Toxicol 1995;77:225–30. [DOI] [PubMed] [Google Scholar]
- 15.Pessione F, Ramond MJ, Njapoum C, et al. Cigarette smoking and hepatic lesions in patients with chronic hepatitis C. Hepatology 2001;34:121–5. [DOI] [PubMed] [Google Scholar]
- 16.The French METAVIR cooperative study group. Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. Hepatology 1994;20:15–20. [PubMed] [Google Scholar]
- 17.Bedossa P, Poynard T for the METAVIR cooperative study group. An algorythm for the grading of activity in chronic hepatitis C. Hepatology 1996;24:289–93. [DOI] [PubMed] [Google Scholar]
- 18.Knodell RG, Ishak KG, Black WC, et al. Formulation and application of numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology 1981;1:431–5. [DOI] [PubMed] [Google Scholar]
- 19.Baum GL, Wolinsky E. Textbook of pulmonary disease, 5th Edn, vol 11. Boston: Little Brown, 1994:257.
- 20.Doll R, Peto R. Cigarette smoking and bronchial carcinoma: dose and time relationships among regular smokers and lifelong non-smokers. J Epidemiol Commun Health 1978;32:303–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gogo AR, Cho CH, Yuen ST, et al. The cytotoxic effect of nicotine on the liver and its modulation of hepatotoxicity induced by carbon tetrachloride in rats. Eur J Gastroenterol Hepatol 1993,5:859–65. [Google Scholar]
- 22.Bagchi M, Bagchi D, Hassoun EA, et al. Smokeless tobacco induced increases in hepatic lipid peroxidation, DNA damage and excretion of urinary lipid metabolites. Int J Exp Pathol 1994;75:197–202. [PMC free article] [PubMed] [Google Scholar]
- 23.Panda K, Chattopadhyay R, Chattopadhyay DJ, et al. Vitamin C prevents cigarette smoke-induced oxidative damage in vivo. Free Radic Biol Med 2000,29:115–24. [DOI] [PubMed] [Google Scholar]
- 24.Baskaran S, Lakshmi S, Prasad PR. Effect of cigarette smoke on lipid peroxidation and antioxidant enzymes in albino rat. Indian J Exp Biol 1999,37:1196–200. [PubMed] [Google Scholar]
- 25.Barbaro G, Di Lorenzo G, Ribersani M, et al. Serum ferritin and hepatic glutathione concentrations in chronic hepatitis C patients related to the hepatitis C virus genotype. J Hepatol 1999;30:774–82. [DOI] [PubMed] [Google Scholar]
- 26.Klatsky AL, Armstrong MA. Alcohol, smoking, coffee, and cirrhosis. Am J Epidemiol 1992;136:1248–57. [DOI] [PubMed] [Google Scholar]
- 27.Yu MW, Hsu FC, Sheen IS, et al. Prospective study of hepatocellular carcinoma and liver cirrhosis in asymptomatic chronic hepatitis B virus carriers. Am J Epidemiol 1997;145:1039–47. [DOI] [PubMed] [Google Scholar]