Abstract
Background and aims: Radiofrequency energy (RFe) treatment to the lower oesophageal sphincter (LOS) and gastric cardia is a new luminally delivered therapy proposed as an alternative treatment for gastro-oesophageal reflux disease (GORD). However, it is unclear how RFe achieves its antireflux effect. This study investigated the effects of RFe on mechanisms of spontaneous reflux in patients with GORD.
Methods: Twenty patients with GORD underwent endoscopy, symptom evaluation, and combined postprandial oesophageal manometry and pH monitoring before and six months after RFe, and 24 hour ambulatory pH monitoring before and at six and 12 months after treatment.
Results: RFe reduced the rate of postprandial transient LOS relaxations from 6.8 (5.7–8.1) (median (interquartile range) per hour to 5.2 (4.2–5.8) per hour (p<0.01), and increased mean basal LOS pressure from 5.2 (SEM 0.3) mm Hg to 8.0 (SEM 0.4) mm Hg (p<0.01). The number of reflux events was reduced from 10 (2–15.3)/3 hours to 5 (3.5–8.5)/3 hours (p<0.05) and there was an associated significant reduction in acid exposure time from 5.4% (0.4–14.7) to 3.9% (0.4–6.6) (p<0.05). RFe significantly reduced ambulatory oesophageal acid exposure from 10.6% (7.8–13.0) to 6.8% (3.1–9.1) (p<0.01) at six months and 6.3% (4.7–10.9) (p<0.05) at 12 months. All patients required acid suppressant medication for symptom control before RFe. Six months after treatment, 15 patients (75%) were in symptomatic remission and 13 (65%) at 12 months.
Conclusions: RFe has significant effects on LOS function that are associated with improvement in the antireflux barrier. Uncontrolled clinical data also suggest a beneficial effect in the control of reflux symptoms in these patients.
Keywords: endoscopy, gastro-oesophageal reflux disease, lower oesophageal sphincter, oesophageal motility, oesophageal pH monitoring
Currently, drug therapy and antireflux surgery are the main options for the long term treatment of gastro-oesophageal reflux disease (GORD). These treatments, although effective, have limitations. Long term drug therapy is associated with issues of compliance and very long term safety. A significant proportion of patients continue to elect to have antireflux surgery as “definitive” treatment. However, this necessitates general anaesthesia, has a mortality of approximately 0.2%, and can be associated with significant morbidity, including dysphagia, gas-bloat syndrome, and postprandial fullness.
Radiofrequency energy (RFe), delivered to the lower oesophageal sphincter (LOS) and gastric cardia,1,2 is one of several new luminally delivered therapies that have been proposed as an alternative treatment for GORD. Delivery of RFe into tissue causes a circumscribed thermal coagulative necrosis that heals by fibrosis. Delivery of RFe has been used to ablate aberrant cardiac conduction pathways,3 and to treat prostatic hypertrophy,4 solid tumours,5 sleep apnoea,6 and lax joint capsules.7
Recent clinical data suggest that RFe treatment to the LOS and gastric cardia reduces gastro-oesophageal reflux. Uncontrolled clinical trials have reported a reduction in oesophageal acid exposure and reflux symptoms.1,8 However, it is unclear how RFe achieves its antireflux effect. Gastro-oesophageal reflux occurs by two main mechanisms. The majority of reflux episodes occur during transient LOS relaxations (TLOSRs) while an important minority of episodes occur because of defective basal LOS pressure.9 The relative mix of these mechanisms varies according to the presence and severity of reflux oesophagitis. In anaesthetised pigs, RFe treatment reversed much of the reduction of basal LOS pressure induced by botulinum toxin injection, as well as significantly increasing gastric yield pressure.2 Preliminary data in dogs10 suggest that RFe may also reduce the rate of TLOSRs. It has been reported that RFe treatment does not reduce the rate of TLOSRs provoked by gastric distension with air in patients with reflux disease, although a trend was observed.8
Inhibition of TLOSRs as well as increases in basal LOS tone may both be important mechanisms for the control of reflux after RFe treatment. The effects of RFe on mechanisms of spontaneous reflux in patients with GORD have not been formally investigated. This was the aim of the current study.
METHODS
Subjects
Twenty patients (10 males, median age 51.2 years (range 32–69)) were recruited for the study. The criteria for entry were symptomatic heartburn responsive to acid suppressive therapy and abnormal 24 hour ambulatory pH monitoring (percentage time with pH <4 of >4%). At entry, all patients had either no erosions or mild-moderate reflux oesophagitis (Los Angeles grade A, B, or C)11 and all required daily acid suppressant therapy to control their heartburn. Most patients however had been documented previously to have had more severe oesophagitis (table 1 ▶). Patients were excluded if they had severe oesophagitis (Los Angeles grade D), previous oesophagogastric surgery, hiatus hernia >2 cm, ⩾3 cm of columnar lined oesophagus, systemic conditions known to be associated with abnormal oesophageal motor function (for example, scleroderma), and an increased risk of endocarditis. Each patient gave written informed consent, and the protocol was approved by the research ethics committee of the Royal Adelaide Hospital.
Table 1.
