Abstract
Background and aims: Chronic bowel disturbances resembling irritable bowel syndrome (IBS) develop in approximately 25% of patients after an episode of infectious diarrhoea. Although we have previously shown that psychosocial factors operating at the time of, or prior to, the acute illness appear to predict the development of post-infectious IBS (PI-IBS), our finding of an increased inflammatory cell number in the rectum persisting for at least three months after the acute infection suggested that there is also an organic component involved in the development of PI-IBS. To evaluate this further, we measured expressions of interleukin 1β (IL-1β) and its receptor antagonist (IL-1ra) in these patients to provide additional evidence that the pathogenesis of PI-IBS is underpinned by an inflammatory process.
Methods: Sequential rectal biopsy samples were prospectively obtained during and three months after acute gastroenteritis, from eight patients who developed post-infectious IBS (INF-IBS) and seven patients who returned to normal bowel habits after acute gastroenteritis (infection controls, INF-CON). Eighteen healthy volunteers who had not suffered from gastroenteritis in the preceding two years served as normal controls (NOR-CON). IL-1β and IL-1ra gene expressions were assayed by reverse transcriptase-polymerase chain reaction, and their levels of expression were quantitated by optical densitometry after electrophoresis on agarose gel.
Results: INF-IBS patients exhibited significantly greater expression of IL-1β mRNA in rectal biopsies than INF-CON patients both during and three months after acute gastroenteritis. Moreover, IL-1β mRNA expression had increased in biopsies taken from INF-IBS patients at three months after the acute infection but no consistent change was observed in INF-CON patients. IL-1β mRNA expression of INF-IBS patients at three months post gastroenteritis was significantly greater than NOR-CON whereas that of INF-CON patients was not significantly different from NOR-CON. Despite these differential changes in IL-1β mRNA expression, no significant changes were observed in IL-1ra mRNA expression among the three groups.
Conclusions: These findings indicate that those patients who develop IBS post infection exhibit greater IL-1β mRNA expression, both during and after the infection, compared with individuals who do not develop PI-IBS. We conclude that such patients may be susceptible to inflammatory stimuli, and that inflammation may play a role in the pathogenesis of PI-IBS.
Keywords: interleukin 1β, irritable bowel syndrome, post-infectious irritable bowel syndrome
There is growing acceptance that acute bacterial gastroenteritis may precipitate irritable bowel syndrome (IBS) in susceptible individuals. Indeed, a large cohort study has shown that bacterial gastroenteritis imparts a relative risk of 11.9 for the development of IBS.1 Prospective studies have shown that patients who developed IBS post infection exhibited more anxiety, depression, and stressful life events at the time of the actual illness than those whose bowel habits returned to normal.2–4 This observation supports the hypothesis that behavioural factors are important determinants of the expression of IBS post infection.
Studies in an animal model have shown that transient and superficial infection of the gut leads to long term dysfunction.5 This was associated with cytokine expression in the mucosal compartment and in the deeper neuromuscular layers.6,7 The state of persistent dysfunction could be reversed by corticosteroids, indicating that it was actively maintained by inflammatory mediators. Studies in patients with post-infectious IBS (PI-IBS) have shown increased inflammatory cells in the mucosa3,8 and similar observations have been made in IBS patients without a history suggestive of a precipitating enteric infection,9 prompting speculation that inflammation may play a role in the pathogenesis of a broader segment of the IBS population.10–12 A preliminary report has suggested that some IBS patients may be genetically predisposed to inflammation because of low secretion of the counter-inflammatory cytokines interleukin 10 and transforming growth factor β.13 Thus it is possible that an inability to efficiently downregulate an inflammatory response may predispose patients to IBS post infection.
The cytokine interleukin 1β (IL-1β) is an important modulator of the inflammatory process.14,15 Its biological activity is inhibited by its naturally occurring antagonist (IL-1ra) and it is the balance of these cytokines that determines the bioavailability of IL-1β and hence its contribution to inflammation.16–18 A preliminary study from our laboratory showed that a subset of IBS patients with diarrhoea exhibited elevated levels of IL-1β.19 Therefore, we chose to measure IL-1β and IL-1ra mRNA levels in rectal biopsies obtained from patients during and after gastroenteritis to test the hypothesis that patients who develop PI-IBS have evidence of a greater inflammatory response to infection and that this persists after infection.
