Abstract
Background: Peginterferon α-2b plus ribavirin therapy in previously untreated patients with chronic hepatitis C yields the highest sustained virological response rates of any treatment strategy but is expensive.
Aims: To estimate the cost effectiveness of treatment with peginterferon α-2b plus ribavirin compared with interferon α-2b plus ribavirin for initial treatment of patients with chronic hepatitis C.
Methods: Individual patient level data from a randomised clinical trial with peginterferon plus ribavirin were applied to a previously published and validated Markov model to project lifelong clinical outcomes. Quality of life and economic estimates were based on German patient data. We used a societal perspective and applied a 3% annual discount rate.
Results: Compared with no antiviral therapy, peginterferon plus fixed or weight based dosing of ribavirin increased life expectancy by 4.2 and 4.7 years, respectively. Compared with standard interferon α-2b plus ribavirin, peginterferon plus fixed or weight based dosing of ribavirin increased life expectancy by 0.5 and by 1.0 years with incremental cost effectiveness ratios of €11 800 and €6600 per quality adjusted life year (QALY), respectively. Subgroup analyses by genotype, viral load, sex, and histology showed that peginterferon plus weight based ribavirin remained cost effective compared with other well accepted medical treatments.
Conclusions: Peginterferon α-2b plus ribavirin should reduce the incidence of liver complications, prolong life, improve quality of life, and be cost effective for the initial treatment of chronic hepatitis C.
Keywords: cost effectiveness analysis, hepatitis C, interferon α-2b, peginterferon α-2b, ribavirin, decision making
Worldwide, 170 million subjects have chronic hepatitis C virus infection with four million in the USA and five million in Western Europe.1–3 Because of its prevalence and its progression to cirrhosis (albeit slowly and not for all individuals), it is the most common reason for liver transplantation.1 Although interferon α-2b (Intron; Schering-Plough, Kenilworth, New Jersey, USA; ESSEX Pharma GmbH, Munich, Germany) and ribavirin (Rebetol; Schering-Plough; ESSEX Pharma GmbH) has been considered the standard of care,3–5 a recent multinational randomised controlled clinical trial6 showed that peginterferon α-2b (PegIntron; Schering-Plough; ESSEX Pharma GmbH) and ribavirin yielded a higher sustained virological response rate. In particular, on the basis of a secondary analysis showing that peginterferon plus ribavirin dosed at >10.6 mg/kg had the highest viral response rates, the European Medicine Evaluation Agency approved peginterferon at 1.5 μg/kg/week plus weight based dosing of ribavirin for the treatment of chronic hepatitis C.
However, peginterferon plus ribavirin is relatively expensive. In the past, demonstrated safety and higher efficacy would have been sufficient for the adoption of a new treatment. With rising medical costs and limited health care budgets, increasing attention is being focused on the economic impact of new drugs. Prior studies have shown that standard combination interferon α-2b plus ribavirin treatment of chronic hepatitis C is “cost effective” compared with other well accepted medical interventions.7–10 Peginterferon plus ribavirin however costs more than standard combination therapy, raising the question of whether its additional clinical benefit (higher response rates and convenient once a week dosing) supports this additional cost. The aim of this study was to determine the cost effectiveness of peginterferon plus ribavirin as initial treatment for patients with chronic hepatitis C.
PATIENTS AND METHODS
Individual patient level data for 1530 adult patients enrolled in an international randomised controlled clinical trial comparing peginterferon α-2b plus ribavirin to interferon α-2b plus ribavirin were used to determine virological treatment response rates, adverse events, actual dosages of drugs received, and actual duration of treatment. The details of this study have been published previously.6 Briefly, inclusion criteria required no prior treatment, RNA positivity for the hepatitis C virus, elevated transaminases, and a recent liver biopsy. Study population characteristics included mean age 44 years, 66% men, 32% genotype 2/3, 15% mild hepatitis, 78% moderate hepatitis or bridging fibrosis, and 7% cirrhosis. Patients were randomised to the following groups: (1) interferon α-2b (3 million units subcutaneously three times weekly) plus ribavirin (1000–1200 mg daily) for 48 weeks; (2) peginterferon α-2b (1.5 μg/kg/week for four weeks and then 0.5 μg/kg/week for the next 44 weeks subcutaneously) plus ribavirin (1000–1200 mg daily) for 48 weeks; and (3) peginterferon α-2b (1.5 μg/kg/week subcutaneously) plus ribavirin (800 mg daily). For the first two groups, ribavirin dosing was based on weight (1000 mg for weight <75 kg or 1200 mg otherwise) and for the third group dosing of ribavirin was fixed.
Patients in the 1.5 μg peginterferon plus ribavirin arm achieved a significantly higher sustained virological response (54%) compared with patients treated with standard combination therapy (47%) or with 0.5 μg peginterferon plus ribavirin (47%). For the subgroup of patients in the 1.5 μg peginterferon plus ribavirin arm who received >10.6 mg/kg of ribavirin daily (weight based dosing), sustained virological response was 61%. We included 48 weeks of peginterferon α-2b (1.5 μg/kg once weekly subcutaneously) plus weight based ribavirin in our primary analysis to be consistent with European drug approved labelling in which ribavirin dosing is weight based. We also projected outcomes for no antiviral therapy.
Decision-analytic model
To extrapolate the long term outcomes resulting from each treatment, we applied a previously published and externally validated Markov model11,12 to estimate the future clinical benefits and economic costs. In a Markov model, the natural history of a disease is represented by a set of predefined health states. Time was represented by annual cycles during which cohort members may or may not progress or die from liver disease or other causes.13,14 Health states were defined by clinical symptoms, liver histology, and virological condition. Histology was defined by the modified histology activity index of Knodell.15,16 In the absence of cirrhosis, a Knodell periportal inflammation score of 0–1 defined mild chronic hepatitis and a score of 3–10 defined moderate chronic hepatitis. Regardless of the Knodell inflammation score, a Knodell fibrosis score of 4 defined chronic hepatitis with cirrhosis. The likelihood of histological progression, clinical decompensation, the mode of decompensation, hepatocellular carcinoma, liver transplantation, and mortality were estimated from international studies of the natural history of chronic hepatitis C and have been published in detail elsewhere.8,11 A German epidemiological expert panel reviewed these data for the appropriateness of applying them to the German health care context. The annual likelihood of liver transplantation for decompensated cirrhosis was reduced to 2.2% (from 3.1%) to reflect the lower per capita liver transplantation rates in Germany compared with the USA.17,18 This figure agrees well with other European estimations.17,19,20 By tracking the survival status, quality of life related to the current health state, and annual health care costs, the computer simulations estimated the average life expectancy, quality adjusted life expectancy, and lifetime direct cost for identical cohorts of patients who received each treatment. Table 1 ▶ shows the transition probabilities, sustained virological response rates, quality of life weights, and costs in the model.