Oesophagitis grade and medication use
| Patient No | Most severe endoscopic grade* pre-entry | Endoscopic grade† at entry | Medications at entry (mg/day) | Endoscopic grade † at 6 months |
Medications at 6 months (mg/day) | Medications at 12 months (mg/day) |
| 1 | Normal | 0 | RAN 600 | 0 | None | None |
| 2 | Normal | 0 | OME 80 | 0 | None | None |
| 3 | II | 0 | OME 20 | A | OME 20 | OME 20 |
| 4 | II | A | OME 20 | A | None | None |
| 5 | Normal | 0 | FAM 80 | A | None | None |
| 6 | I | 0 | OME 80 | 0 | None | None |
| 7 | II | 0 | OME 20 | A | None | RAN 150 |
| 8 | II | A | RAN 300 | A | None | None |
| 9 | II | 0 | OME 20 | 0 | RAN 150 | RAN 150 |
| 10 | III | 0 | OME 20 | 0 | None | None |
| 11 | III | B | OME 80 | A | RAN 300 | RAN 300 |
| 12 | II | 0 | OME 20 | A | None | None |
| 13 | II | 0 | OME 20 | 0 | None | None |
| 14 | III | B | OME 20 | A | RAN 300 | None |
| 15 | III | 0 | OME 20 | A | None | None |
| 16 | II | 0 | OME 20 | 0 | None | None |
| 17 | II | C | OME 20 | A | None | None |
| 18 | Normal | 0 | RAN 300 | 0 | None | RAN 150 |
| 19 | Normal | 0 | PAN 80 | 0 | PAN 40 | None‡ |
| 20 | II | 0 | OME 20 | 0 | None | RAN 300 |
*Savary-Miller classification.
† Los Angeles classification.
OME, omeprazole; PAN, pantoprazole; RAN, ranitidine; FAM, famotidine.
‡Underwent laparoscopic fundoplication at nine months.
Protocol
Before treatment with RFe, patients underwent endoscopy, ambulatory pH monitoring, symptom evaluation, and combined postprandial oesophageal manometry and pH monitoring. All acid suppressive medication was stopped at least five days before the pH monitoring studies. After treatment, medication was continued for three weeks and then stopped. Acid suppressive therapy was restarted only if reflux symptoms recurred, and at the lowest level needed to control symptoms.
After treatment with RFe, postprandial combined oesophageal manometry and pH monitoring were performed at six months. Ambulatory 24 hour pH monitoring was performed at six and 12 months after treatment. Patients were also re-endoscoped at six months and underwent symptom evaluation at 1, 4, 6, and 12 months.
Radiofrequency energy delivery to the lower oesophagus and cardia
All patients were treated as day cases at the Gastrointestinal Investigational Unit of the Royal Adelaide Hospital. Patients were sedated with a combination of midazolam and fentanyl. Flexible gastroscopy was performed to determine the distance from the upper incisors to the squamocolumnar junction. RFe was then delivered using a technique similar to that described previously.1 However, our protocol differed slightly in the number of lesion sets created and the areas that were treated. In the current protocol, two lesion sets (eight RFe lesions) were created at each of seven levels at 0.5 cm intervals between 1.5 cm proximal to 1.5 cm distal to the squamocolumnar junction. An additional six lesion sets were also made at the gastric cardia by inflating the balloon with 22 ml and 25 ml of air, respectively, and withdrawing the catheter until the balloon engaged in the cardia. Thus totals of 20 lesion sets, or 80 RFe lesions, were created in each patient.
All patients were discharged from hospital approximately two hours after treatment. Simple analgesics (acetominophen, acetominophen/codeine) were administered, as needed for pain. Patients were instructed to eat a soft diet for three days, to continue their usual acid suppressive therapy for three weeks, and then to cease it.
Postprandial oesophageal manometry and pH monitoring, and 24 hour ambulatory pH monitoring
Oesophageal manometry was performed with a 4.2 mm multilumen assembly that incorporated a sleeve sensor (Dentsleeve Pty Ltd, Wayville, South Australia). The sleeve sensor monitored LOS pressure. A side hole 1 cm below the distal margin of the sleeve recorded gastric pressure. Side holes at 3 cm intervals, starting at the proximal margin of the sleeve, recorded oesophageal body motility and a side hole in the pharynx recorded swallowing. The sleeve and gastric side hole were perfused with degassed distilled water at 0.3 ml/min, and the oesophageal side holes at 0.15 ml/min by a low compliance manometric perfusion pump. The pharyngeal sidehole was perfused by air at 8 ml/min. Pressures were sensed by external transducers with output to a computerised acquisition system. Oesophageal pH was measured with an antimony electrode (Medtronic Functional Diagnostics A/S, Denmark). Data were digitised with a Macintosh computer (Apple Computer Inc, Cupertino, California, USA) and the digitised signals were displayed and analysed using AcqKnowledge software (Biopac Systems, Goleta, California, USA).
All patients were studied after an overnight fast. After intubation, the manometric assembly was positioned such that the sleeve sensor straddled the lower oesophageal sphincter. The pH electrode was positioned 5 cm above the proximal margin of the lower oesophageal sphincter. Following a 10–15 minute period of accommodation, LOS relaxation and oesophageal peristalsis were assessed in response to 10 water swallows in the recumbent position. Patients then sat up and ate a standard soft mixed nutrient meal (3000 kJ, 55% fat) consisting of savoury mince, mashed vegetables, 150 ml milk, and ice cream. Recordings were then made in the sitting position for three hours. On completion of the postprandial manometric study, the manometric assembly was withdrawn but the pH electrode left in place and connected to a portable data logger electrode (Medtronic Functional Diagnostics A/S, Denmark). Ambulatory pH recordings were then undertaken for 24 hours.