SUBJECTS AND METHODS
Patients and normal controls
Subjects were recruited prospectively from among 228 consecutive patients admitted to our infectious disease ward with acute gastroenteritis between 1993 and 1995. A detailed interview and a validated bowel symptom questionnaire were both administered during their admission to hospital. Patients who fulfilled the criteria for IBS20 or any other chronic bowel diseases prior to their attack of gastroenteritis were excluded from the study. We excluded 67 patients with pre-existing IBS, 12 patients who had previously undergone bowel surgery, and 10 patients who had inflammatory bowel disease. Of 139 eligible patients, 30 declined participation, giving a response rate of 78%. The number of patients who developed PI-IBS was 22. In our earlier study3 we obtained rectal biopsies for paraffin embedded histological sections for cell counting. After we found increased cell counts, consent was obtained from subsequent patients for additional biopsy samples for the purpose of this study. In this way we were able to obtain sequential rectal biopsies of sufficient weight from eight patients who developed PI-IBS (INF-IBS) and from seven patients whose bowel habits returned to normal after their gastroenteritis (infection control group (INF-CON)). Of the other patients who participated in the study, either no rectal biopsy samples were available or samples were available for only one of the two time points that were studied—that is, at acute infection or three months post infection.
These patients were asked to return for a reassessment three months after their gastroenteritis. During this follow up visit, patients again completed the bowel symptom questionnaire. Those who had persistent symptoms were investigated by a gastroenterologist to exclude organic disease, and were subsequently contacted every three months for at least a year to study the symptomatology and natural history of their disease. In this way we identified eight patients who had symptoms after gastroenteritis that were consistent with IBS, and they formed the PI-IBS group (INF-IBS) for the study. All of these patients reported pain relieved by defecation and changes in bowel habit, as described by the Rome criteria.20 Six had diarrhoea, one had constipation, and one had an alternating type of bowel habit. Seven patients, whose bowel habits returned to normal after gastroenteritis, formed the infection control group (INF-CON). Stool cultures, conducted during the acute infection, were positive in seven patients (INF-IBS: four salmonella, one Escherichia coli; INF-CON: one salmonella, one shigella). The majority of patients (all INF-IBS, four INF-CON) received antibiotics during acute gastroenteritis. The normal control group (NOR-CON) consisted of 18 healthy volunteers who did not fulfil the criteria for IBS and who had not had a recent history of gastroenteritis. These were recruited through advertisements placed in the local hospitals and university. Subject characteristics are summarised in table 1 ▶. The infection control group was significantly older than the healthy control group.
Table 1.
Subject characteristics
| INF-IBS (n=8) | INF-CON (n=7) | NOR-CON (n=18) | |
| Age (y)† | 44 (6.8) | 48 (5.2)** | 30 (2.4) |
| Female (No (%)) | 4 (50) | 4 (57) | 14 (78) |
| Stool +ve (No (%)) | 5 (63) | 2 (29) | — |
| Antibiotics (No (%)) | 8 (100) | 4 (57) | — |
†Mean (SEM).
INF-IBS, patients who developed irritable bowel syndrome after acute gastroenteritis; INF-CON, patients who returned to normal bowel habits after acute gastroenteritis; NOR-CON, normal controls.
**p<0.005 versus NOR-CON.
Clinical protocol
Sigmoidoscopy and rectal biopsy was carried out in all patients during the acute admission and at approximately three months after the attack of gastroenteritis. The interval between onset of acute gastroenteritis and the timing of the second rectal biopsy was comparable in the INF-IBS and INF-CON groups (see table 2 ▶). It was also carried out on normal controls. Informed consent was obtained before each sigmoidoscopy.
Table 2.
Rectal biopsy results
| Acute infection biopsy | Post infection biopsy | ||||
| INF-IBS | INF-CON | INF-IBS | INF-CON | NOR-CON | |
| Interval from onset to biopsy (days) | 5 (1) | 4 (1) | 108 (14) | 98 (4) | — |
| IL-1β mRNA:β-actin (×10−2) | 9.8 (1.3)** | 3.6 (0.8) | 16.6 (1.9)††† | 4.4 (0.3) | 6.3 (0.7) |
| IL-1ra mRNA:β-actin (×10−2) | 5.3 (0.4) | 8.6 (1.3) | 7.4 (1.1) | 6.6 (0.4) | 4.5 (0.9) |
INF-IBS, patients who developed irritable bowel syndrome after acute gastroenteritis; INF-CON, patients who returned to normal bowel habits after acute gastroenteritis; NOR-CON, normal controls.
