Abstract
Background: Coeliac disease patients show a number of gastrointestinal motor abnormalities, including a decrease in lower oesophageal sphincter pressure. The prevalence of endoscopic oesophagitis in these subjects however is unknown.
Aim: To evaluate whether untreated adult coeliac patients had an increased prevalence of reflux oesophagitis and, if so, to assess whether a gluten free diet exerted any beneficial effect on gastro-oesophageal reflux disease (GORD) symptoms.
Patients and methods: We retrospectively studied 205 coeliac patients (females/males 153/52, median age 32 years) who underwent endoscopy for duodenal biopsy and 400 non-coeliac subjects (females/males 244/156, median age 37 years) referred for endoscopy for upper gastrointestinal symptoms. Each patient was given a questionnaire for evaluation of GORD symptoms prior to and 4–12 months after endoscopy. Coeliac patients were given a gluten free diet. Oesophagitis patients of both groups, following an eight week course of omeprazole, were re-evaluated for GORD symptoms at four month intervals up to one year. Significance of differences was assessed by Fisher’s exact test.
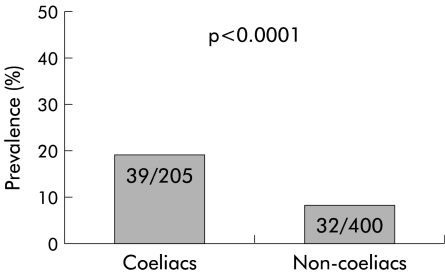
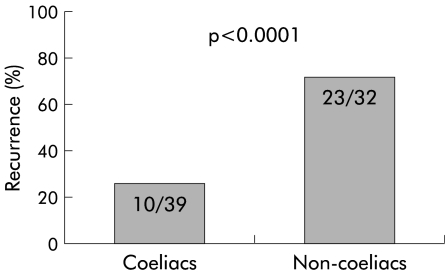
Results: Oesophagitis was present in 39/205 (19%, 95% confidence interval (CI) 13.8–25.0%) coeliac patients and in 32/400 (8%, 95% CI 5.5–11.1%) dyspeptic subjects. At the one year follow up, GORD symptoms relapsed in 10/39 (25.6%, 95% CI 13–42.1%) coeliacs with oesophagitis and in 23/32 (71.8%, 95% CI 53.2–86.2%) non-coeliac subjects with oesophagitis.
Conclusion: Coeliac patients have a high prevalence of reflux oesophagitis. That a gluten free diet significantly decreased the relapse rate of GORD symptoms suggests that coeliac disease may represent a risk factor for development of reflux oesophagitis.
Keywords: coeliac disease, reflux oesophagitis, gluten free diet
Coeliac disease is characterised by damage to the mucosa of the small intestine secondary to ingestion of gluten in sensitive individuals and subsequent malabsorption.1 The severity of coeliac disease depends, at least in part, on the degree and extent of intestinal lesions, and the clinical presentation of the disease, ranging from overt malabsorption to few or no symptoms when only malabsorption of selective nutrients is present.2 Coeliac disease may also present with dyspeptic symptoms which might be related to a number of gastrointestinal motor abnormalities observed in coeliac patients.3,4 In particular, untreated coeliac patients show delayed gastric empting and low values for lower oesophageal sphincter (LOS) pressure.5,6 Whether coeliac disease favours the development of reflux oesophagitis is not known.
To address this issue, we retrospectively studied the prevalence of endoscopic oesophagitis in untreated adult coeliac patients and in a population of control subjects referred to our endoscopy unit because of upper gastrointestinal symptoms. We also evaluated the effect of a gluten free diet on the relapse rate of gastro-oesophageal reflux disease (GORD) related symptoms.