Assessment of symptoms, medication use, and oesophagitis
Quality of life was assessed with both a generic quality-of-life (QoL) scale (SF-36) and a disease specific QoL questionnaire (GERD-HRQL).12 Dysphagia was measured with a validated dysphagia scoring system.13 These evaluations were made before treatment, and at 1, 4, 6, and 12 months after treatment. Medication use was recorded before treatment and at monthly intervals during follow up. Endoscopic grade of oesophagitis at entry into the study was classified using the Los Angeles grading system.11 All patients had undergone previous endoscopic evaluation prior to study entry (table 1 ▶). For these endoscopies, the severity of oesophagitis had been documented using the Savary-Miller classification and no attempt was made to reclassify these grades of oesophagitis into the Los Angeles system to avoid bias.
Data analysis
The three hour postprandial manometric data were analysed by two investigators (WCET, QZ) blinded to the pre/post treatment status. End expiratory basal LOS pressure was referenced to end expiratory intragastric pressure, and was determined at 10 minute intervals as one minute visual means. Basal LOS pressure was determined separately for the fasting and postprandial periods.
TLOSRs were defined according to the criteria published.14 LOS relaxations that lasted more than 15 seconds, which were associated with a swallow within four seconds before or two seconds after the onset of an LOS relaxation, were also included as TLOSRs.15,16
Acid reflux events were defined as a drop in oesophageal pH below 4 for at least four seconds or, if basal oesophageal pH was already below 4, as a further drop in pH of at least 1 pH unit. Infrequently, oesophageal pH drifted downwards during a period of several minutes and dropped below pH 4. These pH drifts were included in the analysis of the duration of oesophageal acid exposure but were not scored as acid reflux events. For analysis of the occurrence of acid reflux during TLOSRs, acid reflux was deemed to have occurred if there was a drop in pH of at least 1 pH unit.
The time of onset of the drop in oesophageal pH was used as the reference time for analysis of the motor events associated with reflux. For each reflux event, the mechanism of reflux was determined from the pattern of LOS pressure and its relationship to swallowing, and the occurrence of abdominal straining.17,18 These mechanisms were classified as TLOSR, swallow induced reflux (including multiple swallowing),19 absent basal LOS pressure, and straining. When pressures were obscured by movement induced artefacts, these episodes were listed as uninterpretable. The presence or absence of oesophageal body common cavity pressure elevation, a manometric indicator of gastro-oesophageal reflux, was also determined during TLOSRs. The rate of spontaneous swallowing was determined by counting the pharyngeal pressure waves.
The 24 hour ambulatory pH data were analysed automatically with the software provided by Medtronic Functional Diagnostics A/S (Denmark). Total, recumbent, and upright acid exposure times, as well as number of reflux events and DeMeester scores, were recorded.
Statistical analysis
The rate of TLOSRs, proportions of TLOSRs associated with acid reflux and oesophageal body common cavities, rate of reflux episodes, acid exposure time, mean acid clearance time, and the proportion of reflux episodes associated with TLOSRs were determined for each patient. In the 24 hour ambulatory pH study, calculations of acid exposure time, number of acid reflux events, and DeMeester scores were derived automatically utilising the software program EsopHagram (Medtronic Functional Diagnostics A/S). Group data were analysed by paired non-parametric (Wilcoxon signed rank) tests and are presented as median (interquartile range). All other data are presented as mean (SEM). Paired comparisons of LOS pressures were made using repeated measures analysis of variance (ANOVA; Abacus Concepts Inc, Berkeley, California, USA). A p value of less than 0.05 was accepted as indicating statistical significance.
RESULTS
All patients completed six months of follow up. One patient withdrew from the trial at nine months and underwent laparoscopic fundoplication, leaving 19 patients with 12 month follow up data.
Postprandial oesophageal manometry and pH monitoring
Lower oesophageal sphincter function
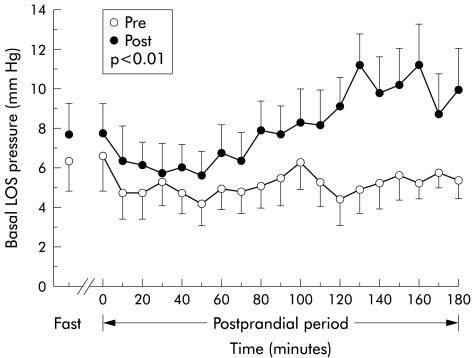
RFe treatment was associated with an increase in mean postprandial basal LOS pressure from 5.2 (SEM 0.3) mm Hg to 8.0 (0.4) mm Hg (p<0.01) (fig 1 ▶). This difference in basal LOS pressure was most marked in the third postprandial hour, during which LOS pressure was 10.2 mm Hg post-treatment versus 5.3 mm Hg pretreatment. RFe treatment had no significant effect on fasting basal LOS pressure (pre: 6.3 (SEM 1.5) mm Hg v post: 7.7 (1.6) mm Hg). RFe treatment also had no effect on LOS relaxation with swallowing, with mean nadir pressure post-treatment (0.1 (SEM 0.1) mm Hg) being similar to that pretreatment (0.1 (0.2) mm Hg).
