Abstract
Background and aim: Clinical data on spontaneous hepatitis B e antigen (HBeAg) seroconversion and acute exacerbation of chronic hepatitis B (CHB) virus infection from large population studies are lacking. In the present study we examined the clinical features and significance of HBeAg seroconversion and acute exacerbation in 3063 Chinese CHB patients.
Methods: Clinical assessment, liver biochemistry, hepatitis B virus (HBV) serology and HBV DNA, time of HBeAg seroconversion, and acute exacerbation were monitored.
Results: Median age at HBeAg seroconversion was 34.5 years. The cumulative HBeAg seroconversion rate significantly increased with alanine aminotransferase (ALT) levels on presentation (p<0.0001). For patients with ALT levels more than twice the upper limit of normal (ULN) on presentation, the HBeAg seroconversion rate at the fifth year of follow up was 72.4%. After HBeAg seroconversion, 65.2% (73/110) of patients had undetectable HBV DNA levels by the Digene Hybrid Capture assay. Of these, 78.1% still had HBV DNA levels detectable by the Amplicor HBV Monitor Test. We found that 37.5% antibody to HBeAg (anti-HBe) positive patients had undetectable HBV DNA levels by the Digene Hybrid Capture assay before acute exacerbation. Acute exacerbations of longer duration, with higher peak ALT, bilirubin, and α fetoprotein levels were associated with an increased HBeAg seroconversion rate (p<0.0001–0.045). Acute exacerbation with peak ALT levels more than five times the ULN carried a 46.4% chance of HBeAg seroconversion within three months. HBeAg seroreversion and mortality occurred in 2.7% and 0.7% of acute exacerbations, respectively.
Conclusion: In the present study we have provided information on HBeAg seroconversion and acute exacerbation, which are important in decision making for CHB treatment and in designing clinical trials.
Keywords: alanine aminotransferase, HBeAg seroconversion, hepatitis B, antiviral therapy
Chronic hepatitis B (CHB) infection is a disease of global importance, with 400 million people being affected.1 Up to 40% of patients may develop cirrhosis related complications and hepatocellular carcinoma (HCC).2
Hepatitis B e antigen (HBeAg) seroconversion, commonly used as a short term end point of treatment,3 can also occur spontaneously.4,5 Many studies have reported the rate of HBeAg seroconversion in patients receiving treatment. The features of spontaneous HBeAg seroconversion have also been reported in various studies.6,7 As these data are important for the understanding of the natural course of CHB infection, and for the design of therapeutic trials, more information from large population studies would be beneficial.
Acute exacerbation occurs frequently in the natural course of CHB infection.8,9 Patients may have repeated episodes of acute exacerbation before eventually achieving HBeAg seroconversion.10 It is well documented that there is a positive correlation between alanine aminotransferase (ALT) levels during acute exacerbation and the chance of HBeAg seroconversion.11 However, there are few studies on other predictive factors in acute exacerbation favouring spontaneous HBeAg seroconversion.12,13 Acute exacerbation also occurs in patients who are antibody to HBeAg (anti-HBe) positive with some patients having HBeAg seroreversion afterwards.14 Acute exacerbation can infrequently lead to hepatic decompensation and mortality.15
In this study, we present epidemiological data from a large population of Chinese CHB patients with respect to HBeAg seroconversion and acute hepatitis B virus (HBV) exacerbation.
PATIENTS AND METHODS
From 1976 to 2000, 3063 patients attending the Hepatitis Clinic, Queen Mary Hospital, University of Hong Kong, Hong Kong, were recruited into the present study. Patients were all hepatitis B surface antigen (HBsAg) positive for at least six months. Patients were excluded from the study if they had any of the following conditions: (1) cirrhosis related complications or HCC on presentation, (2) treatment for CHB at any time, (3) concomitant hepatitis C or D virus infection, (4) evidence of autoimmune hepatitis, Wilson’s disease, or primary biliary cirrhosis, (5) history of heavy alcoholic intake, (6) evidence of fatty liver, and (7) intake of any hepatotoxic drugs. Patients had complete history enquiry and physical examination during the first clinic visit. Blood was taken for liver biochemistry, α fetoprotein (AFP), and HBV serological markers, including HBsAg, HBeAg, and anti-HBe by microparticle enzyme immunoassay (MEIA; Abbott Laboratories, Chicago, Illinois, USA) during the first visit and every subsequent visit which was scheduled at intervals of 3–6 months, or more frequently whenever indicated.
