Abstract
Background and aims: The role of sensory neurones in colitis was studied by chemical denervation of primary sensory neurones as well as antagonism of the vanilloid receptor-1 (VR-1) in rats prior to administration of dextran sulphate sodium (DSS) to induce colitis.
Methods: Neonatal rats were chemically denervated by subcutaneous administration of capsaicin; controls received capsaicin vehicle only. When animals reached maturity, colitis was induced by administration of 5% DSS in drinking water for seven days. Additionally, normal adult rats were treated with a VR-1 antagonist capsazepine (CPZ) or vehicle twice daily via an enema from day 0 to day 6 of the DSS regimen. Control rats were treated with an enema infusion of vehicle and 5% DSS, or without either an enema infusion or DSS in drinking water. For both groups of rats, severity of inflammation was quantitated by disease activity index (DAI), myeloperoxidase (MPO) activity, and histological examination.
Results: DSS induced active colitis in all control rats with resultant epithelial ulceration, crypt shortening, and neutrophil infiltration. Both neonatal capsaicinised rats and normal adult rats treated with CPZ enemas exhibited significantly lower levels of DAI, MPO, and histological damage compared with vehicle treated rats (p< 0.05).
Conclusions: Neonatal capsaicinisation and local administration of CPZ prevents intestinal inflammation in a well established model of colitis indicating that primary sensory neurones possessing VR-1 receptors are required in the propagation of colonic inflammation.
Keywords: vanilloid receptor, capsaicin, capsazepine, sensory neurones, colitis
The enteric nervous system regulates multiple physiological functions in the gut, including inflammation. Stimulation of primary sensory neurones results in neurogenic inflammation, a term referring to the transmission of nociceptive information to the spinal cord as well as stimulation of local inflammatory and immune responses in the peripheral tissues via an axon reflex. This reflex arc is controlled by specific neurotransmitters and their respective receptors. Both our group and others have shown that substance P (SP), an undecapeptide found in the periphery and central nervous system, is critical in the propagation of the inflammatory cascade in acute models of intestinal inflammation.1,2 Specifically, pretreatment of rats with SP receptor antagonists inhibited toxin A induced acute enterocolitis and mice deficient in the SP receptor (neurokinin 1 receptor) were protected from the inflammatory effects of toxin A.3 Additionally, ablation of primary sensory nerves either chemically by administration of capsaicin1 or surgically4 resulted in protection from toxin A mediated inflammation and intestinal secretion. However, it is unclear how intraluminal toxins or bacteria stimulate primary sensory neurones to initiate neurogenic inflammation.
Recently, cDNA found to encode the vanilloid receptor subtype-1 (VR-1) has been cloned and was found to be a non-selective cation channel expressed primarily in primary sensory neurones.5 VR-1 is activated by heat, acid, capsaicin (the active ingredient in hot chili peppers), and possibly through an endogenous ligand. VR-1 stimulation results in the release of SP and other proinflammatory neuropeptides, which results in the initiation of the neurogenic inflammatory response. In addition, capsazepine (CPZ), a synthetic analogue of capsaicin, has been shown to be an effective antagonist of VR-1 in vitro.6 Recently, CPZ has also been proved to inhibit the acute inflammation effects of toxin A induced enterocolitis, implicating VR-1 in the initiation of acute intestinal inflammation.7
In this study, we examined the effect of capsaicin induced primary sensory neurone denervation and local administration of CPZ in a well described colitis animal model. Dextran sulphate sodium (DSS) given in the water of rats for seven days rapidly produces bloody watery diarrhea, and induces a colitis which resembles ulcerative colitis in humans. We propose that chemical denervation of primary sensory nerves of the colon or local antagonism of VR-1 would be protective in this colitis model.
MATERIALS AND METHODS
Sensory denervation by capsaicinisation
Time dated pregnant Sprague-Dawley rats (Charles Rivers Laboratories, Raleigh, North Carolina, USA) were housed with free access to food and water until delivery. On day 1 of life, neonatal pups were weighed and chemically injected subcutaneously with 50 mg/kg capsaicin (n=10) (Sigma Chemical Co., St Louis, Missouri, USA) in phosphate buffered saline (pH 7.4) containing 10% Tween 80 and 10% ethanol, as previously described.8 This technique is a well established method of denervation allowing destruction of over 90% of sensory nerves. A subgroup of animals was injected with vehicle only for control purposes (Tween 80 and 10% ethanol) (n=10). Animals were allowed to develop normally and when they reached 200 g body weight (approximately 40 days old) they were tested for sensory denervation by applying an alkaline solution to the eye; absence of blinking or scratching confirmed sensory denervation. All aspects of this research were approved by the institution’s animal care and use committee.