IL-1β, interleukin 1β; IL-1ra, interleukin 1 receptor antagonist.
Results are expressed as mean (SEM).
**p<0.005 versus INF-CON; †††p<0.001 versus INF-CON and NOR-CON. There were no statistically significant differences between the three groups for IL-1ra mRNA expression.
The rectal mucosa was first examined for inflammatory changes. At least two rectal biopsies were taken from the posterior wall between 6 and 8 cm from the anal verge, away from any areas of obvious inflammation. One sample was snap frozen in liquid nitrogen for subsequent extraction of RNA (method described below). The other sample was placed in formalin and sent off for routine histopathology.
RNA preparation
Total RNA was prepared according to a modified guanidium thiocyanate method.21 Biopsy tissue was homogenised in 4 M guanidium isothiocyanate solution. RNA was extracted in an equal volume of water saturated phenol-chloroform-isoamyl solution (pH 4.0). After centrifugation, the upper aqueous phase was precipitated in two volumes of ethanol overnight at 20°C. The RNA pellet was washed with 75% ethanol and dried in air. The RNA obtained was quantitated by measuring optical density.
RNA was reverse transcribed into complementary DNA (cDNA), as previously described.22 Typically, a 3 μg aliquot of total cellular RNA from each sample was mixed with 500 ng of oligo-deoxythymidylic acid primer (Promega, Madison, Wisconsin, USA) in 20 μl of ribonuclease free water. RNA was then annealed by heating this mixture to 70°C for 10 minutes followed by gradual cooling to 37°C. To the annealed mixture, a cocktail containing 15 units of reverse transcriptase, 1 ml of 10 mM each dATP, dCTP, dGTP, and 25 units of RNasin in commercial RT reaction buffer (Promega) was added. This was then incubated at 42°C for 60 minutes before the reverse transcription reaction was terminated by heating at 90°C for 10 minutes.
Primers for the messages of interest were designed based on the following complementary DNA sequence information: β-actin upstream 5′-CCT TCC TGG GCA TGG AGT CCT G - 3′, downstream 5′-GGA GCA ATG TTG ATC TTC-3′; IL-1β upstream 5′- AGC TTG GTG ATG TCT GGT CCA T-3′, downstream 5′-GAG GTG CTG ATG TAC CAG TTG-3′; and IL-1ra upstream 5′-AGA AGA CCT CCT GTC CTA TG-3′, downstream 5′-TAC TCG TCC TCC TGG AAG TA-3′. The basic polymerase chain reaction (PCR) protocol was the same as described elsewhere.22 cDNA (2 μl) was mixed with 1 μl each of upstream and downstream primers, and 0.5 μl of Thermus aquaticus (Taq) DNA polymerase (Promega). The following cycle parameters were used: denaturation 94°C×30 seconds; annealing 57°C×30 seconds; extension 72°C×60 seconds; and final extension 72°C×five minutes relative target mRNA. Thirty cycles were used for IL-1β, and 32 cycles for IL-1ra and β-actin based on preliminary studies with positive (samples of inflamed mucosa from patients with ulcerative colitis) and negative (deionised water) controls. Each RNA sample was directly applied to PCR to ensure that there was no DNA contamination.
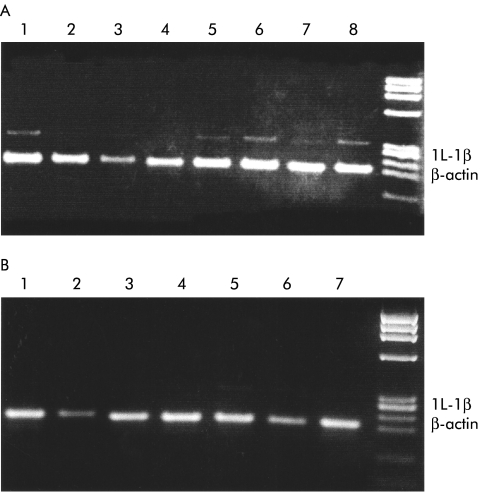
The PCR end product was electrophoresed on 2% agarose gel and stained with ethidium bromide. After drying, the gel was photographed and the negatives were used for semiquantitation by optical densitometry (a photograph of the gel with samples from three months post infection is reproduced in fig 1 ▶). Samples were normalised on the basis of their actin content, as assessed by optical densitometry.