HBeAg seroconversion was defined as loss of HBeAg with development of anti-HBe on at least two consecutive follow ups. In patients who had HBeAg seroconversion, HBeAg seroreversion was defined as subsequent loss of anti-HBe and regaining of HBeAg on at least two consecutive follow ups.
Acute HBV exacerbation was defined as an increase in ALT to more than 1.5 times the upper limit of normal (ULN) after excluding other common causes of ALT elevation, including other viral hepatitis, drug induced hepatitis, alcoholic hepatitis, and steatohepatitis. Time, duration, peak ALT level, peak AFP, peak bilirubin level, and HBeAg/anti-HBe status were all monitored during HBV exacerbation. HBeAg seroconversion was regarded as related to acute exacerbation if HBeAg seroconversion occurred within three months of acute exacerbation.
HBV DNA levels before and after HBeAg seroconversion were measured in 110 patients by Digene Hybrid Capture assay (Digene Corporation, Gaithersburg, Maryland, USA) (lower limit of detection 140 000 copies/ml). For those with undetectable HBV DNA levels by the above assay, HBV DNA levels were determined again by a more sensitive quantitative polymerase chain reaction (PCR) assay (Cobas Amplitor HBV Monitor Test; Roche Diagnostics, Branchburg, New Jersey, USA) (lower limit of detection 200 copies/ml).
Statistical analysis
All statistical analyses were performed using the Statistical Program for Social Sciences (SPSS 10.0 for Windows, SPSS Inc., Chicago, Illinois, USA). Data were analysed by Mann-Whitney test for continuous ordinal data. Differences in paired parameters were tested by the Wilcoxon signed ranks test. The Kaplan-Meier method was applied for calculation of the cumulative rate of HBeAg seroconversion and the cumulative risk of acute HBV exacerbation using the log rank test. A p value of less than 0.05 was considered statistically significant.
RESULTS
Demographic data on presentation for the 3063 patients are listed in table 1 ▶.
Table 1.
Demographic data for the study population on presentation
| No of patients | 3063 |
| Age at presentation (y) | 38 (1–85) |
| Sex (M:F) | 1985: 1078 |
| HBeAg:anti-HBe | 1215: 1826* |
| Albumin (g/l) | 45 (18–58) |
| ALT (U/l) | 42 (4–6820) |
| Bilirubin (μmol/l) | 11 (1–553) |
| AFP (ng/ml) | 4 (1–4991) |
| Duration of follow up (months) | 29 (0.6–291) |
Values are median (range) or number.
*22 patients were negative for both HBeAg and anti-HBe at presentation.
HBeAg, hepatitis B e antigen; anti-HBe, antibody to HBeAg; ALT, alanine aminotransferase; AFP, α fetoprotein.
Of the 1215 patients who were HBeAg positive on presentation, 493 (40.6%) underwent HBeAg seroconversion during the follow up period. Median age at HBeAg seroconversion was 34.5 years (range 3.6–77.4). There was no difference in the cumulative HBeAg seroconversion rate between male and female patients (p=0.53).
The cumulative HBeAg seroconversion rate was studied by stratifying patients according to ALT levels on presentation: group 1 patients had ALT levels ≤0.5×ULN; group 2 patients had ALT levels >0.5–1×ULN; group 3 patients had ALT levels >1–2×ULN; group 4 patients had ALT levels >2–5×ULN; and group 5 patients had ALT levels >5×ULN. Higher ALT levels on presentation were associated with a higher chance of HBeAg seroconversion (overall p<0.0001) (fig 1 ▶). Cumulative HBeAg seroconversion rates according to the different ALT levels on presentation are listed in table 2 ▶.
Figure 1.
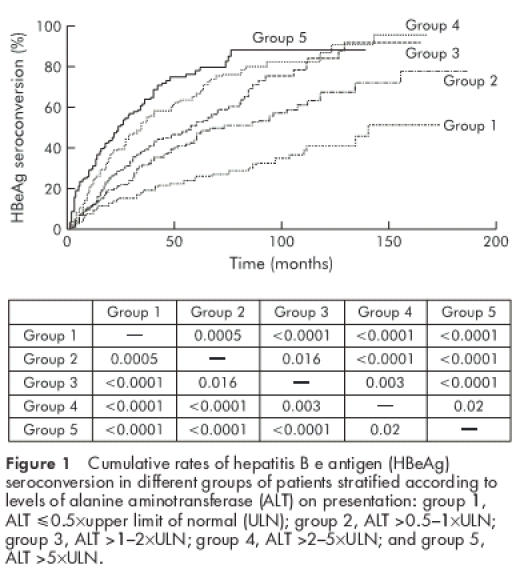
Cumulative rates of hepatitis B e antigen (HBeAg) seroconversion in different groups of patients stratified according to levels of alanine aminotransferase (ALT) on presentation: group 1, ALT ≤0.5×upper limit of normal (ULN); group 2, ALT >0.5–1×ULN; group 3, ALT >1–2×ULN; group 4, ALT >2–5×ULN; and group 5, ALT >5×ULN.