Capsazepine enema
A subset of adult Sprague-Dawley rats (200–250 g) was used to investigate the effects of local application of CPZ (Sigma Chemical Co.). Rats were first lightly anaesthetised with ketamine (40 mg/kg) and xylazine (10 mg/kg). A flexible catheter (OD 4 mm) was then inserted exactly 4 cm from the anus (corresponding to the descending colon) and 1 ml of saline was infused and a massage of the abdomen was performed to remove any faecal matter. Enema administration of 0.5 ml CPZ (100 μg) dissolved in 10% Tween/10% ethanol (n=10) or vehicle (n=10) was performed twice daily from day 0 to 6 of the DSS regimen (see below). Rats were maintained in a head down position to prevent leakage of solution. Control rats were treated with enema infusion of vehicle (n=10), without either enema infusion (n=10).
Induction of colitis
Capsaicinised and vehicle injected rats as well as CPZ and CPZ vehicle-enema animals were exposed to chronic administration of DSS or water alone. Briefly, 5% DSS (molecular weight 5000 kDa; Sigma) was administrated in drinking water for seven days and daily measurements of weight, stool frequency, and guaiac testing were performed. All animals were housed under standard laboratory conditions (23°C, 12 hour light-dark cycles) and fed with regular rodent pellets.
Disease activity index
Disease activity index (DAI) was used to evaluate grade and extent of intestinal inflammation based on a previously published grading system9 (table 1 ▶). The score ranges from 0 to 4 (total score), which represents the sum of scores for weight loss, stool consistency, and rectal bleeding divided by three. DAI has been shown to be well correlated with tissue damage scores and with specific measurements of inflammation such as myeloperoxidase (MPO) activity index.10
Table 1.
Disease activity index (DAI) scoring system
| Score | Weight loss | Stool consistency | Occult/gross rectal bleeding |
| 0 | None | Normal | Normal |
| 1 | 1–5% | ||
| 2 | 5–10% | Loose stools | Haemoccult+ |
| 3 | 10–20% | ||
| 4 | >20% | Diarrhoea | Gross bleeding |
Clinical criteria used to evaluate grade of extent of intestinal inflammation. Scores were tallied for each category and then divided by three to obtain the DAI.
MPO activity index
Segments of proximal and distal colon were removed after euthanasia and stored frozen at −80°C. Specimens were weighed, place in a plastic tube on ice, and 0.5% of hexadecyltrimethyllammonium bromide (HTAB) in 50 mM KH2PO4 (pH 6) (HTAB buffer) was added to each sample. Samples were homogenised on ice using a Polytron tissue homogeniser for 15 seconds followed by three cycles of freeze/thawing. All samples were fortified with additional HTAB buffer to equal 1 ml HTAB/50 mg wet weight. Samples were then vortexed and 500 μl of each were transferred to microfuge tubes. The tubes were centrifuged in an Eppendorf microfuge (12 000 g) at 4°C for two minutes and absorbance of each supernatant read at 460 nm at 0, 30, and 60 seconds after addition of 2.9 ml of 0.167 mg/ml O-dianisidine dihydrochloride to 0.1 ml of supernatant. One unit of MPO activity was defined as degradation of 1 mol of peroxide per minute at 25°C; results are expressed in units per gram of protein.
Histology
Following seven days of 5% DSS administration, rats were killed and sections of caecum, transverse colon, and descending colon were removed and placed in paraformaldehyde. Specimens were then paraffin embedded and subsequently cut into 5 μM sections. Sections were stained with haematoxylin and eosin (H&E) and crypt height was measured using a published technique.11 Briefly, an eyepiece micrometer calibrated with a stage micrometer was used to examine six well oriented crypts from four quadrants of each cross section. The average for each animal was used in computing an average crypt height. Previous results indicate that DSS administration causes a 40% decrease in crypt height.11 A colitis score was also used to determine the extent of the inflammation based on a previously published grading system.1 H&E prepared sections were given a colitis activity score in a blinded fashion. Scores ranged from 0 to 14 (total score), which represents the sum of scores from 0 to 4 for severity of extent, damage, inflammation, and regeneration (table 2 ▶).
Table 2.
Histological colitis scoring system
| Feature score | Score | Description |
| Inflammation severity | 0 | None |
| 1 | Mild | |
| 2 | Moderate | |
| 3 | Severe | |
| Inflammation extent | 0 | None |
| 1 | Mucosa | |
| 2 | Submucosa | |
| 3 | Transmural | |
| Crypt damage | 0 | None |
| 1 | Basal 1/3 damage | |
| 2 | Basal 2/3 damage | |
| 3 | Crypt lost; surface epithelium present | |
| 4 | Crypt and surface epithelium lost | |
| Per cent involvement | 0 | 0% |
| 1 | 1–25% | |
| 2 | 26–50% | |
| 3 | 51–75% | |
| 4 | 76–100% |
Histological criteria to evaluate severity of inflammation. The score ranges from 0 to 14 (total score), which represents the sum of scores from 0 to 4 for severity and extent of inflammation, crypt damage, and per cent of the colon involved. All evaluations were performed by observers unaware of the treatment groups.