Table 1.
Model parameters with lower and upper limits: (a) transition probabilities, (b) treatment efficacy, (c) quality of life data, and (d) costs
| (a) Transition probabilities | Annual probability | ||||
| Initial state | State after transition | Base case | Lower limit | Upper limit | Ref |
| Mild chronic hepatitis C | Viral negative (spontaneous remission) | 0.002 | 0.000 | 0.005 | 54 |
| Moderate chronic hepatitis C | 0.041 | 0.022 | 0.060 | 55−57 | |
| Moderate chronic hepatitis C | Compensated cirrhosis | 0.073 | 0.051 | 0.095 | 55−57 |
| Hepatocellular carcinoma | 0.001 | 0.000 | 0.002 | 58 | |
| Compensated cirrhosis | Diuretic sensitive ascites | 0.025 | 0.018 | 0.032 | 58 |
| Variceal haemorrhage | 0.011 | 0.006 | 0.016 | 58 | |
| Hepatic encephalopathy | 0.004 | 0.001 | 0.007 | 58 | |
| Hepatocellular carcinoma | 0.015 | 0.010 | 0.020 | 58 | |
| Diuretic sensitive ascites | Diuretic refractory ascites | 0.067 | 0.040 | 0.094 | 58 59 |
| Death from liver disease | 0.110 | 0.077 | 0.143 | 59 | |
| Diuretic refractory ascites | Death from liver disease | 0.330 | 0.280 | 0.380 | 59 |
| Variceal haemorrhage (first year) | Death from liver disease | 0.400 | 0.334 | 0.466 | 60 |
| Variceal haemorrhage (subsequent years) | Death from liver disease | 0.130 | 0.085 | 0.175 | 60 |
| Hepatic encephalopathy (first year) | Death from liver disease | 0.680 | 0.659 | 0.701 | 61 |
| Hepatic encephalopathy (subsequent years) | Death from liver disease | 0.400 | 0.378 | 0.422 | 61 |
| Hepatocellular carcinoma | Death from liver disease | 0.860 | 0.837 | 0.883 | 11 |
| Liver transplantation (first year) | Death from liver disease | 0.210 | 0.193 | 0.227 | 62–64 |
| Liver transplantation (subsequent years) | Death from liver disease | 0.057 | 0.047 | 0.067 | 62–64 |
| Decompensated cirrhosis (ascites, variceal haemorrhage, hepatic encephalopathy) | Liver transplantation | 0.022 | 0.011 | 0.033 | 17 18 65 |
| SVR* | ||||
| (b) Treatment efficacy | Base case | Lower limit | Upper limit | Ref |
| Antiviral treatment | ||||
| Interferon 3×3 MU/week+ribavirin 1000–1200 mg/day | 47% | 42% | 51% | 6 |
| Peginterferon 0.5 μg/kg/week+ribavirin 1000–1200 mg/day | 47% | 43% | 52% | 6 |
| Peginterferon 1.5 μg/kg/week+ribavirin 800 mg/day | 54% | 49% | 58% | 6 |
| Peginterferon 1.5 μg/kg/week+ribavirin >10.6 mg/kg/day | 61% | 53% | 68% | 6 |
| Quality of life weight | Source | |||
| (c) Health related quality of life | Base case | Lower limit | Upper limit | |
| Health state | Source | |||
| Mild chronic hepatitis | 0.95 | 0.90 | 1.00 | † |
| Moderate chronic hepatitis | 0.92 | 0.89 | 0.95 | † |
| Compensated cirrhosis | 0.89 | 0.82 | 0.92 | † |
| Decompensated cirrhosis or hepatocellular carcinoma | 0.81 | 0.76 | 0.87 | † |
| Hepatocellular carcinoma | 0.81 | 0.76 | 0.87 | † |
| Liver transplantation | 0.86 | 0.73 | 0.99 | † |
| Death | 0.00 | 0.00 | 0.00 | † |
| Utility multiplier viral positive | 0.98 | 0.93 | 1.00 | † |
| Utility multiplier for interferon+ribavirin | 0.95 | 0.92 | 0.98 | † |
| Utility multiplier for peginterferon+ribavirin | 0.90 | 0.84 | 0.96 | † |
| Annual costs (€) | Source | |||
| (d) Costs | Base case | Lower limit | Upper limit | Source |
| *SVR, sustained virological response rates—that is, proportion of viral negative patients six months after end of treatment (adjusted for histological stage and viral negative rate at 24 weeks in the analysis). | ||||
| †GEHMO quality of life database. | ||||
| ‡GEHMO cost database. | ||||
| Health state | ||||
| Mild chronic hepatitis C | 127 | 63.5 | 254 | ‡ |
| Moderate chronic hepatitis C | 130 | 65 | 260 | ‡ |
| Compensated cirrhosis | 673 | 336.5 | 1346 | ‡ |
| Diuretic sensitive ascites | 1914 | 957 | 3828 | ‡ |
| Diuretic refractory ascites | 12 534 | 6257 | 25 068 | ‡ |
| Hepatic encephalopathy (first year) | 7738 | 3869 | 15 476 | ‡ |
| Hepatic encephalopathy (subsequent years) | 2793 | 1396.5 | 5586 | ‡ |
| Variceal haemorrhage (first year) | 12 314 | 6157 | 24 628 | ‡ |
| Variceal haemorrhage (subsequent years) | 3385 | 1692.5 | 6770 | ‡ |
| Hepatocellular carcinoma | 17 244 | 8622 | 34 488 | ‡ |
| Liver transplantation (first year) | 117 303 | 58 651.5 | 234 606 | ‡ |
| Liver transplantation (subsequent years) | 16 965 | 8482.5 | 33 930 | ‡ |
Health related quality of life
To reflect the morbidity associated with complications resulting from antiviral treatment and from hepatitis C, we also decremented life expectancy for quality of life on a scale from 0 (dead) to 1 (perfect health).21,22 No prior quality of life studies in patients have estimated the effect of the various stages of hepatitis C and treatment using patient preference measures such as visual analogue scales or time trade off,23–26 so we conducted a cross sectional interview based quality of life study in 348 consecutive German patients with chronic hepatitis C at a single centre.27 Health state specific quality of life weights (utilities) were determined using multivariate regression analysis. For the base case, quality of life was based on a transformed visual analogue scale,28,29 but we also examined EuroQoL27 and physician based estimates.8,11 Because ribavirin has been found to be teratogenic in animals, we assumed that ribavirin treated patients with an unplanned pregnancy would have an elective abortion, so we decremented their quality of life by one week.8,30,31 Table 1 ▶ shows the health state specific quality of life estimates for the base case analysis.
