Abstract
Background: Previous studies on the treatment of Helicobacter pylori infection in functional dyspepsia have shown little, if any, effect on dyspeptic symptoms. However, whether such treatment might be of benefit in patients resistant to acid inhibitors has not been formally tested.
Aim: The present study investigated the effect of H pylori treatment in patients with functional dyspepsia resistant to conventional treatment.
Patients: A total of 181 H pylori positive patients with chronic functional dyspepsia who had not responded to a one week antacid run-in and two week double blind antisecretory or placebo treatment were included.
Methods: Patients were randomised to two weeks of treatment with omeprazole 40 mg twice daily combined with amoxicillin 1 g twice daily or omeprazole 20 mg once daily alone. The primary outcome variable (“response”) was defined as no need for further therapy or investigations for dyspeptic symptoms 4–6 months after treatment.
Results: H pylori infection was healed in 10% of patients after omeprazole and in 52% after omeprazole plus amoxicillin. The respective “response” rates were 66% and 62% (NS). H pylori treatment and cure of H pylori infection had no effect on complete resolution of all dyspeptic symptoms, individual symptoms, or various aspects of quality of life.
Conclusion: In functional dyspepsia, H pylori treatment and cure of H pylori are no more effective for symptoms over six months than short term acid inhibition. These results do not support treatment of H pylori in functional dyspepsia.
Keywords: Helicobacter pylori, functional dyspepsia, omeprazole, amoxicillin, randomised clinical trial, gastric acid secretion
Approximately half of patients with functional dyspepsia are infected with Helicobacter pylori.1 The key question in the treatment of H pylori infected patients with functional dyspepsia is whether cure of H pylori infection improves dyspeptic symptoms. In the last few years, several large, randomised, double blind, controlled trials2–8 were performed which produced conflicting results. If any, the symptomatic benefit of H pylori eradication appeared to be very modest, as has also been shown in a meta-analysis of these data.9 However, in contrast with common practice, patients included in these studies did not receive pretreatment with standard drugs such as prokinetic or acid inhibitory drugs. In addition, exclusion of patients who responded to acid reducing drugs may facilitate the detection of a potential effect of H pylori treatment. Thus we have conducted a study in patients with functional dyspepsia who were resistant to standard treatment.
PATIENTS AND METHODS
Study protocol
This investigation was a multicentre, double blind, randomised, clinical trial with parallel groups, carried out according to Good Clinical Practice and the revised Declaration of Helsinki. The ethics committees of all German states approved the protocol, and all patients participating gave written informed consent. Patients were recruited between August 1994 and July 1996.
Selection of patients
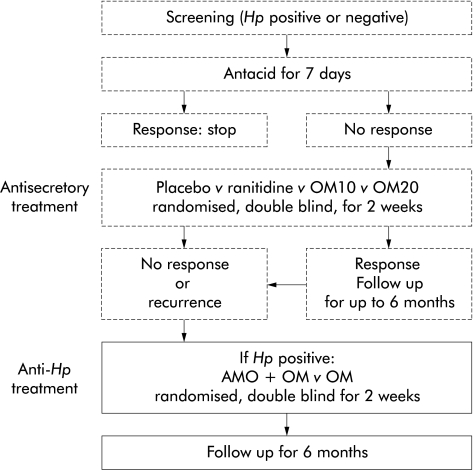
H pylori positive patients, more than 18 years of age, with chronic therapy resistant (see below) functional dyspepsia were recruited from 46 private gastroenterological practices in Germany. H pylori status was ascertained in all patients using both the rapid urease test (HUT; AstraZeneca GmbH, Wedel, Germany) and the 13C urea breath test (13C-UBT). In the event of divergent results, the 13C-UBT test result was decisive. All patients had participated in a previous trial10 on the effect of acid inhibitory treatment in chronic functional dyspepsia (fig 1 ▶). Chronic functional dyspepsia was defined as severe epigastric symptoms, present for the last month, in the absence of organic disease known to produce epigastric symptoms. Organic disease was excluded by means of gastroscopy (normal findings except for hiatal hernia, mucosal erythema, less than 10 gastric erosions, and minor deformation of the pylorus and duodenal bulb), laboratory tests, and sonography (normal findings except for minor hepatic steatosis, small uncomplicated liver cysts, and small haemangiomas). Initial dyspeptic symptoms had to be severe enough to require management (defined as treatment other than liquid antacids and/or endoscopy or other diagnostic tests). In this previous study, patients with antacid resistant severe functional dyspepsia had been randomised to two weeks of treatment with omeprazole 20 mg once daily, omeprazole 10 mg once daily, ranitidine 150 mg at bedtime, or placebo in a double blind, double dummy manner. If at the end of two weeks they still had symptoms requiring therapy, or symptoms reappeared within six months of completing the randomised treatment, they were eligible for the present study (fig 1 ▶). When the period between screening for the previous study and entry into the present study exceeded four weeks, gastroscopy, HUT, and blood tests were repeated.