Statistical analysis
Results are expressed as mean (SEM). Differences among groups were examined by one way ANOVA with Dunnett’s test when comparing treatment versus control groups or the Tukey-Kramer test when comparing all groups. Statistical analysis was done using GraphPad Instat version 3.00 for Windows 95 (GraphPad Software, San Diego, California, USA). Significance was assumed to occur at p<0.05.
RESULTS
Disease activity index (DAI)
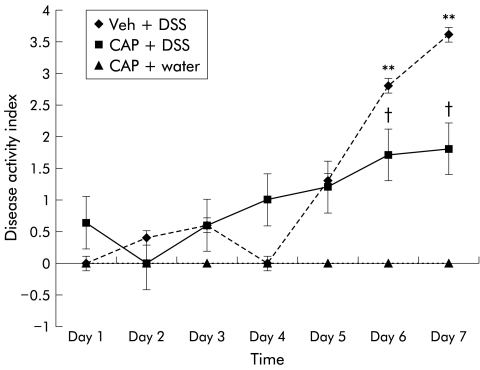
Neonatally capsaicinised rats exhibited significantly lower DAI levels compared with vehicle treated rats (fig 1 ▶) given DSS. The differences between groups reached statistical significance on day 6 of the DSS regimen (1.7 (0.4) v 2.8 (0.1); p<0.05). In addition, higher levels of DAI were observed in vehicle pretreated rats exposed to DSS compared with neonatally capsaicinised rats that received water alone (p<0.01) (fig 1 ▶). The difference between these groups was evident on days 6 and 7 of the study period. Finally, no differences were observed between capsaicinised rats and vehicle treated animals when water alone was dispensed; the latter group has been omitted from the figure for clarity. It should be noted that DAI only increased after four days of DSS exposure, an observation that has been noted in previous reports.12
Figure 1.
Effects of neonatal capsaicisation on disease activity index following administration of 5% dextran sulphate sodium (DSS) in drinking water. Statistical significance was achieved at day 6 between capsaicinised animals and vehicle treated animals (Veh) (†p<0.05). Differences between vehicle treated rats exposed to DSS and capsaicin treated rats exposed to water were highly significant on days 6 and 7 (**p<0.01). Vehicle treated rats that received water alone were omitted for clarity. CAP, capsaicinised rats.
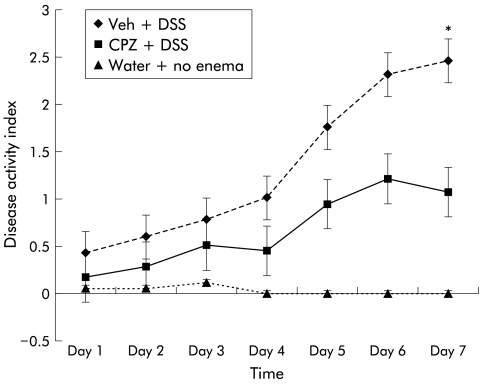
DAI was increased daily in rats treated with vehicle enema plus DSS and CPZ enema plus DSS compared with rats treated with no enema and water. However, the DAI score for CPZ enema plus DSS was significantly less on day 7 compared with vehicle enema plus DSS rats (1.06 (0.2) v 2.4 (0.3); p<0.05) (fig 2 ▶). The only DAI parameter that increased in the DSS+CPZ enema group was bleeding (either haemoccult with or without gross bleeding), which was likely from enema (vehicle or CPZ containing) trauma.
Figure 2.
Disease activity index (DAI) in rats treated with enema application of capsazepine (CPZ). DAI increased daily in rats treated with vehicle enema (Veh) plus dextran sulphate sodium (DSS) so that by day 7 of the DSS regimen, DAI differences were significantly higher compared with rats treated with CPZ and DSS (*p<0.05). The only DAI parameter that increased in the DSS+CPZ enema group was bleeding (either haemoccult with or without gross bleeding), which was likely from enema (vehicle or CPZ containing) trauma.