MATERIALS AND METHODS
Patients
We retrospectively studied 205 coeliac subjects (females/males 153/52, median age 32 years, range 18–66 years) who, from 1996 to 2001, underwent upper endoscopy because of clinical and serological suspicion of coeliac disease. The diagnostic workup included a thorough medical history, routine laboratory measurements, and plasma testing for antibodies against gliadin (AGA, IgA and IgG) and endomysium (EMA). Coeliac disease diagnosis was based on positivity for EMA and on histology showing total or subtotal villus atrophy, crypt hyperplasia, and lymphoplasmacellular infiltration.7 Table 1 ▶ summarises the main clinical features of coeliac patients. Four hundred subjects (females/males 244/156, median age 37 years, range 20–68 years) who consecutively underwent upper endoscopy for upper gastrointestinal symptoms in the same unit, over the same time period, served as a control group. None of these patients showed clinical, serological, or endoscopic features suggestive of coeliac disease. Exclusion criteria were: age <18 or >70 years, severe chronic liver disease, coronary heart disease, alcohol abuse, drug addiction, and use of corticosteroids, non-steroidal anti-inflammatory drugs, antisecretory drugs, drugs affecting gastrointestinal motility, or antibiotics in the previous four weeks. Table 2 ▶ summarises upper gastrointestinal symptoms in the two groups.
Table 1.
Clinical features of the 205 coeliac patients
| Manifestations of coeliac disease | No (%) |
| Sideropenic anaemia | 99 (48) |
| Bloating | 87 (42) |
| Weakness | 76 (37) |
| Sideropenia without anaemia | 68 (33) |
| Chronic diarrhoea | 65 (31) |
| Weight loss | 55 (27) |
| Abdominal distention | 39 (19) |
| Autoimmune thyroid disease | 27 (13) |
| Hypertransaminasaemia | 15 (7) |
| Dermatitis herpetiformis | 5 (2) |
| Depression | 5 (2) |
| Osteoarthropathy | 5 (2) |
| Epilepsy | 2 (1) |
| Recurrent oral aphthous ulcers | 2 (1) |
| Vitiligo | 1 (0.5) |
| Alopecia | 1 (0.5) |
| Psoriasis | 1 (0.5) |
| IgA mesangial nephropathy | 1 (0.5) |
Table 2.
Upper gastrointestinal symptoms in patients with and without coeliac disease
| Coeliac patients (n=205) | Non-coeliac patients (n=400) | |
| Epigastric pain | 67 (32.7%) | 192 (48%) |
| Postprandial fullness | 85 (41.5%) | 153 (38.2%) |
| Nausea | 25 (12.2%) | 34 (8.5%) |
| Belching | 26 (12.7%) | 45 (11.2%) |
| Dysphagia | 4 (1.9%) | 12 (3%) |
| Heartburn | 77 (37.5%) | 121 (30.2%) |
| Regurgitation | 38 (18.5%) | 54 (13.5%) |
Evaluation of GORD related symptoms
In our gastroenterology unit, each patient is routinely administered a questionnaire evaluating a number of gastrointestinal symptoms, including GORD symptoms, before undergoing endoscopy and 4, 8, and 12 months after endoscopy. Heartburn and regurgitation are defined as typical symptoms whereas retrosternal pain, dysphagia, and belching are defined as atypical symptoms of GORD. Each complaint is given a score from 0 (absent) to 1 (mild), 2 (moderate), or 3 (severe). The symptom frequency is graded as 1 for ⩽2 days/week, 2 for 3–5 days/week, and 3 for >5 days/week.