Table 2.
Cumulative hepatitis B e antigen (HBeAg) seroconversion rates in patients with different alanine aminotransferase levels on presentation
| Cumulative HBeAg seroconversion rate (%) | |||||
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| All patients (n=1215) | 18.0 | 31.0 | 41.3 | 47.6 | 53.5 |
| Stratified by ALT levels on presentation | |||||
| ALT <0.5×ULN (n=151) | 9.4 | 16.0 | 19.9 | 23.0 | 26.5 |
| ALT >0.5–1×ULN (n=294) | 11.7 | 22.6 | 32.3 | 39.5 | 47.9 |
| ALT >1–2×ULN (n=284) | 12.8 | 28.9 | 40.1 | 46.2 | 53.0 |
| ALT >2×ULN (n=486) | 28.2 | 44.9 | 58.4 | 66.6 | 72.4 |
| ALT >2–5×ULN (n=243) | 24.9 | 39.5 | 53.5 | 59.8 | 67.5 |
| ALT >5×ULN (n=243) | 31.5 | 50.6 | 63.7 | 74.7 | 78.1 |
ALT, alanine aminotransferase; ULN, upper limit of normal.
In 110 patients with paired sera before and after HBeAg seroconversion, median HBV DNA level was significantly lower after HBeAg seroconversion than before HBeAg seroconversion (<0.142 (range <0.142–309.5×106) v 4.01 (range <0.142–803.9×106) copies/ml; p<0.0001). Seventy three patients (65.2%) had undetectable HBV DNA by the Digene Hybrid Capture assay after HBeAg seroconversion. However, when sera of these 73 patients were retested using the Cobas Amplicor HBV Monitor Test, only 16 (21.9%) had undetectable HBV DNA. The median HBV DNA level of the remaining 57 patients was 3150 copies/ml (range 200–327 000 000). HBV DNA levels were in the range 200–103, >103–104, >104–105, and >105 in 11 (15.1%), 17 (23.3%), 25 (34.2%), and 4 (5.5%) patients, respectively.
A total of 1670 patients (54.5%) had ALT levels less than 1.5×ULN throughout the whole follow up period—that is, more than half of the Chinese CHB patients had near normal ALT levels over a prolonged period of time. The remaining 1393 patients (45.5%) (765 HBeAg positive, 628 anti-HBe positive) had at least one episode of acute exacerbation (929 on presentation and 464 on subsequent follow up). Only 204 patients (14.6%) experienced hepatitis symptoms. Excluding the 929 patients with acute exacerbation on presentation, the cumulative risk of acute exacerbation during follow up is illustrated in fig 2 ▶.
Figure 2.
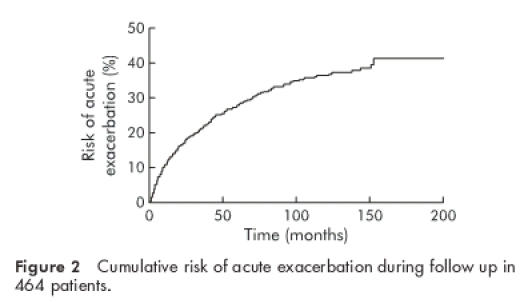
Cumulative risk of acute exacerbation during follow up in 464 patients.
Of the 765 HBeAg positive patients with acute exacerbation, 297 patients (38.8%) had HBeAg seroconversion within three months of acute exacerbation. The differences in the factors of acute exacerbation between patients with and without HBeAg seroconversion are listed in table 3 ▶. (For patients who presented with acute exacerbation, the earliest parameters were taken as peak values if subsequent values were not increasing.) HBeAg seroreversion occurred in 17 of 628 anti-HBe positive patients (2.7%) after acute exacerbation. There were no differences in median peak ALT levels, peak bilirubin levels, peak AFP levels, and duration of acute exacerbation between anti-HBe positive patients with and without HBeAg seroreversion. Ten patients (0.7%) died from acute exacerbation due to hepatic decompensation.