MPO activity
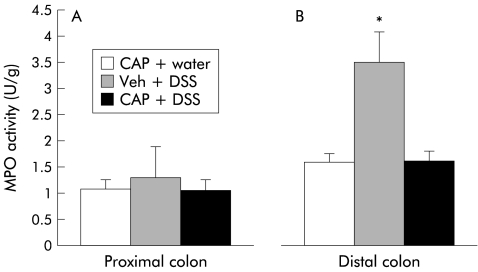
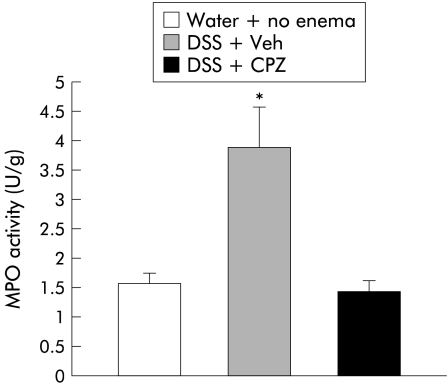
MPO activity is a useful method for evaluating granulocyte infiltration in colonic tissues following induction of colitis.13 Administration of 5% DSS in drinking water induces intestinal inflammation predominantly in the left (distal) side of the colon. No differences were found in the proximal colon between control and DSS treated rats (0.60 (0.06) v 0.68 (0.20) U/g; NS) while MPO activity within the distal colon was significantly higher in animals exposed to DSS (0.93 (0.15 ) v 2.08 (0.31) U/g; p<0.05). With regards to the capsaicin treatment groups, MPO activity in the proximal colon resulted in comparable values among all groups (fig 3A ▶) (CAP+water, 1.08 (0.17) U/g v Veh+DSS, 1.29 (0.59) U/g v CAP+DSS, 1.05 (0.2) U/g; NS). No differences were noted between capsaicinised or vehicle treated animals following DSS or water administration. However, MPO activity in the distal colon was significantly lower in capsaicinised rats in comparison with vehicle treated animals (1.58 (0.24) U/g v 3.49 (0.59) U/g; p<0.05) (fig 3B ▶). As displayed in the figure, no differences were noted in capsaicinised rats when DSS or water alone was dispensed. With respect to CPZ treated rats, MPO activity was significantly increased in vehicle enema plus DSS rats compared with either CPZ enema plus DSS (3.90 (0.69) v 1.45 (0.20); p<0.05) or no enema and water rats (3.90 (0.69) v 1.58 (0.17); p<0.05) (fig 4 ▶).
Figure 3.
Myleoperoxidase (MPO) activity in neonatally capsaicinised rats of proximal and distal colonic segments in a dextran sulphate sodium (DSS) model of colitis. (A) MPO activity in the proximal colon of different treatment groups. Capsaicinisation protected the distal colon of animals exposed to administration of DSS (B). The difference between vehicle treated (Veh) and capsaicin treated rats was statistically significance (*p<0.05). CAP, capsaicinised rats.
Figure 4.
Effects of enema infusion of capsazepine (CPZ) in rats exposed to dextran sulphate sodium (DSS). Animals treated with CPZ vehicle enema (Veh) plus DSS exhibited significantly higher levels of myeloperoxidase (MPO) activity compared with rats who received CPZ and DSS (*p<0.05). No differences were observed between controls (water+no enema) and local application of CPZ and DSS exposure.
Histology
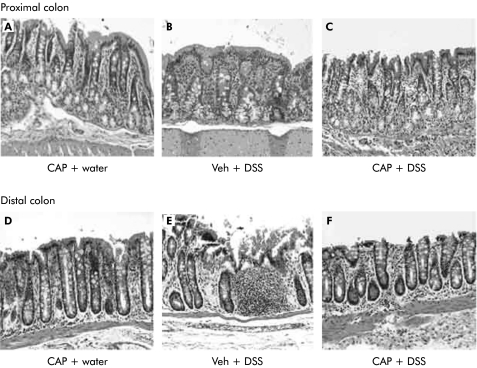
Paraffin embedded proximal and distal segments of the colon were stained with H&E and histologically evaluated for signs of inflammation. Histological examination of the proximal segments of colon revealed mild inflammation in vehicle treated animals provided 5% DSS, manifested by oedema and congestion (fig 5B ▶). Similar patterns were documented in the proximal colons of neonatal capsaicinised rats exposed to either water or 5% DSS (fig 5A, C ▶).
Figure 5.
Paraffin embedded proximal and distal segments of the colon stained with haematoxylin and eosin (10×). Specimens were paraffin embedded and subsequently cut into 5 μM sections. Histological examination of the proximal segments revealed mild inflammation in vehicle treated animals (Veh+dextran sulphate sodium (DSS)(B)), manifested by congestion and oedema. Similar patterns were documented in the proximal colonic segments of neonatal capsaicinised rats (CAP) exposed to either DSS (CAP+DSS (C)) or water (CAP+water (A)). Neonatal capsaicinisation provided protection in the distal colon to rats exposed to DSS (CAP+DSS (F)). However, animals treated with vehicle alone (Veh+DSS (E)) experienced marked intestinal inflammation characterised by focal erosion of the epithelium, oedema, and crypt shortening with inflammatory cells infiltrating the submucosa and lamina propria including neutrophils and lymphocytes.
The protective effects of capsaicinisation were marked in the distal portions of the colon. In vehicle injected rats, the inflammatory patterns observed included focal erosion of the epithelium, oedema and congestion, and crypt shortening with various degrees of inflammatory cells infiltrating the submucosa and lamina propria, including neutrophils and lymphocytes (fig 5E ▶). Neonatal capsaicinisation almost completely abolished the damaging effects of DSS; the histological features observed were similar to those of capsaicinised animals receiving water alone (fig 5D, F ▶).