Figure 1.
Effect of radiofrequency energy treatment on basal lower oesophageal sphincter (LOS) pressure at six months of follow up. Data are displayed as mean (SEM) for each 10 minute interval before (Pre) and after (Post) treatment.
Transient LOS relaxations
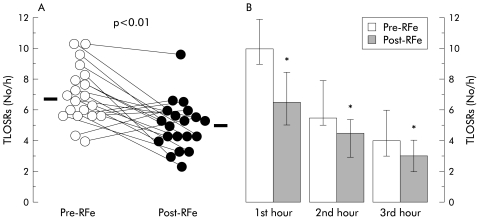
Post-treatment, the rate of TLOSRs was reduced significantly from a median of 6.8 (5.7–8.1) per hour to 5.2 (4.2–5.8) per hour (p<0.01) (fig 2 ▶), and the total number of TLOSRs decreased from 422 to 302. RFe treatment had no effect on the likelihood of acid reflux occurring during a TLOSR, acid reflux occurring with 53% (31–68%) of TLOSRs pretreatment compared with 56% (27–68%) post-treatment. Similarly, RFe had no effect on the proportion of TLOSRs associated with oesophageal body common cavity pressure elevations (pretreatment 98% (88–100%), post-treatment 91% (85–100%)).
Figure 2.
Effect of radiofrequency energy (RFe) treatment on transient lower oesophageal sphincter relaxations (TLOSRs) during the three hour postprandial period after six months of follow up. (A) Individual patient data points before (Pre) and after (Post) RFe treatment. Horizontal bars indicate median values. (B) Histogram showing pooled data for each postprandial hour. Data are displayed as median (interquartile range). *p<0.05 versus Pre-RFe.
Acid reflux events and acid exposure time
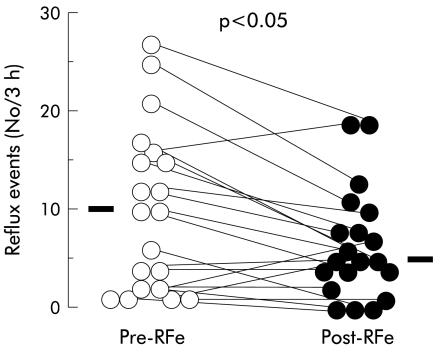
During the three hour postprandial manometric study, the median number of acid reflux events decreased significantly after RFe treatment from 10 (2–15.3)/3 hours to 5 (3.5–8.5)/3 hours (p<0.05) (fig 3 ▶). This corresponds to a fall in the total number of acid reflux events, which fell from 202 to 131 during the three hour postprandial period. There was an associated significant reduction in acid exposure time from 5.4% (0.4–14.7) to 3.9% (0.4–6.6) (p< 0.05). However, acid clearance time was not altered (62 seconds (16–110) pretreatment v 63 seconds (10–99) post-treatment; p>0.05).
Figure 3.
Effect of radiofrequency energy (RFe) treatment on the rate of postprandial acid reflux events during the three hour postprandial period after six months of follow up. Data are displayed as individual patient data points before (Pre-RFe) and after (Post-RFe) treatment. Horizontal bars indicate median values.
Mechanisms of reflux
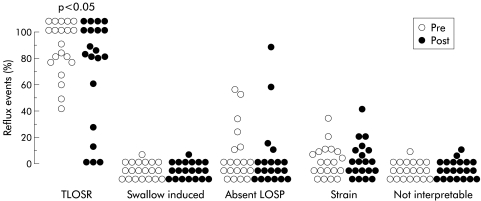
Before treatment, transient lower oesophageal sphincter relaxation was the major mechanism of reflux in the majority (18 of 20) of patients, accounting for 58–100% of acid reflux events in these patients (fig 4 ▶). In the two remaining patients, absent basal LOS pressure was the most prevalent mechanism of reflux, accounting for 52% and 56% of acid reflux events, respectively. In keeping with the effect on TLOSRs, RFe decreased the number of acid reflux events due to TLOSRs from 7.5 (2–10.3)/3 hours to 4 (1.75–7)/3 hours (p<0.05). However, RFe did not change the proportion of acid reflux events due to TLOSRs (pretreatment 95% (76–100%) compared with post-treatment 83% (52–100%); p>0.5).
Figure 4.
Effect of radiofrequency energy treatment on mechanisms of gastro- oesophageal reflux. Each point represents the proportion of reflux events occurring by that mechanism in that patient before (Pre) and after (Post) treatment. LOSP, lower oesophageal sphincter pressure; TLOSR, transient lower oesophageal sphincter relaxation.
Compared with pretreatment, there was also a modest reduction in the number of acid reflux events that occurred during periods of absent basal LOS pressure (39 events pretreatment in six patients, 22 events post-treatment in four patients) but the small numbers of patients in this subgroup precluded meaningful statistical analysis. RFe did not alter the number of acid reflux events that occurred in association with swallow induced LOS relaxation or straining.