Endoscopy
Oesophagitis was defined as one (or more) mucosal break on the top of the folds of the distal oesophagus, identified during partial air inflation. Severity of oesophagitis was assessed according to the Los Angeles classification system.8 Each patient was also evaluated for Helicobacter pylori infection by rapid urease test and histology by a modified Giemsa staining. Diagnosis of H pylori infection was based on positivity on both tests. Hiatal hernia was recognised by dislocation of the normally located squamocolumnar mucosal junction at least 2 cm above the diaphragm during quiet respiration without additional air inflation and by localising the gastric folds above the diaphragmatic hiatus.9
24 hour oesophageal pH monitoring
In 15 coeliacs with oesophagitis who agreed to undergo the procedure, a 24 hour pH recording was performed to detect acid pathological reflux. Patients had not been taking proton pump inhibitors, H2 receptor blocking agents, or prokinetic drugs for at least eight weeks prior to the test. pH monitoring started at 9:00 am after an overnight fast. We used an antimony electrode (Flexilog-TM; Oakfield Instruments Ltd, Eynsham, UK) inserted transnasally with the tip of the probe positioned 5 cm above the manometrically determined LOS. The electrode was connected to a Flexilog 2020 pH-meter (Oakfield Instruments Ltd). Before and after each recording, standard solutions (pH 1.07 and 7.01) were used for calibration. Patients were fed as usual throughout the 24 hour study period. Tracings were stored on a personal computer and analysed by Flexisoft II version 1.4 software (Oakfield Instruments Ltd). Acid reflux was defined as a drop in intraoesophageal pH below 4.10 We analysed the following parameters: percentage of total reflux time as the main parameter, percentage of supine and upright reflux time, number of reflux episodes longer than five minutes, and the longest reflux period.11
Statistical analysis
Significance of differences was assessed by Fisher’s exact test and ANOVA or Student’s t test for unpaired observations, as appropriate. A p value of less than 0.05 was considered to be statistically significant.
RESULTS
Endoscopy
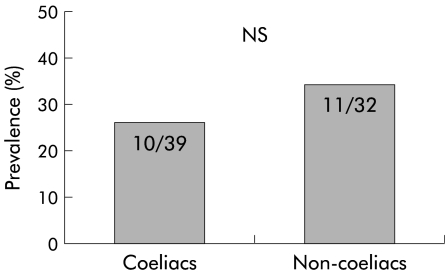
Oesophagitis was found in 39/205 (19%, 95% confidence interval (CI) 13.8–25.0%) coeliac subjects and in 32/400 (8%, 95% CI 5.5–11.1%) dyspeptic patients (p<0.0001) (fig 1 ▶). Hiatal hernia was endoscopically detected in 43/205 (20.9%) coeliacs and in 110/400 (27.5%) non-coeliacs (NS); 10/39 (25.6%) coeliacs with oesophagitis and 11/32 (34.3%) non- coeliac dyspeptics with oesophagitis had a hiatal hernia (NS) (fig 2 ▶). The main features of the patients with oesophagitis, both coeliacs and non-coeliacs, are summarised in table 3 ▶. The prevalence of H pylori infection was comparable in coeliac and non-coeliac patients (75/205=36.6% and 161/400=40.2%, respectively). Moreover, 15/39 (38.5%) coeliac patients with oesophagitis and 13/32 (40.6%) non-coeliac oesophagitis patients were infected with H pylori, as determined by positivity on both histology and the rapid urease test.
Figure 1.
Prevalence of oesophagitis in coeliac patients and in control non-coeliac patients.
Figure 2.
Prevalence of hiatal hernia in coeliac patients and in control non-coeliac patients.
Table 3.
Main features of patients with oesophagitis in the coeliac and non-coeliac groups
| Coeliac patients (n=39) | Non-coeliac patients (n=32) | |
| Age | 38 (6.7) | 45 (7.3) |
| Sex (M/F) | 22/17 | 20/12 |
| Hiatal hernia | 10 | 11 |
| Oesophagitis grade A-B | 39 | 32 |
| H pylori infection | 15 | 13 |
24 hour oesophageal pH monitoring
We performed dynamic pH recording in 15 of 39 coeliac patients with oesophagitis who agreed to undergo this procedure, before treating them with omeprazole. Data analysis showed the presence of GORD (percentage of total time below pH 4 9.1 (2.3)%) in 14/15 patients (table 4 ▶). In particular, supine reflux time was 2.2 (0.7)% and upright reflux time was 12.1 (3.5)%. Also, stationary manometry performed prior to dynamic pH recording to position the tip of the electrode showed that coeliac patients with oesophagitis had LOS pressure values lower than those observed in our control population consisting of 10 healthy volunteers (18.1 (5.2) v 21.5 (6.1) mm Hg, respectively), even though this did not reach statistical significance (table 4 ▶).