Cost data
Estimates of annual direct costs for each health state included the frequency and costs for inpatient and outpatient visits, diagnostic and laboratory testing, medications, and procedures. Itemised costs were based on detailed analyses of the actual variable costs and reimbursement costs in German chronic hepatitis C patients in the German health care system. For ambulatory care costs, reimbursement prices were adjusted using a weighted average for (a) East and West Germany and (b) social and private health insurance. For hospital services, average per diem prices for different types of wards were used. We based the frequency of clinic visits and laboratory testing during antiviral therapy (2, 4, 6, 8, 12, 18, 24, 32, and 48 weeks) on product labelling and expert panel consensus.32 All adverse events requiring a dosage change incurred an additional visit and blood tests. Drug discontinuation necessitated two additional visits and blood tests. Women under the age of 50 years had a qualitative pregnancy test prior to beginning ribavirin and every month thereafter.33 We assumed that ribavirin treated patients with chronic hepatitis C or their partners would use hormone contraception and condoms for six months after discontinuation of ribavirin at €25 per month, based on a patient survey. From trial data,34 we assumed that 1.2% of patients would become pregnant and would incur the costs of an abortion. Drug dosage was as received in the trial, except that we discontinued treatment in patients who were viral positive after 24 weeks of combination therapy because of the low likelihood of a viral negative response with an additional 24 weeks of treatment. As is recommended for pharmacoeconomic analysis,22 drug costs were based on average wholesale costs: €5.15 per ribavirin capsule; €11.01 per million units for interferon; and €1.96 per μg for peginterferon, adjusted for package size and weight of the trial patients. All non-drug costs were inflated to 2000 costs using the medical care component of the Consumer Price Index.35 All costs were converted to Euros using the fixed conversion rate—that is, €1 equals 1.95583 German Mark. Effects of health related quality of life, morbidity, mortality, and patient time on patient preferences were assumed to be incorporated in the quality adjusted life years. To avoid double counting, indirect costs were not included in the numerator of the cost effectiveness ratio.36
The cost effectiveness of each strategy was assessed by determining its incremental cost effectiveness ratio, defined as the incremental discounted cost divided by the incremental discounted quality adjusted life expectancy to yield the cost to increase life expectancy by one quality adjusted life year (€/QALY). This analysis adopted the societal perspective and discounted costs and clinical benefits at an annual rate of 3%.22,37 Most well accepted medical interventions have incremental cost effectiveness ratios falling below about €50 000 per QALY gained,11 so we consider any ratios below this threshold to be cost effective.
Statistical analyses
To assess the robustness of base case results, univariate sensitivity analyses were performed for model parameters using 95% confidence intervals (CIs) or ranges published in the literature. Costs were halved and doubled to obtain lower and upper limits. Antiviral treatment costs were inflated to consider additional costs for side effects and complications. The annual discount rate was varied from 0% to 5%. Multivariate sensitivity analyses were performed on the likelihood of histological progression and on treatment costs for advanced liver disease. A multivariate analysis of utilities was performed based on the limits of the 95% CIs for all utility estimates to bias against or in favour of antiviral treatment. Subgroup analyses examined the effects of age, sex, viral load, initial histology, and genotype. Decision analytic calculations were performed with DATA Professional (TreeAge Software Inc., Williamstown, Massachusetts, USA) and Decision Maker 7.0 (Pratt Medical Group, Boston, Massachusetts, USA). We used SAS 8.1 (SAS Institute Inc., Cary, North Carolina, USA) and Systat 10 (SPSS, Inc, Chicago, Illinois, USA) for statistical analyses.
Assumptions of the model
As in prior analyses,8,11 when data were controversial or incomplete, we attempted to bias model assumptions against antiviral treatment. Although many studies suggest histological improvement or reduced likelihood of hepatocellular carcinoma in relapse patients,38 we assumed no long term benefit from antiviral treatment relapse. We assumed that spontaneous or treatment induced loss of viraemia greatly reduces but does not eliminate the risk of developing progressive liver disease.39 Lastly, serial liver biopsies, which would have increased the cost and morbidity for being viral positive, were not considered.
Role of the funding source
This project was supported in part by a research grant from ESSEX Pharma GmbH (German subsidiary of Schering Plough Inc.) and Schering-Plough Corp. The authors had independence from the funding company in study design, analysis and interpretation of data, report writing, and publication, regardless of results.
RESULTS
Base case analysis
Incidence of liver complications, life expectancy, and quality of life
Because the response rates and model results for the “induction dosing” strategy peginterferon α-2b (1.5 μg/kg/week and then 0.5 μg/kg/week) plus ribavirin (results available from authors) were approximately the same as for interferon α-2b plus ribavirin, for simplicity our text will focus on the remaining strategies. The Markov model projected the sustained response rates into 20 year risks for liver related complications for each strategy (table 2 ▶). Note that the relatively high risks in table 2 ▶ reflect the large proportion of patients with histologically moderate hepatitis, bridging fibrosis, or compensated cirrhosis (84.9%) in the trial. For comparison, if all chronic hepatitis C patients had mild hepatitis, the Markov model projected a 27% 20 year risk of cirrhosis, and if 31% of patients with acute hepatitis C resolve their infection spontaneously,2 the model projected that 19% of patients with post-transfusion hepatitis C would develop cirrhosis after 20 years, which is consistent with published reports.40,41
Table 2.
Twenty year risks of incident liver complications, life expectancy, and quality adjusted life expectancy depending on antiviral treatment (rounded undiscounted values)
| No antiviral treatment | Interferon plus ribavirin | Peginterferon plus fixed ribavirin | Peginterferon plus weight based ribavirin | |
| 20 year risk (%) | ||||
| Compensated cirrhosis | 62 | 34 | 29 | 25 |
| Decompensated cirrhosis | 24 | 14 | 12 | 11 |
| Hepatocellular carcinoma | 9.7 | 5.8 | 5.3 | 4.7 |
| Liver transplantation* | 2.3 | 1.3 | 1.2 | 1.1 |
| Liver-related death | 24 | 14 | 13 | 11 |
| Life expectancy (years) | 25.8 | 29.4 | 30.0 | 30.5 |
| Quality adjusted life expectancy (QALY) | 22.8 | 26.6 | 27.1 | 27.7 |
*Only first transplantations included (no retransplantations).
The model translated higher treatment induced sustained response rates into reduced risk for future liver complications (table 2 ▶); antiviral treatment reduced the 20 year absolute risk of dying from liver complications by at least 10%. The model also translated these reduced risks into gains in life expectancy and quality adjusted life expectancy; antiviral therapy increased life expectancy by at least 3.6 years. Compared with standard combination therapy, peginterferon plus fixed ribavirin increased life expectancy by 0.5 years, and peginterferon plus weight based ribavirin increased life expectancy by 1.0 year.