24 hour ambulatory pH monitoring
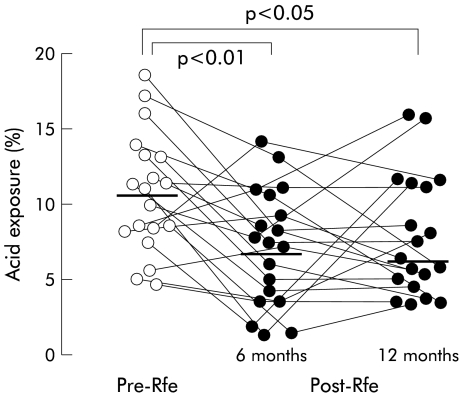
At six months, RFe treatment had significantly reduced the number of reflux episodes, both for the total 24 hours as well as for the upright and recumbent periods separately (table 2 ▶). However, by 12 months, this effect seemed to have been partially lost as only the rate of recumbent reflux episodes remained statistically lower. Median acid exposure time was reduced compared with pretreatment. Overall, 24 hour acid exposure decreased from 10.6% (7.8–13.0) to 6.8% (3.1–9.1) (p<0.01) (fig 5 ▶, table 2 ▶). This reduction was due largely to a reduction in recumbent acid exposure as RFe had no significant impact on acid exposure during the upright posture, although a trend was observed (10% (6.3–14.5) v 6.5%(3.8–11.5); p=0.08). At six months, 7 of 20 (35%) patients had normal 24 hour acid exposure. RFe treatment also reduced the DeMeester score and the number of acid reflux events.
Table 2.
Results of 24 hour pH monitoring
| Pretreatment | 6 months | 12 months | Prev 6 months | Prev 12 months | |
| Oesophageal acid exposure (%) | |||||
| Total 24 h | 10.6 (7.8–13.0) | 6.8 (3.1–9.1) | 6.3 (4.7–10.9) | p<0.01 | p<0.05 |
| Upright | 10.0 (6.3–14.5) | 6.5 (3.8–11.5) | 8.7 (5.6–12.3) | NS | NS |
| Supine | 7.4 (1.1–16.9) | 0.6 (0–6.9) | 0.6 (0–7.6) | p<0.01 | p<0.05 |
| Reflux events (No) | |||||
| Total 24 h | 66.5 (51.8–76.5) | 41.0 (29.3–61.5) | 62 (32.5–56.5) | p<0.01 | NS |
| Upright | 62.0 (43.0–68.3) | 34.5 (25.0–61.0) | 47.0 (28.5–70) | p=0.01 | NS |
| Supine | 6.0 (2.8–12.0) | 1.0 (0–3.3) | 4.0 (1.0–6.0) | p<0.05 | p<0.05 |
| Reflux events >5 min (No/24 h) | 6.5 (4.0–11.5) | 4.5 (2.0–10.0) | 4.0 (2.0–8.0) | NS | NS |
| DeMeester score | 38.8 (28.2–53.3) | 22.7 (11.9–33.6) | 24.1 (17.9–34.4) | p<0.01 | p<0.05 |
Data are expressed as median (interquartile range).
Figure 5.
Effect of radiofrequency energy (RFe) treatment on 24 hour ambulatory oesophageal acid exposure after six and 12 months of follow up before (Pre-RFe) and after (Post-RFe) RFe treatment. Horizontal bars indicate median values.
At 12 months, median total 24 hour acid exposure time was similar to that at six months and remained significantly lower than that pretreatment (table 2 ▶). Four patients had normal acid exposure. The significant reduction in supine acid exposure demonstrated at six months was also sustained at 12 months (p<0.05) (table 2 ▶). RFe treatment had no impact on upright acid exposure at 12 months of follow up. The improvement in DeMeester scores was also maintained at 12 months.
Clinical assessment
Medication use
All patients were on acid suppressant therapy before RFe treatment (table 1 ▶). Six months after RFe treatment, 15 of 20 patients (75%) were happy to remain off acid suppression and were on either no therapy or intermittent antacids only. Of these, four patients were completely asymptomatic and the remainder had only mild and intermittent symptoms. Of the five patients who remained on regular acid suppressant medications, three were able to control their symptoms on a lower level of acid suppression than that before RFe therapy.
At 12 months of follow up, 13 of 20 (65%) patients remained off acid suppression therapy. Two patients were completely asymptomatic and 11 had only mild occasional symptoms for which they did not feel the need to take any medication. Of the remaining patients, one is on proton pump inhibitor therapy and five are on histamine H2 receptor antagonists. One patient who remained symptomatic despite proton pump inhibitor therapy withdrew from the trial at nine months and underwent successful laparoscopic fundoplication.
Grade of oesophagitis
At time of entry into the trial, five patients had erosive or ulcerative oesophagitis (A, 2; B, 2; C, 1) while 15 had no visible erosions on medications (table 1 ▶). Most of the patients had been documented to have had more severe oesophagitis in the past by endoscopists not involved in the present study. Six months after treatment with RFe, 10 patients had no macroscopic mucosal breaks and 10 patients had mild erosive reflux oesophagitis (Los Angeles grade A).
Symptom and quality of life assessment
Both the SF-36 scale and GERD-HRQL showed significant improvement in physical and mental health as well as heartburn severity at six months (table 3 ▶). These improvements were sustained after 12 months. In addition, there was no dysphagia after RFe therapy.
Table 3.