Table 4.
pH metric and manometric parameters in 14 coeliac patients with oesophagitis
| Acid reflux time (%) | 9.1 (2.3) |
| No of acid reflux episodes >5 min | 6.2 (2.8) |
| Longest reflux episode (min) | 18.0 (3.7) |
| LOS pressure (mm Hg) | 18.1 (5.2) |
Values are mean (SD).
LOS, lower oesophageal sphincter.
GORD related symptoms
The prevalence of typical GORD symptoms (that is, heartburn and/or regurgitation) was comparable in coeliac patients and in control dyspeptic non-coeliac patients (table 2 ▶). Patients with oesophagitis, both coeliacs and non-coeliacs, were treated with omeprazole 20 mg twice daily for eight weeks. Moreover, coeliac oesophagitis patients were given a gluten free diet. The GORD related symptom score significantly improved following omeprazole therapy, irrespective of the presence/absence of coeliac disease (data not shown). Twelve months after endoscopy (that is, 10 months after stopping omeprazole therapy), recurrence of GORD symptoms was significantly lower in coeliac than in non-coeliac subjects with oesophagitis (25.6%, 95% CI 13–42.1% v 71.8%, 95% CI 53.2–86.2%; p<0.0001) (fig 3 ▶). The majority (8/10) of coeliac patients with oesophagitis who experienced a recurrence of their GORD related symptoms where those with a hiatal hernia.
Figure 3.
Recurrence of gastro-oesophageal reflux disease related symptoms at one year in coeliac oesophagitis patients on a gluten free diet and in non-coeliac oesophagitis patients.
DISCUSSION
Untreated coeliac disease has been shown to be associated with a number of motor abnormalities of the upper gastrointestinal tract.3 In particular, oesophageal motor abnormalities and manometrical abnormalities were observed in 50% and 30% of 30 untreated coeliac patients, respectively.12 Moreover, untreated coeliac subjects show a significant decrease in LOS pressure.6 However, whether adult coeliac patients are more susceptible to reflux oesophagitis is still unknown. To address this issue, we retrospectively studied 205 adult coeliac disease patients and 400 non-coeliac adult subjects with upper gastrointestinal symptoms as a control group. We found a twofold increase in the prevalence of endoscopic oesophagitis in adult patients who had been diagnosed with coeliac disease compared with control non-coeliac subjects. The increased prevalence of oesophagitis was associated with an increased prevalence of GORD related symptoms even though this did not reach statistical significance. The prevalence of GORD related symptoms in our control population of dyspeptic subjects (that is, 30.2%) was similar to that reported in the literature. In fact, Locke et al and Valle et al described a prevalence of heartburn ranging from approximately 18% to 45%, depending on whether symptoms were experienced at least weekly or only occasionally, respectively.13,14 Moreover, the prevalence of oesophagitis found in our control group (that is, 8%) is comparable with that described in an Italian multicentre survey of a large patient population undergoing routine endoscopy (that is, 8.6%).15
Interestingly, the prevalence of hiatal hernia was comparable in the two groups of patients, thus making it unlikely that the increased prevalence of oesophagitis in coeliacs might be due to alteration of the physical barrier to acidic reflux from the stomach. We also studied the 24 hour pH metry profile in 15 coeliac patients with oesophagitis without hiatal hernia and found pathological reflux in 14/15 subjects.
Based on these results, the question arose as to whether the increased prevalence of oesophagitis in coeliac patients might be related to the underlying disease. To this end we retrospectively evaluated the relapse rate of GORD related symptoms in oesophagitis patients both with and without coeliac disease, after they had been treated with omeprazole 20 mg twice daily for eight weeks. Oesophagitis patients with coeliac disease were also given a gluten free diet. Approximately 70% of oesophagitis patients without coeliac disease compared with 25% of oesophagitis patients with coeliac disease showed recurrence of GORD related symptoms (p<0.0001). The decreased recurrence of GORD related symptoms in oesophagitis patients with coeliac disease on a gluten free diet compared with oesophagitis non-coeliac patients suggests that coeliac disease may play a role in the pathogenesis of GORD.