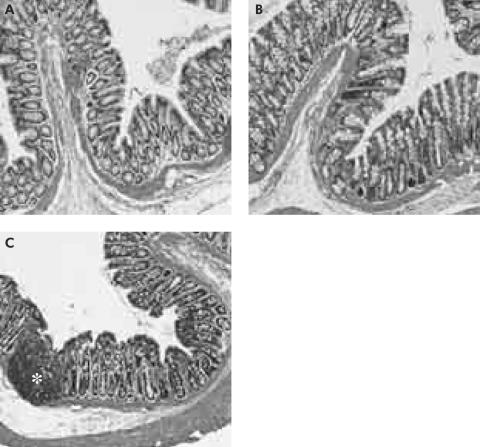
In addition, CPZ enemas provided local protection of DSS induced colitis. Epithelial damage, oedema, neutrophil infiltration, and crypt shortening in the distal colon were reduced by enema infusion of CPZ in comparison with DSS and vehicle enema administration (fig 6 ▶).
Figure 6.
Effect of local capsazepine (CPZ) on distal colon of dextran sulphate sodium (DSS) rats (100×). CPZ enemas provided local protection of DSS induced colitis (A, B). Epithelial damage, oedema, neutrophil infiltration (*), and crypt shortening in the distal colon were reduced by enema infusion of CPZ in comparison with DSS and vehicle enema administration (C).
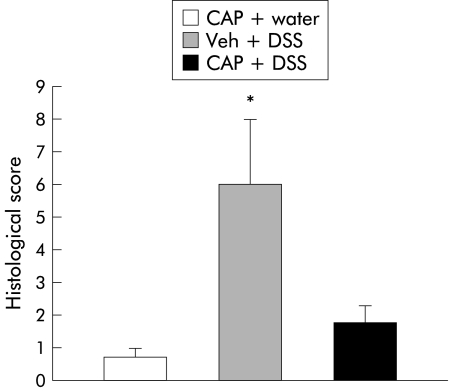
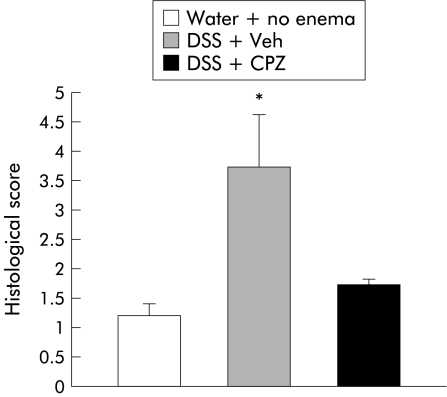
In order to quantitate the severity of inflammation, a previously published grading system1 was employed. Blinded observers unaware of the treatment groups scored H&E stained sections of both capsaicinised and CPZ treated animals. Scores ranged from 0 to 14 (total score), which represents the sum of scores from 0 to 4 for severity of extent, damage, inflammation, and regeneration (table 2 ▶). Results expressed as mean (SEM) are displayed in figs 7 and 8 ▶ ▶. Higher levels of distal colonic inflammation severity were found in vehicle injected rats compared with capsaicinised animals that received either DSS or water (2.66 (0.57) v 0.43 (0.20), 0.44 (0.13); p<0.05). Although a higher trend in crypt damage was observed in the vehicle-DSS group, values did not reach statistical significance. Inflammation severity, extent, or crypt damage patterns were similar among groups in the proximal segments of the colon. Finally, the total colitis score was also significantly greater in vehicle treated animals (6.04 (1.98) v 1.82 (0.52), 0.75 (0.25); p<0.05) (fig 7 ▶). Histology in vehicle enema plus DSS rats demonstrated signs of active colitis resulting in a significant increased colitis score compared with rats treated with CPZ enemas plus DSS (3.7 (0.9) v 1.7 (0.1); p<0.05) or no enemas and water (3.7 (0.9) v 1.2 (0.2); p<0.05) (fig 8 ▶).
Figure 7.
Neonatal capsaicin denervation protected animals from the damaging effects of dextran sulphate sodium (DSS) administration. Histological scores based on a well established scoring system were higher in rats who received capsaicin vehicle (Veh) plus DSS in comparison with those neonatally denervated that received either water or DSS (*p<0.05). CAP, capsaicinised rats.
Figure 8.
Protective actions of local capsazepine (CPZ) infusion in rats exposed to dextran sulphate sodium (DSS). Histological score levels were significantly elevated in vehicle treated animals (Veh) compared with animals treated with CPZ that were exposed to DSS (*p<0.05). Although higher histological scores were observed in animals administered CPZ plus DSS compared with control animals (water+no enema), the differences did not reach significance. This trend might have been due to colonic trauma related to the enema.