Costs
To achieve these clinical benefits, peginterferon plus weight based ribavirin costs about €4700 more than standard combination therapy (table 3 ▶). Although a complete 48 week course of treatment would cost €21 601 for fixed and €23 716 for weight based ribavirin plus peginterferon, expected costs were 33% lower at €14 528 and €15 938, respectively, because of dose reductions or discontinuations for side effects, as occurred in the trial, or for the absence of a viral response after 24 weeks. Table 3 ▶ shows the lifetime cost of each strategy, including future costs due to hepatitis C complications. Compared with standard combination therapy, savings from reduced future liver complications with peginterferon plus fixed ribavirin therapy offset 47% of the higher initial treatment cost. Over a lifetime, peginterferon plus fixed ribavirin therapy would cost €1800 more than standard combination therapy and would increase life expectancy by 0.5 years. Similarly, 66% of the higher cost of peginterferon plus weight based ribavirin therapy was offset by reductions in future liver complications, so life expectancy was increased by 1.0 year at an additional lifetime cost of €1600.
Table 3.
Undiscounted cost of treatment in € (base case analysis). All treatments were for 48 weeks using the following stop criteria: stop antiviral therapy if viral positive after 24 weeks of treatment
| No antiviral treatment | Interferon plus ribavirin | Peginterferon plus fixed ribavirin | Peginterferon plus weight based ribavirin* | |
| Cost of antiviral drugs† | ||||
| Interferon α-2b | 3324 | — | — | |
| Peginterferon α-2b | — | 8029 | 8185 | |
| Ribavirin | 6508 | 5056 | 6295 | |
| Total cost of antiviral drugs | 9832 | 13 085 | 14 480 | |
| Initiation of treatment costs‡ | 473 | 473 | 473 | |
| Office visits+laboratory testing§ | 483 | 546 | 557 | |
| Reproductive services¶ | 411 | 424 | 428 | |
| Total treatment related cost | 11 199 | 14 528 | 15 938 | |
| Total lifetime cost including treatment of future complications (rounded values) | 25 500 | 25 900 | 27 600 | 27 400 |
*The amount of peginterferon and ribavirin used in the subgroup receiving >10.6 mg/kg ribavirin was increased to match the weight distribution in the entire peginterferon plus fixed ribavirin treatment arm (that is, peginterferon doses were identical and ribavirin capsule use in the subgroup were multiplied by 1.26).
†Based on the actual dosages administered in the trial (including dose reductions and discontinuations for side effects or for absence of viral response after 24 weeks).
‡Includes pre-therapeutic diagnostics (pregnancy test, quantitative HCV-RNA, thyroid stimulating hormone, thyroxine, liver biopsy), and partial inpatient cost for initiation of treatment.
§Includes office visits and laboratory tests for routine visits and adverse events as well as periodic thyroid stimulating hormone, thyroxine, and qualitative HCV-RNA.
¶Includes pregnancy tests, condoms, hormone contraception, and elective abortions.
Discounting and cost effectiveness
Discounting reflects the higher value of money spent now as opposed to in the future. Similarly, discounting also weights quality of life deficits experienced now (for example, antiviral therapy) more heavily than those experienced in the future (hepatitis C complications). Applying an annual discount rate of 3%, table 4 ▶ shows that peginterferon plus weight based ribavirin and peginterferon plus fixed ribavirin had incremental cost effectiveness ratios of €6600 and €11 800 per QALY gained, respectively, compared with interferon plus ribavirin. Moving from fixed to weight based dosing of ribavirin would cost €2100 per QALY gained. Because €2100 is lower than the €11 800 with fixed dose ribavirin, peginterferon plus weight based ribavirin is a more efficient use of resources.
Table 4.
Base case analysis: life expectancy, quality adjusted life expectancy, and direct lifetime costs discounted at 3% (rounded values).
| No antiviral treatment | Interferon plus ribavirin | Peginterferon plus fixed ribavirin | Peginterferon plus weight based ribavirin | |
| Costs (€) | 14 100 | 19 300 | 21 800 | 22 400 |
| Life expectancy (y) | 17.0 | 18.6 | 18.8 | 19.1 |
| Quality-adjusted life expectancy (QALY) | 15.1 | 16.8 | 17.0 | 17.3 |
Sensitivity analysis
We examined the effect of applying results observed for clinical subgroups from the trial and of varying each parameter used in the analysis or multiple parameters simultaneously over a wide range to determine their effect on cost effectiveness ratios. The tornado diagram in fig 1 ▶ presents the most important results. These sensitivity analyses showed that peginterferon plus weight based ribavirin remained the best treatment and was cost effective compared with standard combination therapy, even if the sustained response rate was only 50% or if the likelihood of liver disease progression was reduced such that the 20 year cirrhosis incidence was halved. Peginterferon plus fixed ribavirin remained cost effective except for the subgroups genotype 2/3 and high viral load. Peginterferon plus weight based ribavirin however remained cost effective, even for traditionally difficult to treat subgroups such as patients with high viral load. Although antiviral treatment of younger patients was more cost effective than treatment of older patients, peginterferon plus fixed ribavirin remained cost effective for patients up to age 60 years and peginterferon plus weight based ribavirin remained cost effective for patients up to age 69.
Figure 1.