Figure 1.
Design of the previous (broken lines)10 and present (continuous line) studies. Hp, H pylori; OM10, omeprazole 10 mg once daily; OM20, omeprazole 20 mg once daily; AMO+OM, amoxicillin 1 g twice daily plus omeprazole 40 mg twice daily; OM, omeprazole 20 mg once daily. Ranitidine was given as 150 mg at bedtime.
Patients were excluded for the following reasons: heartburn/acid regurgitation without concomitant epigastric symptoms; predominant symptoms suggesting irritable bowel syndrome, in particular pain in the lower abdomen, flatulence, diarrhoea, or constipation; previously documented erosive or ulcerative oesophagitis; peptic ulcer; previous abdominal surgery (except for inguinal hernia, appendectomy, hysterectomy, and Caesarean section); “alarm” symptoms during the previous three months (for example, dysphagia, involuntary weight loss, gastrointestinal bleeding, unexplained fever, abdominal mass, and jaundice); proton pump inhibitors and/or antibiotic treatment within one month prior to the screening period; regular treatment one week prior to the screening period with drugs which might interfere with symptom assessment (for example, non-steroidal anti-inflammatory drugs or bismuthates); and more than 80 ml of ethanol daily.
Treatment
Patients were treated for two weeks with omeprazole 40 mg twice daily plus amoxicillin 1 g twice daily (OM+AMO) or omeprazole 20 mg once daily plus placebo (OM) (all from AstraZeneca Pharmaceutical Production AB, Södertälje, Sweden). Drug intake was monitored by counting returned medication. No other treatment for dyspepsia was allowed during the treatment period (for example, H2 receptor antagonists, prokinetic drugs, or spasmolytics).
Follow up
All patients were followed for six months regardless of their symptoms. They were given a supply of a weak antacid (bags of 10 ml suspension of aluminium hydroxide gel 3.13 g, magnesium oxide 0.27 g, and aluminium hydroxide magnesium carbonate gel 0.63 g) and were permitted to take up to 30 ml daily for up to three consecutive days in case of occasional dyspepsia. Follow up visits were scheduled at the end of the treatment period, and one and six months later. Unscheduled visits were encouraged at any time when more severe dyspeptic symptoms recurred. These symptoms were treated at the discretion of the investigator, but H pylori treatment with antibiotics or bismuth was not allowed. Patients who required management for their symptoms at scheduled or unscheduled visits during the last three months of the study were classed as treatment failures.
Primary and secondary outcome criteria
The main outcome criterion was dyspepsia during the last three months of follow up; treatment success was defined as lack of dyspeptic symptoms requiring management (defined as treatment other than liquid antacids and/or diagnostic tests including endoscopy). A clinically relevant difference in response rates on the primary outcome criterion at the end of the six months of follow up was defined as 20% (60% without, 80% with H pylori treatment). In order to confirm such a difference, accepting a β error of 0.20 and an α error of 0.05 (Fisher’s exact test, two sided), the required number of patients per group in an intention to treat analysis was 91.
Secondary outcome variables included: time until relapse—that is, lack of overall response during the six months of the follow up period; gastrointestinal symptoms according to the investigator’s judgement as well as the patient’s opinion; and quality of life parameters. At each visit, specific symptoms were elicited: epigastric pain or burning, epigastric pressure or fullness, heartburn, acid regurgitation, nausea and/or vomiting, pain in the lower abdomen, flatulence, diarrhoea, and constipation. The severity of individual symptoms during the previous week was graded according to a scale from 0 to 3 (0=no complaints, 1=complaints not interfering with daily activities and not requiring treatment, 2=complaints requiring treatment but not interfering with daily activities, and 3=complaints interfering with daily activities and requiring treatment). In addition, patients answered the question “How were your symptoms in the area of the oesophagus and stomach?” and “How was your general condition during the last seven days?” by making a mark on a 10 cm visual analogue scale (best possible condition 10 cm, worst condition 0 cm). Quality of life during the previous week was assessed using a validated questionnaire adapted to German lifestyle.11,12 This form contained 40 general items relating to physical strength, ability to enjoy and relax, positive mood, absence of negative mood, social contacts, and social well being. An additional questionnaire consisted of nine questions that had been validated in Germany by Eypasch and colleagues,13 and which related to impairment of quality of life by dyspeptic symptoms. They assessed the influence of dyspeptic symptoms on eating, other daily activities, social contacts, sleep, and fears of serious disease. Finally, the patient’s time spent off work and/or in hospital was recorded.