DISCUSSION
In this study, we present direct evidence that primary sensory neurones containing VR-1 are required to propagate a well known model of colitis. Rats receiving continuous oral administration of 5% DSS reproducibly developed epithelial ulceration, crypt shortening, and neutrophil infiltration, reminiscent of active ulcerative colitis. Rats neonatally denervated with subcutaneous injection of capsaicin, a neurotoxin specific to primary sensory neurones, and rats treated with local CPZ enemas, a VR-1 competitive antagonist, were protected from the damaging effects of administration of DSS. Taken together, these data provide strong evidence that an animal model of colitis requires primary afferent neurones containing VR-1.
The enteric nervous system appears to be pivotal in intestinal secretory and inflammatory states including cholera toxin, Escherichia coli heat labile toxin, and heat stable toxin.14 Additionally, enterocolitis caused by toxin A, the enterotoxin secreted by Clostridium difficile, is effectively inhibited by lidocaine, hexamethonium, and capsaicin,14 indicating the role of primary sensory neurones in the propagation of inflammation. Both our group and others have used the highly reproducible and rapid model of toxin A induced acute enteritis to dissect the pathway of neurogenic inflammation.1,15 Previously, we demonstrated that neonatal abolition of primary sensory neurones using capsaicin protected the adult rat from toxin A enteritis. Additionally, pretreatment of adult rats with an SP (a proinflammatory and nociceptive peptide found in primary sensory neurones) receptor antagonist also prevented toxin A induced intestinal inflammation. Using confocal microscopy, we directly demonstrated that toxin A causes agonist induced internalisation of the SP receptor, which was abolished by both an SP receptor antagonist as well as neonatal capsaicin treatment.1 Further confirmation of this mechanism of intestinal neurogenic inflammation was provided by the protective effects of surgical denervation of the primary sensory neurones to a loop of intestine injected with toxin A.16 Finally, mice genetically deficient in the SP receptor were protected from the secretory and inflammatory effects of toxin A.3 Collectively, these studies suggested that an intraluminal bacterial toxin binds to villus enterocytes, which stimulate primary sensory neurones to release proinflammatory peptides such as SP. However, the mechanism by which a bacterial toxin may stimulate sensory neurones to release proinflammatory peptides remains elusive.
Recently, VR-1, a non-selective cation channel expressed in primary sensory neurones, has been sequenced and cloned.5 Stimulation of VR-1 results in excitation of primary sensory neurones and release of SP, which in turn results in nociception and inflammation.17 Additionally, CPZ, a synthetic analogue of capsaicin, was developed and effectively blocked agonist stimulation of VR-1. A recent report demonstrated that pretreatment with CPZ protected rats from the toxin A induced acute ileitis in a dose dependent manner.18 Similar studies on a chronic model of inflammation have not been previously performed.
DSS induced colitis is a reliable animal model of experimental colitis to study acute and chronic inflammation changes, which resembles chronic ulcerative colitis in both symptoms and histology.19–21 This model was chosen over a variety of other chemicals used to induce colonic inflammation such as 2,4,6-trinitrobenzenesulphonic acid,22–24 acetic acid, phorbol ester, sulphated polysaccharides,25 and formalin26,27 which are limited by the lack of chronicity and rapid colonic healing. The DSS induced colitis model provides inflammation limited to the mucosa layer and it is closely related to ulcerative colitis in humans.28 DSS is toxic to the basal crypt cells and causes slow regeneration of the colonic epithelium, and impairs phagocytosis from saturated macrophages enabling toxic bacterial products to further perpetuate intestinal inflammation.29 Histological features in the acute phase are mucosal erosions, crypt shortening, oedema, and infiltration of neutrophils in the mucosa and lamina propria, and in the chronic phase are lymphoid aggregates, ulcerations, and mucosal atrophy,19 which at least in the rat are mainly confined to the distal colon. DSS initially induces acute colonic injury, characterised by non-inflammatory epithelial damage, which causes acute inflammation in the mucosa. The most intense inflammation, as measured by the DAI, occurs after seven days of DSS administration.10 Colonic regeneration and concomitant chronic colitis develop after stopping administration of DSS. It is assumed that the extent of acute inflammation affects the following chronic inflammatory changes.
A recent publication demonstrated that DSS colitis could be significantly attenuated by pretreatment with an SP receptor antagonist,12 again suggesting a neurogenic component to this model of colitis. In the present study, ablation of primary sensory neurones by neonatal capsaicin at a dose (50 mg/kg) which selectively ablates primary afferent nerves while leaving intrinsic neurones intact28 resulted in a significant reduction in DSS induced DAI and MPO activity in the distal colon. The reduction in MPO in capsaicinised animals to control levels correlated with H&E stained sections where little to no neutrophil infiltration was seen in capsaicinised rats given DSS. These results taken collectively again argue that DSS colitis is modulated by primary sensory neurones.