Figure 1.
Interleukin 1β (IL-1β) mRNA expression in (A) post-infectious irritable bowel syndrome patients (INF-IBS) and in (B) post infection controls (INF-CON) (2% agarose gel stained with ethidium bromide; 30 polymerase chain reaction cycles). INF-IBS patients developed IBS after acute gastroenteritis and INF-CON patients returned to normal bowel habits after acute gastroenteritis
Statistical analysis
Analysis of variance was performed in the first instance, and if this showed a statistically significant difference, then the independent sample t test was used to compare the results between the two groups. The χ2 test was used to compare categorical data. In view of multiple comparisons, α was set at 0.01.
RESULTS
No histological features of inflammatory bowel disease were noted in any of our subjects. Minor inflammatory changes were observed during acute gastroenteritis in six patients (three INF-IBS and three INF-CON). At three months after gastroenteritis, sigmoidoscopy was normal in all patients and neither inflammatory bowel disease nor microscopic colitis was reported in any of the biopsies sent to our histopathology laboratory.
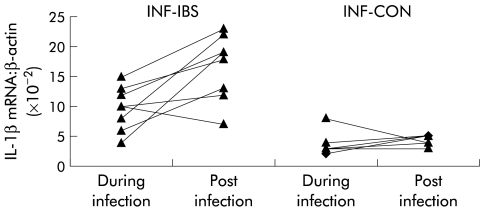
During acute gastroenteritis, IL-1β mRNA expression was significantly greater in INF-IBS than in INF-CON patients (see table 2 ▶). This difference was accentuated three months later by a significant increase in IL-1β mRNA expression in biopsies taken from INF-IBS but not INF-CON patients (figs 1 ▶, 2 ▶). At three months, IL-1β mRNA expression in INF-IBS patients, but not that in INF-CON patients, was significantly greater than that found in normal controls.
Figure 2.
Changes in interleukin 1β (IL-1β) mRNA expression after infection in INF-IBS patients (who developed irritable bowel syndrome after acute gastroenteritis) and in INF-CON patients (who returned to normal bowel habits after acute gastroenteritis).
There were no significant differences between IL-1ra mRNA expression in biopsies taken from any of the three groups during or after gastroenteritis (table 2 ▶, fig 3 ▶).
Figure 3.
Changes in interleukin 1 receptor antagonist (IL-1ra) mRNA expression after infection in INF-IBS patients (who developed irritable bowel syndrome after acute gastroenteritis) and in INF-CON patients (who returned to normal bowel habits after acute gastroenteritis).
DISCUSSION
The results of this study have shown that patients with acute gastroenteritis, who go on to develop IBS, have a higher expression of IL-1β mRNA compared with infected patients who do not develop IBS. This is in keeping with the clinical observation that patients with a more severe infective gastroenteritis are more likely to develop IBS post infection.3,4 A correlation is also available from animal studies showing that attenuation of the host response to the initial infection reduces the magnitude of post-infective gut dysfunction.6 Taken together, these observations suggest that, in addition to previously identified behavioural determinants,2 organic factors are important in the development of IBS post infection.
A recent preliminary report suggested that some IBS patients have a genotypic profile that is similar to that seen in patients with inflammatory bowel disease.13 Specifically, some IBS patients are low secretors of the counter-inflammatory cytokines interleukin 10 and transforming growth factor β. Such patients may downregulate an inflammatory response inefficiently, and might be expected to exhibit a stronger profile of inflammatory markers than controls. The present finding of increased expression of IL-1β mRNA during acute gastroenteritis in those patients who subsequently developed IBS supports the notion that an enhanced acute inflammatory response may be a determinant of IBS post infection and invites speculation that this may be genetically determined.