Data management and statistics
Data were transferred to and analysed by an independent statistical institute (Institute for Numerical Statistics, Cologne, Germany). Both an intention to treat (ITT) and per protocol (PP) analysis were performed, and all outcome variables were analysed by treatment group and by the 13C-UBT result (four weeks after the end of treatment).
The following 13 characteristics were selected prospectively for univariate and logistic regression analyses using the main outcome criterion as the dependent variable: age, sex, foreign nationality (country of birth outside of Germany), overweight (body mass index >27.8 kg/m2 for males and >27.3 kg/m2 for women), daily smoking, daily alcohol drinking, previous dyspepsia (dyspeptic symptoms before the present painful episode), duration of dyspepsia (>2 years), presence of reflux symptoms (acid regurgitation and/or regurgitation), severity of dyspepsia (visual analogue scale), general condition (visual analogue scale), cure of H pylori infection, and treatment group. The same independent variables (except 13C-UBT result) were used for a second logistic regression analysis using cure of H pylori infection as the dependent variable.
All analyses were based on SAS (version 6.11) and SPSS (version 7.5) for Windows.
Assignment
The randomisation list was computer generated in blocks of two; the block size was kept secret until the code was broken for analysis. The allocation sequence was concealed in sequentially numbered sealed opaque envelopes. Complete sets of envelopes were kept at Astra Hässle AB and at the Institute for Numerical Statistics. The blocks were consecutively ordered by random number. Each centre received complete blocks together with the corresponding sealed envelopes containing the treatment allocation; in no case was the code broken prematurely. Patients fulfilling all entry criteria (with the exception of the 13C-UBT result, which became available to the investigators with a few days delay) were allocated a random number, and treatment was started. Patients with a negative 13C-UBT result were excluded from analysis.
Blinding
The randomised study treatment was given in a double blind double dummy manner using matching placebo preparations. Patients in the OM+AMO group received omeprazole capsules (40 mg twice daily) plus amoxicillin tablets (1 g twice daily) and those in the OM group received omeprazole capsules (20 mg once daily) plus omeprazole placebo capsules, and amoxicillin placebo tablets (twice daily). Placebo and active medications as well as omeprazole 20 and 40 mg capsules were similar in appearance and taste. The treatment code was broken after clean filing and allocation of individual patients to ITT and PP analyses.
RESULTS
Participant flow and follow up
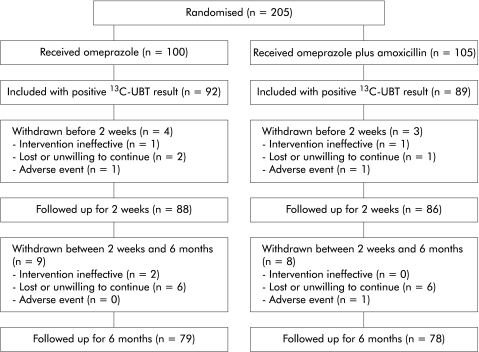
A full trial profile is given in fig 2 ▶. The ITT analysis included 181 patients (OM 92, OM+AMO 89). Patients in the two groups were well matched with regard to demographic characteristics and dyspeptic symptoms (table 1 ▶). Intake of less than 75% of the study medication, a reason for exclusion from the ITT analysis, was found in only two patients in the OM+AMO group, indicating good compliance. The PP analysis included 144 patients who completed the study without major deviations from the protocol: 74 in the omeprazole only group and 70 in the combination group (table 2 ▶).
Figure 2.
Trial profile showing progress through the various stages of the study. 13C-UBT, 13C urea breath test.
Table 1.
Comparison of treatment groups. Results are given according to intention to treat
| Presenting characteristic | OM (n=92) | OM+AMO (n=89) |
| Age (y) (mean (SD)) | 46 (15) | 49 (16) |
| Sex (% male) | 42% | 40% |
| Body mass index (kg/m2) (mean (SD)) | 25 (4) | 25 (4) |
| Current smoker (%) | 21 | 21 |
| Alcohol consumption, current (%) | 44 | 51 |
| Treatment in previous study (%) | ||
| Omeprazole 10 mg | 27 | 20 |
| Omeprazole 20 mg | 17 | 20 |
| Ranitidine 150 mg | 34 | 24 |
| Placebo | 22 | 36 |
| Entering this study from previous study (%) | ||
| Immediately after treatment period | 86 | 87 |
| During follow up | 14 | 14 |
| History of dyspepsia (%) | ||
| <1 y | 10 | 15 |
| 1–5 y | 52 | 49 |
| >5 y | 38 | 36 |
| Previous unsuccessful treatment of H pylori (%) | 2 | 7 |
| Dyspeptic symptoms (VAS) | 50.5 | 51.0 |
| General condition (VAS) | 48.0 | 46.0 |
OM, omeprazole; OM+AMO, omeprazole plus amoxicillin; VAS, median of visual analogue scale values, determined by the patient.