Table 3.
Parameters of acute exacerbation in patients with and without hepatitis B e antigen (HBeAg) seroconversion
| Acute exacerbation with HBeAg seroconversion (n=297) | Acute exacerbation without HBeAg seroconversion (n=468) | p Value | |
| Peak ALT (U/l) | 279 (75–3990) | 191 (75–3955) | <0.0001 |
| Peak bilirubin (μmol/l) | 17 (4–390) | 15 (2–868) | 0.045 |
| Peak AFP (ng/ml) | 10 (1–3958) | 7 (1–2015) | 0.001 |
| Duration of acute exacerbation (weeks) | 24 (1–236) | 17 (1–436) | 0.018 |
All values are median (range).
ALT, alanine aminotransferase; AFP, α fetoprotein.
The chance of HBeAg seroconversion within three months of acute exacerbation in patients with peak ALT levels 1.5–2×ULN, >2–5×ULN, and > 5×ULN were 27.2%, 35.6%, and 46.4%, respectively. The chance of HBeAg seroconversion within three months of acute exacerbation in patients with peak AFP levels of ≤20, >20–100, and >100 ng/ml were 35.2%, 43.6%, and 50.5%, respectively. Of 336 patients with peak ALT levels >5×ULN and 91 patients with peak AFP levels >100 ng/ml, 70 patients had both of the above parameters. Forty of these 70 patients (57.1%) had HBeAg seroconversion within three months of acute exacerbation.
Compared with anti-HBe positive patients, HBeAg positive patients had significantly higher median peak ALT levels (148 (range 75–8000) v 218 (75–3990) U/l, respectively; p<0.0001), higher median peak AFP levels (5 (range 1–2867) v 8 (1–3958) U/l, respectively; p<0.0001), and longer median duration of acute exacerbation (17 (range 3–416) v 21 (2–436) weeks, respectively; p=0.011).
HBV DNA levels within three months prior to acute exacerbation were measured in 46 HBeAg positive patients (14 with HBeAg seroconversion and 32 without HBeAg seroconversion after acute exacerbation) and 32 anti-HBe positive patients. In HBeAg positive patients, there was a trend for patients with HBeAg seroconversion to have lower HBV DNA levels before acute exacerbation compared with patients who did not achieve HBeAg seroconversion (29.0 (range 0.26–817.7×106) v 241.8 (range 0.27–1700×106) copies/ml; p=0.073). The median HBV DNA level was significantly higher in HBeAg positive patients compared with that of anti-HBe positive patients (182.2 (range 0.26–1700) v 0.31 (<0.142–407.0)×106 copies/ml, respectively; p<0.0001). All 46 HBeAg positive patients had detectable HBV DNA levels above 0.142×106 copies/ml (lower limit of detection of Digene Hybrid Capture assay) whereas 12 anti-HBe positive patients (37.5%) had HBV DNA levels below 0.142×106 copies/ml within three months prior to acute exacerbation. Of these 12 patients, 11 (91.7%) had detectable HBV DNA, ranging from 537 to 45 400 copies/ml, and one patient (8.3%) had undetectable HBV DNA by the Amplicor HBV Monitor Test.
DISCUSSION
This large population study has provided essential information on the probability of HBeAg seroconversion when assessing patients on presentation. The overall HBeAg seroconversion rate was 18% and 53.5% for the first and fifth years, respectively (table 2 ▶). In general, the higher the ALT level on presentation, the higher the chance of HBeAg seroconversion during subsequent follow up. Table 2 ▶ provides a guide for the design of therapeutic trials whereby one can balance the chance of spontaneous HBeAg seroconversion and the chance of HBeAg seroconversion induced by treatment.
Even when mild elevation of ALT levels to above 1.5×ULN was taken as acute exacerbation, 54% of patients still did not have any exacerbation throughout their follow up period. Therefore, unlike in Caucasian CHB patients, Chinese CHB patients often had normal or near normal ALT levels. Various factors during acute exacerbation were identified as predisposing patients to HBeAg seroconversion. Patients with peak ALT levels > 5×ULN and peak AFP levels >100 ng/ml had a 57.1% chance of HBeAg seroconversion within three months of occurrence of acute exacerbation. These two parameters may reflect, respectively, the degree of immune mediated attack on infected hepatocytes and hepatocyte regeneration afterwards. Additional information that has not been well studied in the past was duration of acute exacerbation. We found that patients with a longer duration of acute exacerbation had a higher chance of HBeAg seroconversion subsequently (table 3 ▶).