Our finding of increased IL-1β mRNA expression in the rectal mucosa of patients with IBS symptoms, but not in asymptomatic individuals post infection, suggests that inflammation may contribute to expression of PI-IBS. This is in keeping with our previous results3 and those of others8 which demonstrate an increased inflammatory cell number in the mucosa of patients with PI-IBS. Taken together, these observations contribute to a growing literature showing that there is low grade inflammation and immune activation in the colorectum of a subset of IBS patients.11 As these findings are not restricted to patients with a history of overt infection, it would appear that gastroenteritis is one of several triggers that may precipitate inflammation based IBS in susceptible individuals.
We chose IL-1β mRNA expression simply as a ubiquitous marker of inflammation and we attach no mechanistic importance to the increased expression of this cytokine mRNA in rectal biopsies from PI-IBS patients. It is unlikely that IL-1β, restricted to the mucosal compartment, contributes to the changes in sensory or motor activity that are considered to be the basis of symptom generation in IBS. Studies in animals have shown that superficial infection of the intestinal mucosa is accompanied by inflammation and expression of proinflammatory cytokines in the mucosal compartment and that this in turn leads to expression of cytokines in the deeper neuromuscular layers.7 In the animal model, expression of Th2 cytokines IL-4 and IL-13 in the muscularis externa are critical for the induction of changes in neuromuscular function.6 Thus mucosal expression of IL-1β may be viewed as a surrogate marker for changes that occur in deeper tissues.
A key question that needs to be addressed is whether anti-inflammatory therapies might be useful in IBS patients with evidence of ongoing low grade inflammation. While treatment with parenterally administered corticosteroid or local cyclooxygenase inhibitors reversed post-infective gut dysfunction in an animal model, a preliminary report suggests that this may not be the case in PI-IBS.23 The window of therapeutic opportunity for reversal of gut dysfunction in PI-IBS may differ from that seen in the animal model, as may the mediators and their accessibility to orally administered anti-inflammatory treatment.
In conclusion, the main finding of this study that an enhanced peripheral inflammatory response occurs in patients with gastroenteritis who subsequently go on to develop IBS, contrasts with previous work identifying behavioural factors that predict the development of IBS post infection. We do not regard these pathways as being mutually exclusive. On the contrary, there is growing evidence that behavioural and peripheral inflammatory processes converge to influence disease expression, and supporting examples are available in animal based studies. It has been shown that prior stress enhances the response to a subsequent inflammatory stimulus24 and that stress may reactivate quiescent colitis25 and the accompanying changes in gut physiology.26 Taken together, these observations underscore the importance of both behavioural and inflammatory processes in the development of IBS following enteric infection.
Acknowledgments
Supported in part by a grant from the Special Trustees for the Former United Sheffield Hospitals and by travel awards from the Royal College of Physicians and Surgeons of Glasgow, the British Digestive Foundation-Hunt and Hurst Travel Award, and Wellcome UK-CIHR Canada. K-AG was supported by scholarships from the Association of Commonwealth Universities, the National University of Singapore, and the Physiological Society.
Abbreviations
IBS, irritable bowel syndrome
PI-IBS, post-infectious IBS
IL-1β, interleukin 1β
IL-1ra, interleukin 1 receptor antagonist
PCR, polymerase chain reaction
REFERENCES
- 1.Rodriguez LAG, Ruigomez A. Increased risk of irritable bowel syndrome after bacterial gastroenteritis: cohort study. BMJ 1999;318:565–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gwee KA, Graham JC, McKendrick MW, et al. Psychometric scores and development of irritable bowel after infectious diarrhoea. Lancet 1996;347:150–3. [DOI] [PubMed] [Google Scholar]