There was no significant difference between groups for any of the characteristics.
Table 2.
Major protocol deviations and number of patients in the intention to treat (ITT) and per protocol (PP) analyses
| OM | OM+AMO | Total | |
| Patients in ITT analysis | 92 | 89 | 181 |
| Discontinuation due to adverse event | 1 | 2 | 3 |
| Lost/refused to continue | 7 | 7 | 14 |
| Non-compliance | |||
| Study medication* | 0 | 2 | 2 |
| Use of prohibited medication | 5 | 4 | 9 |
| Day of visit | 2 | 3 | 5 |
| Other | 3 | 1 | 4 |
| Total patients with major protocol deviation | 18 | 19 | 37 |
| Patients in PP analysis | 74 | 70 | 144 |
*More than 25% of study medication was returned.
OM, omeprazole; OM+AMO, omeprazole plus amoxicillin.
There was no significant difference between the treatment groups.
Analysis
Effect of treatment on cure of H pylori
Four weeks after the end of treatment, 13C-UBT values were negative in nine of 92 (10%) patients after omeprazole and in 46 of 89 (52%) patients after omeprazole and amoxicillin treatment (table 3 ▶, ITT analysis). Analysing only patients with known 13C-UBT results, the respective H pylori cure rates were 9/78 (12%) and 33/81 (57%).
Table 3.
Patients with no need for further treatment and/or diagnostic tests for dyspepsia during the last three months of the six month follow up according to assigned treatment and to Helicobacter pylori status after treatment
| H pylori status after treatment | OM | OM+AMO | Total |
| Cured | 8/9 (89%) | 33/46 (72%) | 41/55 (75%) |
| Not cured | 51/69 (74%) | 20/35 (57%) | 71/104 (68%) |
| Unknown | 2/14 (14%)** | 2/8 (25%)* | 4/22 (18%)** |
| Total | 61/92 (66%) | 55/89 (62%) | 116/181 (64%) |
*p=0.03, **p<0.0001 compared with patients in the same column with known H pylori status.
OM, omeprazole; OM+AMO, omeprazole plus amoxicillin.
There was no significant difference between treatment groups (intention to treat analysis).
Symptomatic response during follow up
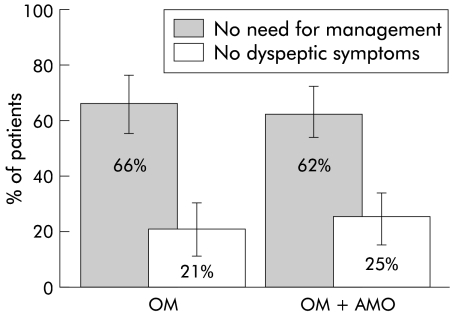
The results for the primary outcome variable—no need for further management of dyspepsia 4–6 months after the end of treatment—are shown in fig 3 ▶. On an ITT basis, 61 of 92 patients (66%; 95% confidence interval (CI) 56–76%) were considered to be “responders” after OM treatment compared with a similar number after OM+AMO treatment (55 of 89 (62%); 95% CI 51–72%; p=0.54, Fisher’s exact test). In addition, no difference was seen when the proportions of patients who were free of any dyspeptic symptoms were compared (fig 3 ▶). These results were similar to those of the PP analysis. As the cure rate of H pylori after OM+AMO treatment was only 46/89 (52%, 95% CI 41–62%), the response rates were also calculated according to the 13C-UBT results after therapy (table 3 ▶). The response rate did not differ significantly between patients in whom H pylori infection was cured (41/55 (75%); 95% CI 61–85%) and those with persistent infection (71/104 (68%); 95% CI 58–77%) (table 3 ▶). The seemingly high failure rate of patients with unknown post-treatment H pylori status is explained by early withdrawal from the study.
Figure 3.
Effect of omeprazole (OM) and omeprazole plus amoxicillin (OM+AMO) on symptomatic response 4–6 months after the treatment period (intention to treat analysis). The proportion of patients who did not need further management of dyspepsia (treatment and/or diagnostic tests) and the proportion of patients who did not complain of any dyspeptic symptoms are shown. Bars indicate 95% confidence intervals.