The results of our study are apparently in contrast with those of Oderda et al who, in a paediatric population, found a decreased prevalence of oesophagitis in coeliac children compared with control non-coeliac subjects.16 The apparent discrepancy with our study might be due, at least in part, to the fact that they included all coeliac children, irrespective of whether they were on a gluten containing diet or a gluten free diet. In fact, coeliac children on a gluten free diet had a very low prevalence of mucosal damage and the prevalence of peptic oesophagitis was significantly higher in coeliac children on a gluten containing diet compared with coeliac children on a gluten free diet (15% v 4%, respectively). This supports our findings, including the beneficial effect exerted by a gluten free diet on GORD symptoms in coeliac subjects with oesophagitis.
The mechanism whereby coeliac disease may predispose to the development of oesophagitis is only hypothetical. An increase in plasma levels of enteroglucagon and neurotensin, which are known to decrease LOS pressure and delay gastric emptying, has been demonstrated in coeliac patients.17,18 One may therefore hypothesise that untreated coeliac disease patients are more susceptible to the development of oesophagitis due to a derangement of the gastrointestinal hormone profile19 which may eventually lead to a decrease in LOS pressure together with delayed gastric emptying. That LOS pressure values in coeliac patients with oesophagitis were lower than in healthy control subjects is in partial support of our hypothesis.
Other factors might be involved in the increased prevalence of oesophagitis in coeliac patients. Recently, reflux oesophagitis has been shown to be associated with a Th1-type proinflammatory response as opposed to a Th2 predominant response observed in Barrett’s oesophagus.20 Similarly, small bowel damage in the course of coeliac disease is believed to be caused by a Th1/Th0-type proinflammatory response developed by CD4+ gluten sensitive T cells.21 This suggests that specific gastrointestinal mucosa immune responses to a noxious agent may influence disease development and progression and may explain the increased prevalence of oesophagitis in coeliac patients.
In conclusion, coeliac patients have a high prevalence of endoscopic oesophagitis which does not seem to be accounted for by alteration of the physical barrier to acidic reflux. That a gluten free diet decreased the relapse rate of GORD related symptoms suggests that oesophagitis might be directly related to the underlying coeliac disease through a mechanism as yet not identified. A prospective study is underway in our unit to confirm our retrospective observation and to correlate small bowel lesions and relapse of GORD related symptoms in coeliac patients with oesophagitis. Based on our study, a thorough examination of the duodenum at endoscopy is warranted in subjects with reflux oesophagitis.
Acknowledgments
This study was supported by grants from Centro Interdipartimenale per la Ricerca su Alimenti Nutrizione ed Apparato Digerente (CIRANAD), Second University of Napoli, Italy, and from Ministero della Istruzione della Università e della Ricerca (MIUR), Italy
Abbreviations
GORD, gastro-oesophageal reflux disease
LOS, lower oesophageal sphincter
EMA, endomysium antibodies
REFERENCES
- 1.Dicke WK, Weijers NA, van der Kamer JH. Coeliac disease. The presence in wheat of a factor having a deleterious effect in cases of coeliac disease. Acta Paediatr 1953;42:34. [DOI] [PubMed] [Google Scholar]
- 2.Catassi C, Ratsch IM, Fabiani E, et al. Coeliac disease in the year 2000: Exploring the iceberg. Lancet 1994;343:200–3. [DOI] [PubMed] [Google Scholar]