Mortality due to acute exacerbation was uncommon, occurring in only 0.7% of patients. These data do not suggest that treatment with lamivudine or interferon-alpha for patients with acute HBV exacerbation is indicated routinely. In fact, the high rate of spontaneous HBeAg seroconversion should be borne in mind when one is considering initiation of treatment for patients with very high ALT levels. Evans et al have commented from their study of 454 Asian-Americans that “high rate of spontaneous seroconversion should be weighted in decisions to treat HBV carriers with interferon-alpha”.6 For patients with ALT levels >2×ULN, the cumulative spontaneous HBeAg seroconversion rate was 72.4% by the end of five years (table 2 ▶). This is similar to the HBeAg seroconversion rate for patients on five years of continuous lamivudine.16 It is unfortunate that the lamivudine study did not have a five year placebo arm, but with the data from the present study it is difficult to conclude that lamivudine therapy is of benefit, at least as far as long term cumulative HBeAg seroconversion is concerned. Lamivudine is however useful in decreasing necroinflammation and improving fibrosis.17–19 In addition, this study also showed that HBeAg seroreversion occurred in only 2.7% of anti-HBe positive patients undergoing acute exacerbation. This suggests that spontaneous HBeAg seroconversion was very stable in Chinese CHB patients.
Although all HBeAg positive patients with available serum tested for HBV DNA with acute exacerbation had HBV DNA levels of more than 105 copies/ml within three months of acute exacerbation, 37.5% anti-HBe positive patients had HBV DNA levels lower than 105 copies/ml within three months of acute exacerbation (91.7% still had detectable HBV DNA level by the Amplicor PCR assay). Acute exacerbation could therefore occur in anti-HBe positive patients with a very low HBV DNA titre. This also suggests that Chinese anti-HBe positive patients with low HBV DNA levels are not necessarily in the quiescent phase of HBV infection. The study of Hsu et al confirms that a proportion of anti-HBe positive patients continue to have disease progression. Hepatitic flares associated with low HBV DNA levels in anti-HBe positive patients would lead to continuing liver damage, resulting in the development of cirrhosis and cirrhosis related complications in these patients.7,20 That these exacerbations are equally likely to be due to wild-type virus and precore mutants has been reported by us and other groups previously.21–23 Anti-HBe positive patients should be monitored as carefully as HBeAg positive patients for the development of cirrhosis related complications and HCC. In addition, the majority of patients after HBeAg seroconversion still had detectable HBV DNA levels measured by the PCR assay. HBV DNA was detectable by the Cobas Amplicor HBV Monitor Test in 78.1% of patients with HBV DNA undetectable by the Digene Hybrid Capture assay. This raises the issue of whether the end point of CHB treatment should be HBeAg seroconversion. A more prolonged and maximal suppression of HBV DNA, even after HBeAg seroconversion, to maintain improvement in necroinflammation and fibrosis would appear to be a more logical approach.
In conclusion, the present large population study has provided essential epidemiological and clinical data on HBeAg seroconversion and acute HBV exacerbation in chronic hepatitis B infection. This information may be important in decision making for the treatment of HBV and in designing clinical trials.
Abbreviations
CHB, chronic hepatitis B
HBeAg, hepatitis B e antigen
anti-HBe, antibody to HBeAg
HBsAg, hepatitis B surface antigen
HCC, hepatocellular carcinoma
ALT, alanine aminotransferase
HBV, hepatitis B virus
AFP, α fetoprotein
ULN, upper limit of normal
PCR, polymerase chain reaction
REFERENCES
- 1.Lee WM. Hepatitis B virus infection. N Eng J Med 1997;337:1733–45. [DOI] [PubMed] [Google Scholar]
- 2.Maynard JE, Kare MA, Alter MJ, et al. Control of hepatitis B by immunization: global perspective. In: Zukerman AJ, ed. Viral hepatitis and liver disease. New York: Alan R Liss, 1988:967–9.