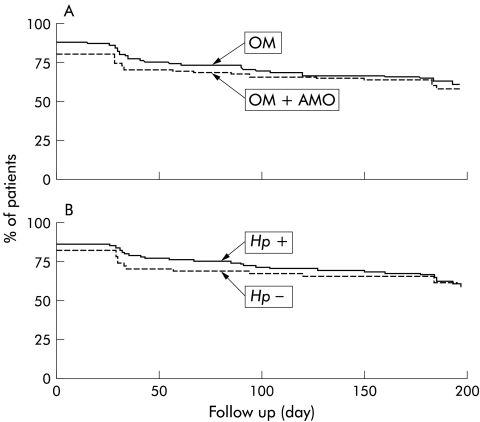
The life table curves shown in fig 4 ▶ comprise the proportions of patients whose dyspepsia was judged not to require further management at two weeks. The curves indicate the proportion of patients without a first recurrence of dyspepsia requiring management. There was no significant difference between the two treatment groups (fig 4A ▶) or between patients with were or were not cured of H pylori infection (fig 4B ▶).
Figure 4.
Life table analysis of patients in remission (no need for further management for dyspepsia) according to the randomised treatment (A) and to Helicobacter pylori status after treatment (B). Intention to treat analysis. OM, omeprazole (n=89); OM+AMO, omeprazole plus amoxicillin (n=92). There was no significant difference between treatment groups or between H pylori positive (n=104) and negative (n=55) patients. Note that the onset (day 0) of the life table curves is below 100% because of the fraction of patients who did not reach remission during the two week treatment period.
Impairment of general condition, suffering caused by dyspepsia, and individual symptoms
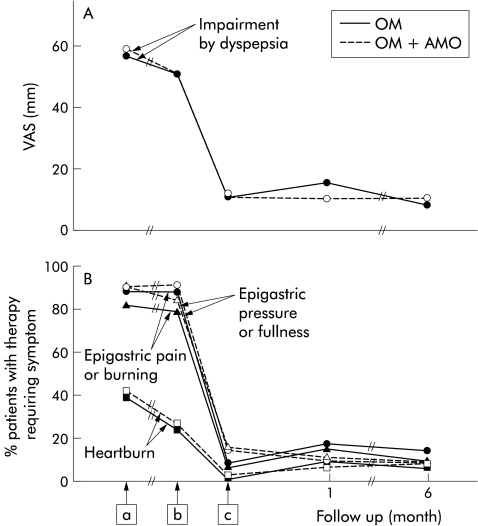
The time course of impairment by dyspepsia and the three most frequent dyspeptic symptoms (epigastric pain or burning, epigastric pressure or fullness, and heartburn) showed little change between entry into the double blind antisecretory study and entry into the present study (fig 5 ▶). However, regardless of treatment group, a dramatic improvement in all parameters (p<0.0001) was seen during the treatment phase of the present study, with no further change occurring during the follow up period. The time course of changes within the two treatment groups was almost identical. The prevalence of nausea/vomiting and lower abdominal pain, flatulence, diarrhoea, and constipation was low and did not change during the study.
Figure 5.
Time course of impairment by dyspepsia (A) and of the three most frequent individual dyspeptic symptoms severe enough to require treatment (B) from the beginning of the previous and during the present study. Values in (A) are medians (because of skewed distribution) of visual analogue scales (VAS) and those in (B) are percentage of patients who needed treatment for the given individual symptom. A significant (p<0.0001) improvement in all parameters was noted during the treatment period in the present study but no change occurred during follow up. There was no significant difference between the treatment groups. Intention to treat analysis. OM, omeprazole; OM+AMO, omeprazole plus amoxicillin. a, beginning of previous study; b, start of treatment in the present study; and c, end of treatment in the present study.
Quality of life
One dyspepsia specific and six general aspects of quality of life were assessed. Irrespective of the therapy, every parameter (physical strength, ability to relax and enjoy, positive mood, absence of negative mood, social contact, social well being, and absence of impairment by dyspepsia) improved significantly to normal levels12,13 during the 14 day treatment period, and no further change was observed during the six month follow up period (data not shown). In no instance was there a significant difference between treatment groups.
There was no significant difference in days off work (OM: 77 patients none, seven patients ranging from 1 to 29 days, no data for eight; OM+AMO: 78 patients no days, seven ranging from 2 to 24, no data for four). Only one patient, from the OM+AMO group, was hospitalised for 18 days.
Factors associated with outcome
No significant correlation was found in either the univariate or logistic regression analysis between any of the 13 preselected presenting characteristics. In particular, cure of H pylori infection did not correlate with outcome. A separate analysis, with cure of H pylori infection as the dependent variable and using the same presenting characteristics (except 13C UBT results), showed, as predicted, a correlation with treatment group (OM+AMO; p<0.0001) and a direct correlation with age (p=0.026). Logistic regression analysis revealed that the two factors were independent of each other. In the first step, “treatment group” appeared with a p value of <0.001; the second step identified “age” as an additional significant independent variable that improved the correlation (p=0.04). When the variable “treatment group” was removed from the analysis, “age” remained as the only significant factor (p=0.03), thus indicating that H pylori is more easily treated in older patients.