- 3.Bassotti G, Castellanucci G, Betti C, et al. Abnormal gastrointestinal motility in patients with coeliac sprue. Dig Dis Sci 1994;39:1947–54. [DOI] [PubMed] [Google Scholar]
- 4.Usai P, Bassotti G, Usai Satta P, et al. Esophageal motility in adult coeliac disease. Neurogastroenterol Mot 1995;7:239–44. [DOI] [PubMed] [Google Scholar]
- 5.Perri F, Pastore M, Zicolella A, et al. Gastric emptying of solids is delayed in coeliac disease and normalizes after gluten withdrawal. Acta Paediatr 2000;89:921–5. [DOI] [PubMed] [Google Scholar]
- 6.Iovino P, Ciacci C, Sabbatini F, et al. Esophageal impairment in adult coeliac disease with steatorrhea. Am J Gastroenterol 1998;93:1243–9. [DOI] [PubMed] [Google Scholar]
- 7.Ciclitira PJ, King A, Fraser J. AGA technical review on coeliac sprue. Gastroenterology 2001;120:1526–40. [DOI] [PubMed] [Google Scholar]
- 8.Lundell LR, Dent J, Bennet JR, et al. Endoscopic assessment of esophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut 1999;45:172–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trujillo NP, Slaughter RL, Boyce HW Jr. Endoscopic diagnosis of sliding-type diaphragmatic hiatal hernias. Am J Dig Dis 1968;13:855–67. [DOI] [PubMed] [Google Scholar]
- 10.Scindlbeck NE, Heinrich C, Koenig A, et al. Optimal threshold, sensitivity, and specificity of long term pH-metry for detection of gastroesophageal reflux disease. Gastroenterology 1987;93:85–90. [DOI] [PubMed] [Google Scholar]
- 11.Jamieson JR, Stein HJ, De Meester TR, et al. Ambulatory 24 h esophageal pH monitoring: normal values, optimal thresholds, specificity, sensitivity, and reproducibility. Am J Gastroenterol 1992;87:1102–11. [PubMed] [Google Scholar]
- 12.Usai P, Usai Satta P, Lai M, et al. Autonomic dysfunction and upper digestive functional disorders in untreated adult coeliac disease. Eur J Clin Invest 1997;27:1009–15. [DOI] [PubMed] [Google Scholar]
- 13.Locke GR,III, Talley NJ, Fett SL, et al. Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology 1997;112:1448–56. [DOI] [PubMed] [Google Scholar]
- 14.Valle C, Broglia F, Pistorio A, et al. Prevalence and impact of symptoms suggestive of gastroesophageal reflux disease. Dig Dis Sci 1999;9:1848–52. [DOI] [PubMed] [Google Scholar]
- 15.Baldi F, Crotta S, Penagini R. Guidelines for the diagnostic and therapeutic management of patients with gastro-oesophageal reflux disease. Ital J Gastroenterol Hepatol 1998;30:107–12. [PubMed] [Google Scholar]
- 16.Oderda G, Forni M, Morra I, et al. Endoscopic and histologic findings in the upper gastrointestinal tract of children with coeliac disease. J Pediatr Gastroenterol Nutr 1993;16:172–7. [DOI] [PubMed] [Google Scholar]
- 17.Kilander AF, Dotevall G, Lindstedt G, et al. Plasma enteroglucagon related to malabsorption in coeliac disease. Gut 1984;25:629–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bardella MT, Fraquelli M, Peracchi M, et al. Gastric emptying and plasma neurotensin levels in untreated coeliac patients. Scand J Gastroenterol 2000;35:269–73. [DOI] [PubMed] [Google Scholar]
- 19.Besterman HS, Bloom SR, Sarson DL, et al. Gut-hormone profile in coeliac disease. Lancet 1978;4:786–8. [DOI] [PubMed] [Google Scholar]
- 20.Fitzgerald RC, Onwuegbusi BA, Bajaj-Elliott M, et al. Diversity in the oesophageal phenotypic response to gastro-oesophageal reflux: immunological determinants. Gut 2002;50:451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nilsen EM, Lundin KEA, Hansen T, et al. Gluten specific, HLA-DQ restricted T cells from coeliac mucosa produce cytokines with Th1 and Th0 profile dominated by interferon γ. Gut 1995;37:766–76. [DOI] [PMC free article] [PubMed] [Google Scholar]