- 3.Gwee KA, Leong YL, Graham JC, et al. The role of psychological and biological factors in post infective gut dysfunction. Gut 1999;44:400–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neal KR, Hebden J, Spiller R. Prevalence of gastrointestinal symptoms six months after bacterial gastroenteritis and risk factors for the development of the irritable bowel syndrome: postal survey of patients. BMJ 1997;314:779–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbara G, Vallance BA, Collins SM. Persistent intestinal neuromuscular dysfunction after acute nematode infection in mice. Gastroenterology 1997;113:1224–32. [DOI] [PubMed] [Google Scholar]
- 6.Barbara G, De Giorgio R, Deng Y, et al. Role of immunologic factors and cyclooxygenase 2 in persistent postinfective enteric muscle dysfunction in mice. Gastroenterology 2001;120:1729–36. [DOI] [PubMed] [Google Scholar]
- 7.Khan I, Collins SM. Expression of cytokines in the longitudinal muscle myenteric plexus of the inflamed intestine of rat. Gastroenterology 1994;107:691–700. [DOI] [PubMed] [Google Scholar]
- 8.Spiller RC, Jenkins D, Thornley JP, et al. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut 2000;47:804–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chadwick VS, Chen W, Shu D, et al. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology 2002;122:1778–83. [DOI] [PubMed] [Google Scholar]
- 10.Collins SM, Piche T, Rampal P. The putative role of inflammation in the irritable bowel syndrome. Gut 2001;49:743–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins SM. A case for an immunological basis for irritable bowel syndrome. Gastroenterology 2002;122:2078–80. [DOI] [PubMed] [Google Scholar]
- 12.Mayer EA, Collins SM. Evolving patophysiologic models of functional gastrointestinal disorders. Gastroenterology 2002;122:2032–48. [DOI] [PubMed] [Google Scholar]
- 13.Chan J, Gonsalkorale WM, Perrey C, et al. IL-10 and TGF-β genotypes in irritable bowel syndrome: Evidence to support an inflammatory component? Gastroenterology 2000;118:A1191. [Google Scholar]
- 14.Sartor BF. Cytokines in intestinal inflammation: pathophysiological and clinical considerations. Gastroenterology 1994;106:533–9. [DOI] [PubMed] [Google Scholar]
- 15.Dinarello CA, Wolff SM. The role of interleukin-1 in disease. N Engl J Med 1993;328:106–13. [DOI] [PubMed] [Google Scholar]
- 16.Casini-Raggi V, Kam L, Chong YJT, et al. Mucosal imbalance of IL-1 and IL-1 receptor antagonist in inflammatory bowel disease. A novel mechanism of chronic intestinal inflammation. J Immunol 1995;154:2434–40. [PubMed] [Google Scholar]
- 17.Isaacs KL, Sartor RB, Haskill S. Cytokine messenger RNA profiles in inflammatory bowel disease mucosa detected by polymerase chain reaction amplification. Gastroenterology 1992;103:1587–95. [DOI] [PubMed] [Google Scholar]
- 18.Cominelli F, Nast CC, Duchini LM. Recombinant interleukin-1 receptor antagonist blocks the proinflammatory activity of endogenous interleukin-1 in rabbit immune colitis. Gastroenterology 1992;103:365–71. [DOI] [PubMed] [Google Scholar]
- 19.Khan I, Collins SM. Is there an inflammatory basis for a subset of patients presenting with diarrhoea in the irritable bowel syndrome? Gastroenterology 1994;108:A523. [Google Scholar]
- 20.Thompson WG, Creed F, Drossman DA, et al. Functional bowel disease and functional abdominal pain. Gastroenterol Int 1992;5:75–91. [Google Scholar]
- 21.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987;162:156–9. [DOI] [PubMed] [Google Scholar]
- 22.Khan I, Tabb T, Garfield RE, et al. Polymerase chain reaction assay of mRNA using 28S rRNA as internal standard. Neuroscience Letter 1992;47:114–17. [DOI] [PubMed] [Google Scholar]
- 23.Dunlop S, Jenkins D, Naesdal J, et al. Randomised double-blind placebo-controlled trial of prednisolone in post-infectious irritable bowel syndrome. Gastroenterology 2002;122:499. [DOI] [PubMed] [Google Scholar]
- 24.Gue M, Bonbonne C, Fioramonti J, et al. Stress-induced enhancement of colitis in rats: CRF and arginine vasopressin are not involved. Am J Physiol 1997;272:G84–91. [DOI] [PubMed] [Google Scholar]
- 25.Qiu BS, Vallance BA, Blennerhassett PA, et al. The role of CD4+ lymphocytes in the susceptibility of mice to stress-induced reactivation of experimental colitis. Nat Med 1999;5:1178–82. [DOI] [PubMed] [Google Scholar]
- 26.Collins SM, McHugh K, Jacobson K, et al. Previous inflammation alters the response of the rat colon to stress. Gastroenterology 1996;111:1509–15. [DOI] [PubMed] [Google Scholar]