Adverse events and change of diagnosis
No relevant adverse effects attributable to treatment were observed. At various time points after the first visit, the following diagnoses of organic abdominal diseases were made: duodenal ulcer (one 42 year old female with persistent H pylori after OM, diagnosed on study day 194); erosive oesophagitis (one 62 year old male after OM, day 104); gastric leiomyosarcoma with liver metastases (one 58 year old male after OM, five weeks after the end of follow up); pancreatic carcinoma (one 51 year old male after OM+AMO, day 135); and ovarian carcinoma (one 74 year old female after OM+AMO, day 96).
DISCUSSION
Successful outcome, as predefined by the absence of severe dyspeptic symptoms during the final three months of follow up, was no more frequent after H pylori treatment than after simple short term antisecretory treatment. The same was found for all additional outcome criteria tested in multiple exploratory analyses. If anything, there was a tendency in favour of antisecretory treatment alone compared with the combination with amoxicillin. Hence it is unlikely that a significant or clinically relevant difference in outcome in favour of H pylori treatment would have been reached with a much larger number of patients. The success of simple antisecretory treatment is even more surprising as our patients were not blinded to the result of the 13C-UBT after treatment. Cure of H pylori infection might have fulfilled patients’ expectations and thus have favoured success in the group with treatment of H pylori.
An important distinction between this and other studies2–8 of H pylori therapy in functional dyspepsia was restrictive patient selection. All patients had normal or nearly normal findings at gastroscopy, abdominal sonography, and in laboratory tests, supporting the assumption of functional dyspepsia. In addition, they appeared to be resistant to acid inhibitory therapy in the previous prospective study.10 Hence the remaining management alternatives for functional dyspepsia were prokinetics, whose efficacy is currently being challenged,14 and H pylori therapy, which, in the opinion of many physicians and patients, offers the possibility of cure of dyspepsia. In these patients we expected a greater chance of demonstrating an effect of H pylori therapy than in a general population of patients with functional dyspepsia.2–8
The 52% cure rate of H pylori infection achieved using dual therapy with high dose omeprazole and amoxicillin was rather low. We chose this regimen because initial data suggested very high healing rates.15 The study was already ongoing when subsequent studies showed disappointing results with this combination,16,17 comparable with those in the present trial. This weakens the ITT analysis according to treatment. However, almost identical results were obtained when we compared patients with healed and unhealed infection instead of the two treatment groups.
A major criticism of previous studies on H pylori therapy in functional dyspepsia was the short duration of follow up. We considered the six month follow up period in the present study to be sufficient for the following histopathological and functional reasons. The major improvement in gastritis, in particular regression of infiltrates with polymorphonuclear leucocytes, occurs within a few days after the beginning of effective antimicrobial therapy,18 and superficial epithelial damage recovers within a few weeks,19,20 while it takes longer for disappearance of chronic inflammatory cells.19–22 Recent evidence suggests that chronic inflammatory cells may not disappear even after several years.23 Most importantly, there are no data indicating that a favourable effect after one year is not already present after six months.3,24,25 The favourable effect seen in the study by McCarthy and colleagues24 after one year appears to be weak as the study was not randomised. In a more recent study from the same group,25 a positive effect of H pylori therapy on a subgroup of patients with ulcer-like dyspepsia was, if anything, stronger after six than after 12 months of follow up. Finally, follow up of our patients over six months as well as of those in a similar study4 over one year do not suggest even a trend for improvement of symptoms after cure of H pylori infection. Therefore, there is no rational basis for the hope that longer follow up would change the result.
The surprisingly prompt and long lasting improvement in symptoms during the two week treatment period was unexpected in view of the previous resistance to active therapy demonstrated by this patient group. One explanation is that in the previous study10 only 25% of patients received the stronger acid inhibitory dose of omeprazole (20 mg) that was shown to be effective, the majority having received omeprazole 10 mg, ranitidine 150 mg, or placebo. In contrast, the present treatment consisted of omeprazole at a daily dose of 20 or 80 mg. A more likely explanation implies that there was some bias of both investigators and patients towards more severe symptoms in order to be eligible for H pylori therapy. The dramatic improvement may have been due to the very high expectations that this last resort would be effective. A similar bias was suspected in the preceding study where H pylori positivity correlated with lack of symptomatic response.10
In conclusion, the results of this study indicate that treatment of H pylori does not lead to improvement in functional dyspepsia resistant to conventional management. Together with other trials2–8 that fulfil important quality criteria,26,27 more evidence is accumulating that H pylori treatment is of little, if any, value in the treatment of functional dyspepsia.