Symptom and quality of life scores
| Pretreatment | 6 months | 12 months | Pre v 6 months | Pre v 12 months | |
| SF-36 physical score | 43 (34.5–48.3) | 50 (40.6–53) | 51.5 (46–55.5) | p<0.01 | p<0.05 |
| SF-36 mental score | 50 (40.4–56) | 57.5 (48–59.6) | 56.5 (53.8–61.3) | p<0.05 | p<0.05 |
| GERD-HRQoL score | 19.5 (14–25) | 11.5 (1.5–12.3) | 7 (2.5–11) | p<0.01 | p<0.05 |
| Dysphagia score | 45 (35.6–45) | 45 (34.1–45) | 45 (30.5–11) | NS | NS |
Data are expressed as median (interquartile range).
Effect of change in rate of TLOSRs on symptoms, medication use, and oesophagitis grade at six months
Fifteen patients exhibited a fall in the rate of TLOSRs, the majority of whom (n=12) were in symptomatic remission; three had reflux symptoms controlled with acid suppressant therapy. Half of the patients in symptomatic remission had minor erosive reflux oesophagitis (Los Angeles grade A) at six months, with the remainder showing no evidence of erosive reflux oesophagitis. Two of three patients on acid suppressant medication had Los Angeles grade A reflux oesophagitis at six months. The remaining patient had a normal endoscopy at six months of follow up. Of the five patients who exhibited an increase in the rate of TLOSRs at six months, two had relapsed symptomatically and required acid suppressant medication. Of these, one (on a proton pump inhibitor) had no detectable erosions and the other (on a histamine receptor antagonist) had Los Angeles grade A oesophagitis (grade B pretreatment).
Effect of normalisation of 24 hour acid exposure on symptoms, medication use, and oesophagitis grade
Of the seven patients with normal acid exposure at six months, five were asymptomatic but two remained symptomatic and required regular acid suppressant medications. Four patients had a normal endoscopy post-treatment; three had Los Angeles grade A reflux oesophagitis. At 12 months, one patient continued to use a proton pump inhibitor for characteristic reflux symptoms despite a normal acid exposure while the other three patients were asymptomatic off medication.
Complications
Early in our experience, one patient required readmission for management of post- procedural pain. Subsequent investigations with a computed tomography scan and barium swallow excluded an oesophageal perforation. The clinical findings were consistent with mediastinal inflammation, and he was successfully managed conservatively with analgesic and antibiotic therapy.
DISCUSSION
In this study we investigated the effects of RFe treatment on reflux mechanisms and found that RFe had significant effects on LOS function; the rate of TLOSRs was reduced and postprandial basal LOS pressure was increased. These effects were associated with a reduction in reflux events and oesophageal acid exposure. Thus RFe treatment alters LOS function in a manner that enhances its antireflux capability.
The major finding in our study was that RFe treatment reduced the rate of TLOSRs significantly, by 24% when assessed during a three hour postprandial period. We also found that RFe inhibited TLOSRs in dogs.10 Consistent with previous observations,20 TLOSR was the major mechanism of reflux, both before and after treatment, and the reduction in TLOSRs was the major reason for the reduction in reflux episodes. Although RFe decreased the number of TLOSRs, it did not alter the overall mix of reflux mechanisms, probably because TLOSR was by far the dominant mechanism.
A recent study in humans did not find a statistically significant reduction in the rate of TLOSRs8 after RFe treatment, although a trend was observed. The different findings in the two human studies may be related to different methodologies. Our study used a meal to stimulate TLOSRs. This closely simulates the normal pattern of reflux, which in most patients is predominantly postprandial.21 In contrast, Dibaise and colleagues8 used abrupt gaseous gastric distension as the stimulus for triggering TLOSRs. Not only is this a very potent stimulus that may have overridden any inhibitory effect of RFe, the distension volume may have dissipated well before the end of the 60 minute monitoring period.
The mechanism by which RFe treatment decreased the rate of TLOSRs is not clear and was not specifically investigated by this study. TLOSRs are triggered predominantly by gastric distension and mediated through vagal pathways integrated by a pattern generator located in the vagal complex in the brainstem.20 RFe could interfere with triggering of TLOSRs by several potential mechanisms. Firstly, intramural lesions could interrupt neural signalling of distension, either by ablating mechanoreceptors at the gastric cardia or by interrupting the afferent nerves carrying signals to brainstem control centres. Interference with the efferent motor pathway seems less likely as there was no effect on swallow induced LOS relaxation, although the swallow stimulus might override a partial inhibitory effect. The most important region of the stomach for triggering TLOSRs is around the gastric cardia.22 RFe lesions heal by fibrosis and contraction,2 and could thereby have altered the mechanics of the gastric cardia, resulting in less distension and therefore fewer stimuli to the mechanoreceptors. Each/both mechanism(s) could contribute in an individual. More precise determination of the mechanisms by which RFe treatment decreases the rate of TLOSRs deserves further study.