In order to test the hypothesis that VR-1 containing primary sensory neurones may be pivotal in initiating experimental colitis, we attempted local enema infiltrations of CPZ in the distal colon of rats given DSS. Locally applied CPZ significantly reduced DAI, MPO, and histological scores in DSS colitis. Similar to capsaicinised rats, little or no neutrophils were evident on H&E sections of DSS rats given CPZ enemas.
Other publications have found conflicting data on the effect of denervation of animals with capsaicin. In models of acute inflammation such as 2,4,6-trinitrobenzenesulphonic acid,22,23 acetic acid,30 or formalin,26,27 denervation was attempted by subcutaneous injection of capsaicin in adult animals. In these models, denervation by capsaicin appeared to accentuate inflammation. Additionally, SP or calcitonin gene related peptide (CGRP) levels either did not change23 or were reduced.26,27 The investigators in these studies attributed these effects to reduction of CGRP, a putative protective peptide in inflammation. However, both our group and others have determined that capsaicin appears to denervate sensory neurones most effectively when given to newborn rat pups; adult rats may only have 50% of sensory nerves ablated by a massive capsaicin injection.31 Also, we intentionally chose an animal model of colitis that did not involve an acute toxic effect on the colonic mucosa. Additionally, we chose specific end points of intestinal inflammation that are widely used in the literature such as MPO, DAI, and histological damage, unlike the previously sited articles which employed different parameters (for example, colon wet weight, per cent damaged area, etc). Also, the nearly identical protective effects of both neonatal denervation with capsaicin and local application of CPZ argue strongly that sensory nerves are required for propagation of this model of colitis. Finally, we believe that simulation of VR-1 is a more proximal event in the neurogenic inflammatory cascade that may or may not change SP or CGRP levels.
Sensory neurones appear to be important in the inflammatory response in a variety of organ systems. For example, tachykinins (the peptide family containing SP) and their receptors appear to be critical in bronchoconstriction and neurogenic inflammation in the lung,32 skin,33,34 and bladder.35 In the digestive tract, it appears that the SP receptor is required in experimental pancreatitis36 and Clostridium difficile ileitis.1 However, sensory nerves containing CGRP offer protection in the stomach to acid evoked injury. Specifically, capsaicin denervated rats and CGRP-1 receptor antagonism weakened gastric mucosal defence suggesting that CGRP induced hyperaemia and vasodilatation is protective in the stomach.37 Clearly, sensory neurones may exert either protective or destructive effects when stimulated by an exogenous agent.
In conclusion, these studies indicate that VR-1 containing primary sensory neurones are required in the propagation of DSS colitis. Either local damage or generation of a possible endogenous VR-1 agonist by DSS administration results in sensory neurone activation and subsequent release of proinflammatory peptides such as SP. Inhibition of VR-1 by local administration of small doses of CPZ or other VR-1 antagonists may represent a novel therapeutic pathway to prevent inflammatory bowel disease. Further investigation into the possible protective role of VR-1 antagonists after inflammation has been established is also warranted.
Acknowledgments
Grant support was provided by the American Surgical Association Fellowship Award, Institute for Medical Research Grant (CRM), and NIH grant (R01 DK55808, TT).
Abbreviations
CPZ, capsazepine
DAI, disease activity index
DSS, dextran sulphate sodium
MPO, myleoperoxidase
VR-1, vanilloid receptor subtype-1
CGRP, calcitonin gene related peptide
H&E, haematoxylin and eosin
HTAB, hexadecyltrimethyllammonium bromide
SP, substance P
REFERENCES
- 1.Mantyh CR, Pappas TN, Lapp JA, et al. Substance P activation of enteric neurons in response to intraluminal Clostridium difficile toxin A in the rat ileum. Gastroenterology 1996;111:1272–80. [DOI] [PubMed] [Google Scholar]
- 2.Pothoulakis C, Castagliuolo I, LaMont JT, et al. CP-96,345, a substance P antagonist, inhibits rats intestinal responses to Clostridium difficile toxin A but not cholera toxin. Proc Natl Acad Sci U S A 1994;91:947–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castagliuolo I, Riegler M, Pasha A, et al. Neurokinin-1 (NK-1) receptor is required in Clostridium difficile-induced enteritis. J Clin Invest 1998;101:1547–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mantyh CR, McVey DC, Vigna SR. Extrinsic surgical denervation inhibits Clostridium difficile toxin A-induced enteritis in rats. Neurosci Lett 2000;292:95–8. [DOI] [PubMed] [Google Scholar]