Acknowledgments
We are grateful to Dr Madeline Frame for expert assistance with the manuscript preparation. Supported by Swiss Science Research Foundation, grant No 31-43240.95, and by AstraZeneca GmbH, Wedel, Germany
Abbreviations
13C-UBT
13C urea breath test
ITT, intention to treat
PP, per protocol
Appendix
The FROSCH Study Group
Werner Abels (Nürnberg), Krikor Amdja (Wermelskirchen), Herbert Bock (Frankfurt), Dibor Bojanovski (Hannover), Hilmar Böneke (Lienen), Burkhardt Cyrus (München), Axel Dettmer (München), Reinhard Diedrich (Marburg), Klaus Dietrich (Saarbrücken), Wolfgang Dübel (Berlin), Michael Dudek (Düsseldorf), Dieter Ebbinghaus (Lünen), Wolfgang Fortelny (Waldsassen), Roland Graf (Leutkirch), Wolfgang Güttel (Rastatt), Hans-Jürgen Hagel (Schwabach), Bernt Hawickhorst (Wülfrath), Martin Hell (Salzgitter-Lebenstedt), Rüdiger Hildebrandt (Clausthal-Zellerfeld), Walter Hofmeister (Weiden), Wolfgang Huppertz (Essen), Harvey Jürgens (Ölde), Bernhard Klesser (Ulm), Horst Klewer (Wetzlar), Michael Klöters (Spaichingen), Andreas Kocjan (Lüdenscheid), Justine Kosmowski (Marburg), Joachim Labenz (Essen), Günter Leiber (Marburg), Helmut Lichti (Gladenbach), Andrei Mares (Frankfurt), Peter Mayr (Stockach), Bernd Metscher (Berlin), Heinrich Miks (Hamm), Hubert Mönnikes (Marburg), Michael Müller (Delmenhorst), Claus Nolte (Mettmann), Paul-Peter Pech (Münster), Stefan Pfäffl (Nürnberg), Thomas Rachel (Rastatt), Axel Rambow (Marburg), Gerd Rosprich (Saarbrücken), Thomas Schädlich (Ellefeld), Andreas Schober (Göttingen), Michael Schumacher (Wolmirstedt), Rainer Stroband (Münster), Huschang Toluipur (Schiffweiler), Rüdiger Vogt (Mannheim), Rainer Wack (Berlin), Günter Wilhelms (Goslar), Jürgen Zeus (Erlangen).
REFERENCES
- 1.Pantoflickova D, Blum AL, Koelz HR. Helicobacter pylori and dyspepsia: A real causal link? Baillieres Clin Gastroenterol 1998;12:503–32. [DOI] [PubMed] [Google Scholar]
- 2.Blum AL, Talley NJ, O’Morain C, et al. Lack of effect of treating Helicobacter pylori infection in patients with nonulcer dyspepsia. N Engl J Med 1998;339:1875–81. [DOI] [PubMed] [Google Scholar]
- 3.McColl K, Murray L, El Omar E, et al. Symptomatic benefit from eradicating Helicobacter pylori infection in patients with nonulcer dyspepsia. N Engl J Med 1998;339:1869–74. [DOI] [PubMed] [Google Scholar]
- 4.Talley NJ, Janssens J, Lauritsen K, et al. Eradication of Helicobacter pylori in functional dyspepsia: randomized double blind placebo controlled trial with 12 months’ follow up. BMJ 1999;318:833–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Talley NJ, Vakil N, Ballard II ED, et al. Absence of benefit of eradicating Helicobacter pylori in patient with nonulcer dyspepsia. N Engl J Med 1999; 341:1106–11. [DOI] [PubMed] [Google Scholar]
- 6.Miwa H, Hirai S, Nagahara A, et al. Cure of Helicobacter pylori infection does not improve symptoms in non-ulcer dyspepsia patients—a double-blind placebo-controlled study. Aliment Pharmacol Ther 2000;14:317–24. [DOI] [PubMed] [Google Scholar]
- 7.Froehlich F, Gonvers JJ, Wietlisbach V, et al. Helicobacter pylori eradication treatment does not benefit patients with nonulcer dyspepsia. Am J Gastroenterol 2001; 96:2329–36. [DOI] [PubMed] [Google Scholar]
- 8.Bruley Des Varannes S, Flejou JF, Colin R, et al. There are some benefits for eradicating Helicobacter pylori in patients with non-ulcer dyspepsia. Aliment Pharmacol Ther 2001;15:1177–85. [DOI] [PubMed] [Google Scholar]
- 9.Moayyedi P, Soo S, Deeks J, et al. Systematic review and economic evaluation of Helicobacter pylori eradication treatment for non-ulcer dyspepsia. Dyspepsia Review Group. BMJ 2000;321:659–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blum AL, Arnold R, Stolte M, et al. Short course acid suppressive treatment for patients with functional dyspepsia: results depend on Helicobacter pylori status. The Frosch Study Group. Gut 2000;47:473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siegrist J, Junge A, Fünfstück G. Lebensqualität unter antihypertensiver Therapie: Vergleich von Captopril und Metoprolol. Med Welt 1991;42:133–8. [Google Scholar]