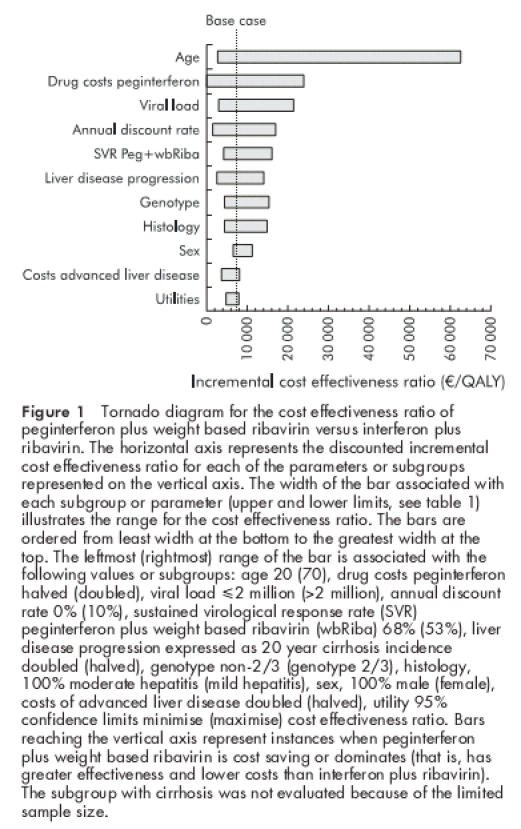
Tornado diagram for the cost effectiveness ratio of peginterferon plus weight based ribavirin versus interferon plus ribavirin. The horizontal axis represents the discounted incremental cost effectiveness ratio for each of the parameters or subgroups represented on the vertical axis. The width of the bar associated with each subgroup or parameter (upper and lower limits, see table 1 ▶) illustrates the range for the cost effectiveness ratio. The bars are ordered from least width at the bottom to the greatest width at the top. The leftmost (rightmost) range of the bar is associated with the following values or subgroups: age 20 (70), drug costs peginterferon halved (doubled), viral load ≤2 million (>2 million), annual discount rate 0% (10%), sustained virological response rate (SVR) peginterferon plus weight based ribavirin (wbRiba) 68% (53%), liver disease progression expressed as 20 year cirrhosis incidence doubled (halved), genotype non-2/3 (genotype 2/3), histology, 100% moderate hepatitis (mild hepatitis), sex, 100% male (female), costs of advanced liver disease doubled (halved), utility 95% confidence limits minimise (maximise) cost effectiveness ratio. Bars reaching the vertical axis represent instances when peginterferon plus weight based ribavirin is cost saving or dominates (that is, has greater effectiveness and lower costs than interferon plus ribavirin). The subgroup with cirrhosis was not evaluated because of the limited sample size.
Using an alternative quality of life metric (the EuroQoL estimated from the same German chronic hepatitis C patients survey), fixed and weight based ribavirin plus peginterferon had discounted incremental cost effectiveness ratios of €14 500 and €8000 per QALY gained compared with standard combination therapy. With previously published physician based quality of life estimates,8,11 these discounted incremental cost effectiveness ratios improved to €8400 for fixed and €4900 for weight based ribavirin plus peginterferon.
Finally, because response rates for weight based dosing for ribavirin were obtained from a subgroup, we analysed the following worst case scenario. If weight based dosing did not improve the sustained response rate in the 323 of 511 patients who received ≤10.6 mg/kg of ribavirin, the cost effectiveness ratio for peginterferon plus weight based ribavirin would be €16 900 per QALY gained compared with standard combination therapy.
DISCUSSION
Because of the high worldwide prevalence of hepatitis C and its slowly progressive natural history, numerous studies suggest that future population morbidity and mortality from hepatitis C may double over the next 10–20 years.42–45 On an individual level, although not all patients develop liver complications, numerous studies have documented quality of life decrements, and in particular for patients who develop decompensated cirrhosis or hepatocellular carcinoma the effects of the disease can be particularly life shortening in the absence of liver transplantation.41,46 Although long term data from randomised clinical trials on hard clinical endpoints such as survival or cirrhosis are lacking, mounting evidence suggests that viral eradication leads to improved histology, decreased risk of hepatocellular carcinoma, decreased liver disease progression, and perhaps improved survival.38,39,47 Thus the higher viral eradication rates of 54–61% seen with peginterferon and ribavirin should decrease future liver complications and extend average life expectancy by over 4–5 years, well beyond the benefit of most medical interventions.48
Accounting for dose reductions and discontinuations, peginterferon plus weight based ribavirin treatment costs on average approximately €15 900 or €4700 more than interferon and ribavirin. To put these treatment costs in perspective with the burden of the disease, 1997 US direct and indirect expenditures for hepatitis C were estimated to be $5.5 billion.49 In the absence of antiviral treatment, annual direct medical care costs for hepatitis C may double over the next 10–20 years.45 When considering lifetime costs, the higher sustained response rate with peginterferon and ribavirin should lead to cost savings from the prevention of future liver related complications and should offset the majority of the higher antiviral costs. Even in subgroup analyses, peginterferon plus weight based ribavirin remained preferred and cost effective. Relative to haemodialysis50 or to coronary artery bypass surgery51 with incremental cost effectiveness ratios of about €66 000 per QALY saved (conversion rate 1.1 €/US$), peginterferon plus weight based ribavirin is about 10 times more “cost effective”—that is, spending €66 000 on hepatitis C treatment would result in 10 healthy life years gained compared with one healthy life year gained with bypass surgery or haemodialysis.
This study has several limitations. Firstly, European labelling permits treatment with peginterferon and ribavirin for 24 weeks based on interferon and ribavirin trial results for patients with genotype 2/3. Because the peginterferon and ribavirin trial began prior to these latter results becoming known, it did not include a 24 week treatment arm. Thus we could not examine a 24 week treatment duration because relapse rates are unknown. Such an approach would reduce initial antiviral drug treatment costs and make antiviral treatment even more “cost effective” although the proportion of patients relapsing and their retreatment costs would reduce those savings. Future studies will need to determine the sustained response rates and cost effectiveness of 24 weeks of treatment. Finally, our results assume that the efficacy of peginterferon plus ribavirin observed in a subgroup of individuals who received >10.6 mg/kg ribavirin daily would be achievable for the entire study population. Although this assumption requires confirmation from ongoing prospective clinical trials, increased viral response rates observed in logistic regression analysis6 support the rationale behind weight based ribavirin dosing in European labelling.
Our economic analysis likely underestimates disease related costs for several reasons. Firstly, we used variable costs (the cost to treat one additional patient with a disease) and did not consider fixed costs (such as buildings, maintenance, and administrative personnel) or indirect or productivity costs. Secondly, we did not consider the cost of future liver biopsies and further therapy for non-responders. Thirdly, we did not consider the reduced incidence of hepatocellular carcinoma in non-responders or histological normalisation in responders. Our analysis applied average age, sex distribution, and histologies to avoid potential biases related to patient level variation in the different treatment groups of the trial and applied a consistent resource utilisation structure in the model and institutional assignment (where different institutions or countries may vary in their economic efficiencies and accounting practices).52,53
Because not all chronic hepatitis C patients will develop progressive liver disease, assessment of the eligibility and appropriateness of peginterferon and ribavirin treatment requires a careful discussion between patients and physicians regarding prognosis and willingness to consider antiviral treatment to prevent potential future liver complications. For chronic hepatitis C patients similar to those in the clinical trial, our analysis suggests that two patients will need to be treated to prevent one case of cirrhosis and four patients will need to be treated to prevent one case of decompensated cirrhosis or one death from liver disease. To achieve these benefits, patients must be willing to accept the risk of side effects and to be monitored for haemolysis and the risk of teratogenic effects. Nevertheless, these results suggest that peginterferon plus either fixed dose or weight based dosing of ribavirin should reduce the incidence of liver complications, prolong life, improve quality adjusted life expectancy, and be cost effective.