- 3.Yuen MF, Lai CL. Treatment for chronic hepatitis B. Lancet Infect Dis 2001;1:232–41. [DOI] [PubMed] [Google Scholar]
- 4.Realdi G, Alberti A, Fugge M, et al. Seroconversion from hepatitis B e antigen to anti-HBe in chronic hepatitis B virus infection. Gastroenterology 1980;79:195–9. [PubMed] [Google Scholar]
- 5.Hoofnagle JH, Dusheiko GM, Seeff LB, et al. Seroconversion from hepatitis B e antigen to antibody in chronic type B hepatitis. Ann Intern Med 1982;94:744–8. [DOI] [PubMed] [Google Scholar]
- 6.Evans AA, Fine M, London WT. Spontaneous seroconversion in hepatitis B e antigen-positive chronic hepatitis B: implications for interferon therapy. J Infect Dis 1997;176:845–50. [DOI] [PubMed] [Google Scholar]
- 7.Hsu YS, Chien RN, Yeh CT, et al. Long-term outcome after spontaneous HBeAg seroconversion in patients with chronic hepatitis B. Hepatology 2002;35:1522–7. [DOI] [PubMed] [Google Scholar]
- 8.Lok AS, Lai CL. Acute exacerbations in Chinese patients with chronic hepatitis B virus (HBV) infection. Incidence, predisposing factors and etiology. J Hepatol 1990;10:29–34. [DOI] [PubMed] [Google Scholar]
- 9.Davis GL, Hoofnagle JH, Waggoner JG. Spontaneous reactivation of chronic hepatitis B virus infection. Gastroenterology 1984;86:230–5. [PubMed] [Google Scholar]
- 10.Liaw YF, Yang SS, Chew TJ, et al. Acute exacerbation in hepatitis B e antigen positive chronic type B hepatitis: a clinicopathological study. J Hepatol 1985;1:227–33. [DOI] [PubMed] [Google Scholar]
- 11.Sznchez-Tapias JM, Costa J, Mas A, et al. Analysis of factors predicting early seroconversion to anti-HBe in HBeAg-positive chronic hepatitis B. J Hepatol 1988;6:15–22. [DOI] [PubMed] [Google Scholar]
- 12.Lok ASF, Lai CL, Wu PC, et al. Spontaneous hepatitis B e antigen to antibody seroconversion and reversion in Chinese patients with chronic hepatitis B virus infection. Gastroenterology 1987;92:1839–43. [DOI] [PubMed] [Google Scholar]
- 13.Liaw YF, Chu CM, Su IJ, et al. Clinical and histological events preceding hepatitis B e antigen seroconversion in chronic type B hepatitis. Gastroenterology 1983;84:216–19. [PubMed] [Google Scholar]
- 14.Lai MY, Chen DS, Lee SC, et al. Reactivation of hepatitis B virus in anti-HBe-positive chronic active type B hepatitis: molecular and immunohistochemical studies. Hepatogastroenterology 1988;35:17–21. [PubMed] [Google Scholar]
- 15.Sheen IS, Liaw YF, Tai DI, et al. Hepatic decompensation associated with hepatitis B e antigen clearance in chronic type B hepatitis. Gastroenterology 1985;89:732–5. [DOI] [PubMed] [Google Scholar]
- 16.Guan R, Lai CL, Liaw YF, et al. Efficacy and safety of 5 years lamivudine treatment of Chinese patients with chronic hepatitis B. J Gastro Hepatol 2001:16(suppl):60A. [DOI] [PubMed] [Google Scholar]
- 17.Lai CL, Chien RN, Leung NWY, et al. A one-year trial of lamivudine for chronic hepatitis B. N Engl J Med 1998;339:61–8. [DOI] [PubMed] [Google Scholar]
- 18.Dienstag JL, Schiff ER, Wright TL, et al. Lamivudine as initial treatment for chronic hepatitis B in the United States. N Engl J Med 1999;341:1256–63. [DOI] [PubMed] [Google Scholar]
- 19.Schiff ER, Heathcote J, Dienstag JL, et al. Improvement in liver histology and cirrhosis with extended lamivudine therapy. Hepatology 2000;32:296A. [Google Scholar]
- 20.Alter HJ. HBe conversion: a near-religious experience for some but not all. Hepatology 2002;36:1–2. [Google Scholar]
- 21.Yuen MF, Sablon E, Yuan HJ, et al. The relationship between the development of precore and core promoter mutations and HBeAg seroconversion in chronic hepatitis B. J Infect Dis 2002;186:1335–8. [DOI] [PubMed] [Google Scholar]
- 22.Chan HLY, Leung NWY, Hussain M, et al. Hepatitis B e antigen-negative chronic hepatitis B in Hong Kong. Hepatology 2000;31:763–8. [DOI] [PubMed] [Google Scholar]
- 23.Ballard AL, Boxall EH. Epidemiology of precore mutants of hepatitis B in the United Kingdom. J Med Virol 2000;62:463–70. [DOI] [PubMed] [Google Scholar]