- 12.Siegrist J, Middeke M, Osterkorn K. Lebensqualität hypertensiver Ärzte unter Hochdrucktherapie. Fortschr Med 1991;109:348–52. [PubMed] [Google Scholar]
- 13.Eypasch E, Wood-Dauphinée S, Williams JI, et al. Der gastrointestinale Lebensqualitätsindex (GLQI). Ein klinimetrischer Index zur Befindlichkeitsmessung in der gastroenterologischen Chirurgie. Chirurg 1993;64:264–74. [PubMed] [Google Scholar]
- 14.Delaney B, Moayyedi P, Deeks J, et al. The management of dyspepsia: a systematic review. Health Technol Assess 2000;4 (iii-v):1–189. [DOI] [PubMed] [Google Scholar]
- 15.Bayerdörffer E, Mannes GA, Sommer A, et al. High dose omeprazole treatment combined with amoxicillin eradicates Helicobacter pylori. Eur J Gastroenterol Hepatol 1992;4:697–702. [Google Scholar]
- 16.Labenz J, Gyenes E, Ruhl GH, et al. Omeprazole plus amoxicillin: efficacy of various treatment regimens to eradicate Helicobacter pylori. Am J Gastroenterol 1993;88:491–5. [PubMed] [Google Scholar]
- 17.Koelz HR, Beglinger C, Inauen W, et al. Double-blind comparison of three different amoxicillin plus omeprazole regimens for eradication of Helicobacter pylori in patients with duodenal ulcer (abstract). Gastroenterology 1995;108:A133. [Google Scholar]
- 18.Plein K, Madisch A, Stolte M, et al. Short-term changes in Helicobacter pylori gastritis and bulbitis during and after 2 weeks of treatment with omeprazole and amoxicillin in duodenal ulcer patients. Z Gastroenterol 2001;39:503–10. [DOI] [PubMed] [Google Scholar]
- 19.Solcia E, Fiocca R, Villani L, et al. Effects of permanent eradication or transient clearance of Helicobacter pylori on histology of gastric mucosa using omeprazole with or without antibiotics. Scand J Gastroenterol 1996;31:105–10. [DOI] [PubMed] [Google Scholar]
- 20.van der Hulst RW, van der Ende A, Dekker FW, et al. Effect of Helicobacter pylori eradication on gastritis in relation to cagA: a prospective 1-year follow-up study. Gastroenterology 1997;113:25–30. [DOI] [PubMed] [Google Scholar]
- 21.Labenz J, Stolte M, Peitz U, et al. Omeprazole/amoxicillin versus triple therapy for Helicobacter pylori in duodenal ulcer disease: two-year follow-up of a prospective randomized study. Z Gastroenterol 1995;33:590–3. [PubMed] [Google Scholar]
- 22.Dixon MF. Histological responses to Helicobacter pylori infection: gastritis, atrophy and preneoplasia. Baillieres Clin Gastroenterol 1995;9:467–86. [DOI] [PubMed] [Google Scholar]
- 23.Kikuchi T, Kato K, Ohara S, et al. The relationship between persistent secretion of RANTES and residual infiltration of eosinophils and memory T lymphocytes after Helicobacter pylori eradication. J Pathol 2000;192;243–50. [DOI] [PubMed] [Google Scholar]
- 24.McCarthy C, Patchett S, Collins RM, et al. Long-term prospective study of Helicobacter pylori in nonulcer dyspepsia. Dig Dis Sci 1995;40:114–19. [DOI] [PubMed] [Google Scholar]
- 25.Gilvarry J, Buckley MJM, Beattie S, et al. Eradication of Helicobacter pylori affects symptoms in non-ulcer dyspepsia. Scand J Gastroenterol 1997;32:535–40. [DOI] [PubMed] [Google Scholar]
- 26.Veldhuyzen van Zanten SJ, Cleary C, Talley NJ, et al. Drug treatment of functional dyspepsia: a systematic analysis of trial methodology with recommendations for design of future trials. Am J Gastroenterol 1996;91:660–73. [PubMed] [Google Scholar]
- 27.Mégraud F, O’Morain C, Malfertheiner P, et al. Guidelines for clinical trials in Helicobacter pylori infection. Working party of the European Helicobacter pylori Study Group. Gut 1997;41(suppl 2):S1–23. [PubMed] [Google Scholar]