Acknowledgments
Further members of the following research groups participated in this study
Participating members of the German Hepatitis C Model (GEHMO) Group: W Greiner and J-M Graf von der Schulenburg (University of Hanover); M Bullinger (University of Hamburg, Berlin); P Aidelsburger, F Hessel, and F Buchner (University of Greifswald); M Corzillius (University of Kiel).
Participating members of the International Hepatitis Interventional Therapy (IHIT) Group: FH Anderson (Vancouver General Hospital, Vancouver, BC); S Arora (University of New Mexico, Albuquerque, NM); B Bacon (St Louis University School of Medicine, St Louis, MO); L Balart (Center for Digestive Diseases, New Orleans, LA); KG Benner (Oregon Health Sciences University, Portland, OR); M-A Bigard (Hopital de Brabois Adultes, Vandoeuvre Les Nancy, France); HC Bodenheimer (Mt Sinai Medical Center, New York, NY); M Bourliere (Hopital Saint Joseph, Marseille, France); C Brechot (Hôpital Necker, Paris, France); H Brunner (KH Lainz der Stadt Wien, Vienna, Austria); S Caldwell (University of Virginia, Charlottesville, VA); W Carey (Cleveland Clinic Foundation, Cleveland, OH); RL Carithers Jr (University of Washington, Seattle, WA); GL Davis (University of Florida, Gainesville, FL); J Dienstag (Massachusetts General Hospital, Boston, MA); J Donovan (University of Nebraska, Omaha, NE); R Esteban-Mur, M Buti (Hospital Valle d’Hebron, Barcelona, Spain); GT Everson (University of Colorado, Denver, CO); S Feinman (Mount Sinai Hospital, Toronto, Ontario); S Flamm (Northwestern Memorial Hospital, Chicago, IL); PR Galle (Klinikum der Johannes-Gutenberg-Universität, Mainz, Germany); R Gish (California Pacific Medical Center, San Francisco, CA); N Gitlin (Emory University, Atlanta, GA); T Goeser (Medizinische Einrichtungen der Universität Köln, Germany); S Gordon (William Beaumont Hospital, Royal Oak, MI); H Greten (Universitäts-Krankenhaus Eppendorf, Hamburg, Germany); S Hadzyiannis (Hippokration Hospital, Athens, Greece); I Hokeberg (Akademiska Hospital, Uppsala, Sweden); I Jacobson (Cornell University, New York, NY); P Kwo (Indiana University School of Medicine, Indianapolis, IN); DR LaBrecque (University of Iowa Hospital and Clinic, Iowa City, IA); WM Lee (University of Texas Southwestern Medical Center, Dallas, TX); S Lindgren (University Hopital MAS, Malmo, Sweden); KL Lindsay (University of Southern California, Los Angeles, CA); MP Manns (Medizinische Hochschule Hannover, Hannover, Germany); P Marcellin (Hôpital Beaujon, Clichy France); P Marotta (London Health Sciences Centre, University, London, Ontario); T McGarrity (Pennsylvania State University, Hershey, PA); JG McHutchison (Scripps Clinic, La Jolla, CA); R Moreno (Hospital de la Princesa, Madrid, Spain); TR Morgan (VA Medical Center, Long Beach, CA); R Perrillo (Ochsner Clinic, New Orleans, LA); M Poliquin (Centre Universitaire de l’Universite de Mtl, Montreal, Quebec); Thierry Poynard (Hôpital Pitié-Salpiêtriére, Paris, France); J Rakela (University of Pittsburgh, Pittsburgh, PA); R Reindollar (Charlotte Clinic for GI and Liver Diseases, Charlotte, NC); JL Rodriguez-Agullo (Hospital Clinico Universitario San Carlos, Madrid, Spain); R Rouzier-Panis (Centre CAP, Nime, France); V Rustgi (Metropolitan Research, Fairfax, VA); JM Sanchez-Tapias (Hospital Clinic I Provincial, Barcelona, Spain); ER Schiff (University of Miami School of Medicine, Miami, FL); D Schuppan (Klinikum der Universität Erlangen-Nürnberg, Erlangen, Germany); M Sherman (The Toronto Hospital, Toronto, Ontario); ML Shiffman (Medical College of Virginia, Richmond, VA ); M Silva (Fundacion Favaloro, Buenos Aires, Argentina); C Smith (Minnesota Clinical Research Center, St Paul, MN); H Tanno (Clinica del Higado, Rosario, Argentina); C Trepo (Hospital Hotel Dieu, Lyon, France); W Vogel (Leopold-Franzens-University Innsbruck, Innsbruck, Austria); T Wright (University of California San Francisco, San Francisco, CA); S Zeuzem (Klinikum der JW Goethe Universität, Frankfurt, Germany).
Panel of expert German hepatologists participating in the survey of German practice: Ch Antoni (University Hospital, Mannheim); Th Berg (University Hospital Charite, Berlin); N Demmel (Teaching Hospital of the University of Munich, Städtisches Krankenhaus München-Neuperlach); D Hüppe (Herne); B Kallinowski (University of Heidelberg); C Kölbel (Teaching Hospital of the University of Mainz, Trier); S Mauss (Düsseldorf); B Moeller, (Berlin); M K Müller (Marienhospital, Osnabrück); C Niederau (Academic Teaching Hospital of the University of Essen, St Josefs Hospital, Oberhausen); G Teuber (University Hospital, Frankfurt); L Theilmann, (Städtisches Klinikum, Pforzheim); E Will (Mannheim); R Zachoval (Groβhadern Medical Center, University of Munich); A Zipf, (Mannheim).
The authors also acknowledge the advice of members of the Advisory Board of the German Hepatitis C Model (GEHMO) Group: R Holle (Institute of Health Economics and Health Care Management, GSF-National Research Centre for Environment and Health, Neuherberg, Germany); N Mühlberger, (University of Munich, Germany); B Gibis (Institute of Health Economics, Edmonton, Alberta, Canada).
Grant support: This study was supported in part by a research grant from ESSEX Pharma GmbH (German subsidiary of Schering Plough Inc.), Munich, Germany, and Schering-Plough Corporation, Kenilworth, NJ, USA.