RFe treatment was associated with an increase in postprandial basal LOS pressure. Similar to previous studies1 however we did not find any significant effect on fasting basal LOS pressure. The reasons for the apparently different effects on fasting and postprandial LOS pressure are not clear but may relate to the effects of the meal on basal LOS pressure and perhaps also differences in the timing and approach to measurement of LOS pressure. We measured LOS pressure throughout a three hour period whereas in the previous studies measurements were taken at only one point in time. In absolute terms, the increase in basal LOS pressure was relatively small, only about 3–5 mm Hg. However, this modest increase may nevertheless be functionally significant. Relatively low basal LOS pressure is sufficient to control reflux.23,24 Thus a small increase in basal LOS pressure could transform a sphincter from being functionally incompetent during straining to one that is competent, with resultant significant impact on reflux that occurs as a consequence of absent LOS pressure, particularly if there is concurrent inhibition of TLOSRs. While a reduction in the number of postprandial reflux episodes due to absent basal LOS pressure was seen, the small number of events precluded statistical analysis. Definition of the mechanism for the increase in basal LOS pressure was not an aim of the study. RFe causes thermal injury and subsequent healing and fibrosis of sphincteric muscle. A recent endoscopic ultrasonographic study in a porcine model suggests that the LOS muscle layer is significantly thickened after RFe treatment,25 which may in turn result in altered mechanics of the gastro-oesophageal junction which could improve antireflux function. The effect on basal LOS pressure was evident only in the second and third postprandial hours. The reasons for this are not clear. It is possible that the normal drop in LOS pressure that occurs postprandially obscured or lessened the increase caused by RFe, and that as pressure increased in the late postprandial period the effect became more apparent. However, this does not explain why basal LOS pressure before the meal was similar before and after treatment.
This study also evaluated the patterns of reflux with ambulatory 24 hour pH monitoring. RFe treatment significantly reduced the rate of reflux events and oesophageal acid exposure at six months, and normalised levels in 35% of patients. The significant reduction in total and recumbent oesophageal acid exposure at six months was sustained after 12 months of follow up. The reduction in acid exposure was less marked, perhaps because a major determinant of this variable is acid clearance, which can vary considerably among patients and episodes and which was not altered by RFe treatment. The mechanisms responsible for the reduction in ambulatory reflux were not specifically assessed. However, by extrapolation from the postprandial studies, it is likely that both a decrease in the rate of TLOSRs and an increase in basal LOS pressure may be involved. An increase in basal LOS pressure is likely to be more important in the reduction in recumbent reflux as TLOSRs are relatively infrequent during this period.
Although it was not the primary aim of the study and the study design did not include appropriate controls for this aspect, we also assessed the effect of RFe treatment on clinical measures of reflux disease. Consistent with previous reports1 RFe treatment resulted in a substantial reduction in reflux symptoms. At follow up, 75% of patients were in clinical remission at six months and 65% at 12 months. This was reflected in a reduced need for acid suppressant medications as well as improvement in general quality of life scores. All patients had found acid suppressant therapy necessary before treatment; most (75%) were off all therapy at six months. The corresponding endoscopic findings are difficult to assess as all patients were on acid suppression at time of entry into the trial. Nevertheless, despite most patients being off therapy at six months, only mild erosive reflux oesophagitis (Los Angeles grade A) was noted in half of the patients while the remainder had no visible mucosal breaks. Although the data are uncontrolled, we believe that it is unlikely that the symptomatic improvement can be explained entirely by a placebo effect. The level of symptomatic remission in the present study is substantially higher than that reported for the placebo maintenance treatment of patients with reflux oesophagitis (24–52% at six months26,27 and 34% at 12 months).28
RFe treatment was well tolerated by 19 of our 20 patients. One major complication arose in a patient who had a small hiatus hernia which may have contributed to the development of signs of mediastinal inflammation, although there was no sign of visceral perforation on computed tomography scan and barium study. No other complication was seen.
In summary, our study demonstrates that RFe treatment has significant effects on LOS function that are associated with improvement in the antireflux barrier. Uncontrolled clinical data also suggest a beneficial effect in the control of reflux symptoms in these patients. Further studies are needed to evaluate the mechanism of effects on transient lower oesophageal sphincter relaxations, and to gather placebo controlled data with long term follow up.
Abbreviations
RFe, radiofrequency energy
LOS, lower oesophageal sphincter
GORD, gastro-oesophageal reflux disease
TLOSRs, transient LOS relaxations
QoL, quality of life
REFERENCES