Abbreviations
QALY, quality adjusted life year
SVR, sustained virological response rate
REFERENCES
- 1.Anonymous. National Institutes of Health Consensus Development Conference Panel statement: management of hepatitis C. Hepatology 1997;26(suppl 1):2–10S. [Google Scholar]
- 2.Alter MJ, Kruskon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States 1988 through 1994. N Engl J Med 1999;341:556–62. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous. EASL International Consensus Conference on hepatitis C. Paris, 26–27 February 1999. Consensus statement. J Hepatol 1999;31(suppl 1):3–8. [PubMed] [Google Scholar]
- 4.Poynard T, Marcellin P, Lee SS, et al. Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT). Lancet 1998;352:1426–32. [DOI] [PubMed] [Google Scholar]
- 5.McHutchison JG, Gordon SC, Schiff ER, et al. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med 1998;339:1485–92. [DOI] [PubMed] [Google Scholar]
- 6.Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001;358:958–65. [DOI] [PubMed] [Google Scholar]
- 7.Younossi ZM, Singer ME, McHutchison JG, et al. Cost effectiveness of interferon alpha2b combined with ribavirin for the treatment of chronic hepatitis C. Hepatology 1999;30:1318–24. [DOI] [PubMed] [Google Scholar]
- 8.Wong JB, Poynard T, Ling MH, et al. Cost-effectiveness of 24 or 48 weeks of interferon alpha-2b alone or with ribavirin as initial treatment of chronic hepatitis C. International Hepatitis Interventional Therapy Group. Am J Gastroenterol 2000;95:1524–30. [DOI] [PubMed] [Google Scholar]
- 9.Buti M, Casado MA, Fosbrook L, et al. Cost-effectiveness of combination therapy for naive patients with chronic hepatitis C. J Hepatol 2000;33:651–8. [DOI] [PubMed] [Google Scholar]
- 10.Sheperd J, Waugh N, Hewitson P. Combination therapy (interferon alfa and ribavirin) in the treatment of chronic hepatitis C: a rapid and systematic review. Health Technol Assess 2000;41–67. [PubMed] [Google Scholar]
- 11.Bennett WG, Inoue Y, Beck JR, et al. Estimates of the cost-effectiveness of a single course of interferon-alpha 2b in patients with histologically mild chronic hepatitis C. Ann Intern Med 1997;127:855–65. [DOI] [PubMed] [Google Scholar]
- 12.Wong JB, Bennett WG, Koff RS, et al. Pre-treatment evaluation of chronic hepatitis C: Risks, benefits and costs. JAMA 1998;280:2088–93. [DOI] [PubMed] [Google Scholar]
- 13.Beck JR, Pauker SG. The Markov process in medical prognosis. Med Decis Making 1983;3:419–58. [DOI] [PubMed] [Google Scholar]
- 14.Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making 1993;13:322–38. [DOI] [PubMed] [Google Scholar]
- 15.Knodell RG, Ishak KG, Black WC, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology 1981;1:431–5. [DOI] [PubMed] [Google Scholar]
- 16.Desmet VJ, Gerber M, Hoofnagle JH, et al. Classification of chronic hepatitis: diagnosis, grading, and staging. Hepatology 1994;19:1513–20. [PubMed] [Google Scholar]
- 17.Fischer-Fröhlich CL. Die Situation der Organtransplantation in der Bundesrepublik Deutschland und im europäischen Ausland aus medizinischer Sicht-eine Bestandsaufnahme. In: Landeszentrale für politische Bildung Baden-Württemberg, ed. Organentnahme und Transplantation-im Spannungsfeld zwischen Ethik und Gesetz. Stuttgart: Bad Urach, 1997:7–28.
- 18.Deutsche Stiftung Organtransplantation (DSO). http://www.dsa.de. Zahlen und Daten: Gespendete und transplantierte Organe, 1992–2000.
- 19.Persijn G. Cohen B (eds). Eurotransplant Foundation: Annual Report 1994. Eurotransplant Foundation (1994), 1994.
- 20.Anonymous. European Liver Transplant Registry: ELTR Up-dating 30/06/1994. European Liver Transplant Registry. Hopital Paul Brousse Villejuif, 1994.
- 21.Drummond MF, O’Brien B, Stoddart GL, et al. Methods for the Economic Evaluation of Health Care Programmes. 2nd edn. New York: Oxford University Press, 1997.
- 22.Gold MR, Siegel JE, Russell LB, et al. Cost-effectiveness in Health and Medicine. New York: Oxford University Press, 1996.
- 23.Carithers RL Jr, Sugano D, Bayliss M. Health assessment for chronic HCV infection: results of quality of life. Dig Dis Sci 1996;41(suppl 12):75–80S. [DOI] [PubMed] [Google Scholar]
- 24.Foster GR, Goldin RD, Thomas HC. Chronic hepatitis C virus infection causes a significant reduction in quality of life in the absence of cirrhosis. Hepatology 1998;27:209–12. [DOI] [PubMed] [Google Scholar]
- 25.Bonkovsky HL, Woolley JM. Reduction of health-related quality of life in chronic hepatitis C and improvement with interferon therapy. The Consensus Interferon Study Group. Hepatology 1999;29:264–70. [DOI] [PubMed] [Google Scholar]
- 26.Ware JE Jr, Bayliss MS, Mannocchia M, et al. Health-related quality of life in chronic hepatitis C: impact of disease and treatment response. The Interventional Therapy Group. Hepatology 1999;30:550–5. [DOI] [PubMed] [Google Scholar]
- 27.Siebert U, Ravens-Sieberer U, Greiner W, et al. Patient-based health-related quality of life in different health stages of chronic hepatitis C. Hepatology 2001;44:222A. [Google Scholar]
- 28.Torrance GW, Feeny DH, Furlong WJ, et al. Multiattribute utility function for a comprehensive health status classification system. Med Care 1996;34:701–22. [DOI] [PubMed] [Google Scholar]
- 29.Torrance GW, Feeny D, Furlong W. Visual analog scales: do they have a role in the measurement of preferences for health states? Med Decis Making 2001;21:329–34. [DOI] [PubMed] [Google Scholar]
- 30.Heckerling PS, Verp MS. Amniocentesis or chorionic villus sampling for prenatal genetic testing: a decision analysis. J Clin Epidemiol 1991;44:657–70. [DOI] [PubMed] [Google Scholar]
- 31.Kuppermann M, Shiboski S, Feeny D, et al. Can preference scores for discrete states be used to derive preference scores for an entire path of events? An application to prenatal diagnosis. Med Decis Making 1997;17:42–55. [DOI] [PubMed] [Google Scholar]
- 32.Wasserman Y. Physicians’ Fee Reference 1995, 12th edn. West Allis: Medical Publishers Ltd, 1995.
- 33.Anonymous. St Anthony’s DRG Guidebook 1995. Alexandria, VA: St Anthony Publishing, 1996.
- 34.Maddrey WC. Safety of combination interferon alfa-2b/ribavirin therapy in chronic hepatitis C-relapsed and treatment-naive patients. Semin Liver Dis 1999;19(suppl 1):67–75. [PubMed] [Google Scholar]
- 35.StatistischesBundesamt. Preisindex für Arzt-, Krankenhaus-und sonstige Dienstleistungen für die Gesundheitspflege, 2000.
- 36.Luce BR, Manning WG, Siegel JE, et al. Estimating costs in cost-effectiveness analysis. In: Gold MR, Siegel JE, Russell LB, eds. Cost-effectiveness in Health and Medicine. New York: Oxford University Press, 1996:176–213.
- 37.Weinstein MC Siegel JE, Gold MR, et al. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA 1996;276:1253–8. [PubMed] [Google Scholar]
- 38.Bonis PA, Ioannidis JP, Cappelleri JC, et al. Correlation of biochemical response to interferon alfa with histological improvement in hepatitis C: a meta-analysis of diagnostic test characteristics. Hepatology 1997;26:1035–44. [DOI] [PubMed] [Google Scholar]
- 39.Reichard O, Glaumann H, Fryden A, et al. Two-year biochemical, virological, and histological follow-up in patients with chronic hepatitis C responding in a sustained fashion to interferon alfa-2b treatment. Hepatology 1995;21:918–22. [PubMed] [Google Scholar]
- 40.Liang TJ, Rehermann B, Seeff LB, et al. Pathogenesis, natural history, treatment, and prevention of hepatitis C. Ann Intern Med 2000;132:296–305. [DOI] [PubMed] [Google Scholar]
- 41.Alter HJ, Seeff LB. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin Liver Dis 2000;20:17–35. [DOI] [PubMed] [Google Scholar]
- 42.Davis GL, Albright J, Cook S, et al. Projecting the future healthcare burden from hepatitis C in the United States. Hepatology 1998;28:390A. [Google Scholar]
- 43.Deuffic S, Buffat L, Poynard T, et al. Modeling the hepatitis C virus epidemic in France. Hepatology 1999;29:1596–601. [DOI] [PubMed] [Google Scholar]
- 44.Armstrong GL, Alter MJ, McQuillan GM, et al. The past incidence of hepatitis C virus infection: implications for the future burden of chronic liver disease in the United States. Hepatology 2000;31:777–82. [DOI] [PubMed] [Google Scholar]
- 45.Wong JB, McQuillan GM, McHutchison JG, et al. Estimating future hepatitis C morbidity, mortality, and costs in the United States. Am J Public Health 2000;90:1562–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seeff LB, Hollinger FB, Alter HJ, et al. Long-term mortality and morbidity of transfusion-associated non-A, non-B, and type C hepatitis: A National Heart, Lung, and Blood Institute collaborative study. Hepatology 2001;33:455–63. [DOI] [PubMed] [Google Scholar]
- 47.Nishiguchi S, Kuroki T, Nakatani S, et al. Randomised trial of effects of interferon-alpha on incidence of hepatocellular carcinoma in chronic active hepatitis C with cirrhosis. Lancet 1995;346:1051–5. [DOI] [PubMed] [Google Scholar]
- 48.Wright JC, Weinstein MC. Gains in life expectancy from medical interventions—Standardizing data on outcomes. N Engl J Med 1998;339:380–6. [DOI] [PubMed] [Google Scholar]
- 49.Leigh JP, Bowlus CL, Leistikow BN, et al. Costs of hepatitis C. Arch Intern Med 2001;161:2231–7. [DOI] [PubMed] [Google Scholar]
- 50.Weinstein MC. High-priced technology can be good value for money. Ann Intern Med 1999;130:857–8. [DOI] [PubMed] [Google Scholar]
- 51.Wong JB, Sonnenberg FA, Salem DN, et al. Myocardial revascularization for chronic stable angina. Analysis of the role of percutaneous transluminal coronary angioplasty based on data available in 1989. Ann Intern Med 1990;113:852–71. [DOI] [PubMed] [Google Scholar]
- 52.Drummond MF, Davies L. Economic analysis alongside clinical trials. Revisiting the methodological issues. Int J Technol Assess Health Care 1991;7:561–73. [DOI] [PubMed] [Google Scholar]
- 53.Ellwein LB, Drummond MF. Economic analysis alongside clinical trials. Bias in the assessment of economic outcomes. Int J Technol Assess Health Care 1996;12:691–7. [DOI] [PubMed] [Google Scholar]
- 54.Yousuf M, Nakano Y, Sodeyama T, et al. Persistence of viremia in patients with type-C chronic hepatitis during long-term follow-up. Scand J Gastroenterol 1992;27:812–16. [DOI] [PubMed] [Google Scholar]
- 55.Tremolada F, Casarin C, Alberti A, et al. Long-term follow-up of non-A, non-B (type C) post-transfusion hepatitis. J Hepatol 1992;16:273–81. [DOI] [PubMed] [Google Scholar]
- 56.Mattsson L. Outcome of acute symptomatic non-A, non-B hepatitis: a 13-year follow-up study of hepatitis C virus markers. Liver 1993;13:274–8. [DOI] [PubMed] [Google Scholar]
- 57.Takahashi M, Yamada G, Miyamoto R, et al. Natural course of chronic hepatitis C. Am J Gastroenterol 1993;88:240–3. [PubMed] [Google Scholar]
- 58.Fattovich G, Giustina G, Degos F, et al. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology 1997;112:463–72. [DOI] [PubMed] [Google Scholar]
- 59.Salerno F, Borroni G, Moser P, et al. Survival and prognostic factors of cirrhotic patients with ascites: a study of 134 outpatients. Am J Gastroenterol 1993;88:514–19. [PubMed] [Google Scholar]
- 60.Anonymous. Sclerotherapy for male alcoholic cirrhotic patients who have bled from esophageal varices: results of a randomized, multicenter clinical trial. The Veterans Affairs Cooperative Variceal Sclerotherapy Group. Hepatology 1994;20:618–25. [PubMed] [Google Scholar]
- 61.Christensen E, Krintel JJ, Hansen SM, et al. Prognosis after the first episode of gastrointestinal bleeding or coma in cirrhosis. Survival and prognostic factors. Scand J Gastroenterol 1989;24:999–1006. [DOI] [PubMed] [Google Scholar]
- 62.Ascher N, Lake J, Emond J, et al. Liver transplantation for hepatitis C virus-related cirrhosis. Hepatology 1994;20(suppl 1):24–7S. [DOI] [PubMed] [Google Scholar]
- 63.Detre K, Belle S, Lombarddero M. Liver transplantation for chronic viral hepatitis. Viral Hepatitis Rev 1996;2:219–28. [Google Scholar]
- 64.Kilpe V, Krakauer H, Wren R. An analysis of liver transplant experience from 37 transplant centers as reported to Medicare. Transplantation 1993;56:554–61. [DOI] [PubMed] [Google Scholar]
- 65.Berg J, Bechstein WO, Mueller A, et al. Lebertransplantation. Internist (Berl) 1998;39:1237–45. [DOI] [PubMed] [Google Scholar]