- 1.Triadafilopoulos G, Dibaise JK, Nostrant TT, et al. Radiofrequency energy application to the gastroesophageal junction for the treatment of GERD. Gastrointest Endosc 2001;53:407–15. [DOI] [PubMed] [Google Scholar]
- 2.Utley DS, Kim M, Vierra MA, et al. Augmentation of lower esophageal sphincter pressure and gastric yield pressure after radiofrequency energy delivery to the esophagogastric junction: a porcine model. Gastrointest Endosc 2000;52:81–6. [DOI] [PubMed] [Google Scholar]
- 3.Morady F. Drug therapy: radio-frequency energy ablation as treatment for cardiac arrhythmias. N Engl J Med 1999;340:534–44. [DOI] [PubMed] [Google Scholar]
- 4.Issa M, Oesterling J. Transurethral needle ablation (TUNA): an overview of radiofrequency thermal therapy for the treatment of benign prostatic hyperplasia. Curr Opin Urol 1996;6:20–7. [Google Scholar]
- 5.LeVeen H, Wapnick S, Piccone V, et al. Tumour eradication by radiofrequency energy therapy: response in 21 patients. JAMA 1976;253:2198–200. [PubMed] [Google Scholar]
- 6.Powell NB, Riley RW, Troell RW, et al. Radiofrequency volumetric tissue reduction of the palate in subjects with sleep-disordered breathing. Chest 1998;113:1163–74. [DOI] [PubMed] [Google Scholar]
- 7.Hecht P, Hayashi K, Cooley AJ, et al. The thermal effect of monopolar radiofrequency energy on the properties of joint capsule. An in vivo histologic study using a sheep model. Am J Sports Med 1998;26:808–14. [DOI] [PubMed] [Google Scholar]
- 8.DiBaise JK, Brand RE, Quigley EM. Endoluminal delivery of radiofrequency energy to the gastroesophageal junction in uncomplicated GERD: efficacy and potential mechanism of action. Am J Gastroenterol 2002;97:833–42. [DOI] [PubMed] [Google Scholar]
- 9.Holloway RH. The anti-reflux barrier and mechanisms of gastroesophageal reflux. Baillieres Clin Gastroenterol 2000;14:681–91. [DOI] [PubMed] [Google Scholar]
- 10.Kim MS, Holloway RH, Dent J, et al. Radiofrequency energy delivery to the gastric cardia inhibits triggering of transient lower esophageal sphincter relaxation in a canine model. Gastrointest Endosc 2003;57:17–22. [DOI] [PubMed] [Google Scholar]
- 11.Armstrong D, Bennett JR, Blum AL. The endoscopic assessment of esophagitis: a progress report on observer agreement. Gastroenterology 1996;111:85–92. [DOI] [PubMed] [Google Scholar]
- 12.Velanovich V. Comparison of generic (SF-36) vs. disease-specific (GERD-HRQL) quality-of-life scales for gastroesophageal reflux disease. J Gastrointest Surg 1998;2:141–5. [DOI] [PubMed] [Google Scholar]
- 13.Dakkak M, Bennett J. A new dysphagia score with objective evaluation. J Clin Gastroenterol 1992;14:99–100. [DOI] [PubMed] [Google Scholar]
- 14.Holloway RH, Penagini R, Ireland AC. Criteria for objective definition of transient lower esophageal sphincter relaxation. Am J Gastroenterol 1995;268:G128–33. [DOI] [PubMed] [Google Scholar]
- 15.Sifrim D, Holloway RH, Missotten T, et al. Swallow-induced abnormally prolonged lower esophageal sphincter relaxations (SAPLESRs) (abstract). Gastroenterology 1998;114:A838. [Google Scholar]
- 16.van Herwaarden MA, Samsom M, Smout AJ. Excess gastroesophageal reflux in patients with hiatus hernia is caused by mechanisms other than transient LES relaxations. Gastroenterology 2000;119:1439–46. [DOI] [PubMed] [Google Scholar]
- 17.Dent J, Holloway RH, Toouli J, et al. Mechanisms of lower esophageal sphincter incompetence in patients with symptomatic gastroesophageal reflux. Gut 1988;29:1020–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Penagini R, Schoeman MN, Dent J, et al. Motor events underlying gastro-oesophageal reflux in ambulant patients with reflux esophagitis. Neurogastroenterol Motil 1996;8:131–41. [DOI] [PubMed] [Google Scholar]
- 19.Kawahara H, Dent J, Davidson G. Mechanisms responsible for gastroesophageal reflux in children. Gastroenterology 1997;113:399–408. [DOI] [PubMed] [Google Scholar]
- 20.Mittal RK, Holloway RH, Penagini R, et al. Transient lower esophageal sphincter relaxation. Gastroenterology 1995;109:601–10. [DOI] [PubMed] [Google Scholar]
- 21.Johnsson L, Adlouni W, Johnsson F. Timing of reflux symptoms and esophageal acid exposure. Gullet 1992;2:58–62. [Google Scholar]
- 22.Franzi SJ, Martin CJ, Cox MR, et al. Response of canine lower esophageal sphincter to gastric distension. Am J Physiol 1990;259:G380–5. [DOI] [PubMed] [Google Scholar]
- 23.Dent J, Dodds WJ, Hogan WJ, et al. Factors that influence induction of gastroesophageal reflux in normal human subjects. Dig Dis Sci 1988;33:270–5. [DOI] [PubMed] [Google Scholar]
- 24.Sloan S, Rademaker AW, Kahrilas PJ. Determinants of gastroesophageal junction incompetence: Hiatal hernia, lower esophageal sphincter, or both? Ann Intern Med 1992;117:977–82. [DOI] [PubMed] [Google Scholar]
- 25.Chang KJ, Utley DS. Endoscopic ultrasound (EUS) in-vivo assessment of radiofrequency (RF) energy delivery to the gastroesophageal junction in a porcine model. Gastrointest Endosc 2001;53:AB165. [Google Scholar]
- 26.Laursen LS, Havelund T, Bondesen S, et al. Omeprazole in the long-term treatment of gastro-oesophageal reflux disease. Scand J Gastroenterol 1995;30:839–46. [DOI] [PubMed] [Google Scholar]
- 27.Hatlebakk JG, Johnsson F, Vilien L, et al. The effect of cisapride in maintaining symptomatic remission in patients with gastro-oesophageal reflux disease. Scand J Gastroenterol 1997;32:1100–6. [DOI] [PubMed] [Google Scholar]
- 28.Bate VM, Booth SN, Crowe JP, et al. Omeprazole 10 mg or 20 mg once daily in the prevention of recurrence of reflux oesophagitis. Gut 1995;36:492–8. [DOI] [PMC free article] [PubMed] [Google Scholar]