- 5.Caterina MJ, Schumacher MA, Tominaga M, et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 1997;389:816–24. [DOI] [PubMed] [Google Scholar]
- 6.Bevan S, Hothi S, Hughes G, et al. Capsazepine:a competitive antagonist of the sensory neurone excitant capsaicin. Br J Pharmacol 1992;107:544–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McVey DC, Vigna SR. The capsaicin VR-1 receptor mediates substance P release in toxin A-induced enteritis in rats. Peptides 2001;22:1439–46. [DOI] [PubMed] [Google Scholar]
- 8.Meller ST, Lewis SJ, Ness TJ, et al. Neonatal capsaicin treatment abolishes the nociceptive responses to intravenous 5-HT in the rat. Brain Res 1991;542:212–18. [DOI] [PubMed] [Google Scholar]
- 9.Stevceva L, Pavli P, Husband A, et al. Dextran sulfate sodium-induced colitis is ameliorated in interleukin 4 deficient mice. Gen Immun 2001;2:309–16. [DOI] [PubMed] [Google Scholar]
- 10.Cooper HS, Murthy SN, Shah RS, et al. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest 1993;69:249–83. [PubMed] [Google Scholar]
- 11.Tessner TG, Chon SM, Schoolman S, et al. Prostaglandins prevent decreased epithelial cell proliferation associated with dextran sodium sulfate injury in mice. Gastroenterology 1998;115:874–82. [DOI] [PubMed] [Google Scholar]
- 12.Stucchi AF, Shofer S, Leeman S, et al. NK-1 antagonist reduces colonic inflammation and oxidative stress in dextran sulfate-induced colitis in rats. Am J Physiol Gastrointest Liver Physiol 2000;279:G1298–306. [DOI] [PubMed] [Google Scholar]
- 13.Takizawa H, Shintani N, Natsui M, et al. Activated immunocompetent cells in rat colitis mucosa induced by dextran sodium sulfate and not complete partial suppression of colitis by FK506. Digestion 1995;56:259–64. [DOI] [PubMed] [Google Scholar]
- 14.Jodal M. Neuronal influences on intestinal transport. J Intern Med 1990;732(suppl 1):125–32. [DOI] [PubMed] [Google Scholar]
- 15.Castagliuolo I, LaMont JT, Letourneau R, et al. Neuronal involvement in the intestinal effects of Clostridium difficile toxin A and Vibrio cholerae enterotoxin in rat ileum. Gastroenterology 1994;107:657–65. [DOI] [PubMed] [Google Scholar]
- 16.Mantyh CR, McVey DC, Vigna SR. Extrinsic surgical denervation inhibits Clostridium difficile toxin A-induced enteritis in rats. Neurosci Lett 2000;292:95–8. [DOI] [PubMed] [Google Scholar]
- 17.Caterina MJ, Leffler A, Malmberg AB, et al. Impaired nociceptive and pain sensation in mice lacking the capsaicin receptor. Science 2000;288:306–13. [DOI] [PubMed] [Google Scholar]
- 18.McVey DC, Vigna SR. The capsaicin VR-1 receptor mediates substance P release in toxin A-induced enteritis in rats. Peptides 2001;22:1439–46. [DOI] [PubMed] [Google Scholar]
- 19.Renzi D, Pellegrini B, Tonelli F, et al. Substance P (neurokinin-1) and neurokinin A (neurokinin-2) receptor gene and protein expression in the healthy and inflamed human intestine. Am J Pathol 2000;157:1511–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holzer P, Holzer-Petsche U. Tachykinins in the gut. Part I. Expression, release and motor function. Pharm Ther 1997;73:173–217. [DOI] [PubMed] [Google Scholar]
- 21.Tessner TG, Chon SM, Schoolman S, et al.. Prostaglandins prevent decreased epithelial cell proliferation associated with dextran sodium sulfate injury in mice. Gastroenterology 1998;115:874–82. [DOI] [PubMed] [Google Scholar]
- 22.Evangelista S, Tramontana M. Involvement of calcitonin gene-related peptide in rat experimental colitis. J Physiol Paris 1993;87:277–80. [DOI] [PubMed] [Google Scholar]
- 23.Reinshagen M, Patel A, Sottili M, et al. Action of sensory neurons in an experimental at colitis model of injury and repair. Am J Physiol 1996;270:G79–86. [DOI] [PubMed] [Google Scholar]
- 24.McCafferty DM, Wallace JL, Sharkey KA. Effects of chemical sympathectomy and sensory nerve ablation on experimental colitis in the rat. Am J Physiol 1997;272:G272–80. [DOI] [PubMed] [Google Scholar]
- 25.Kim HS, Berstad A. Experimental colitis in animal models. Scand J Gastroenterol 1992;27:529–37. [DOI] [PubMed] [Google Scholar]
- 26.Eysselein VE, Reinshagen M, Cominelli F, et al. Calcitonin gene related peptide and substance P decrease in the rabbit colon during colitis. Gastroenterology 1991;101:1211–19. [DOI] [PubMed] [Google Scholar]
- 27.Reinshagen M, Patel A, Sottili M, et al. Protective function of extrinsic sensory neurons in acute rabbit experimental colitis. Gastroenterology 1994;106:1208–14. [DOI] [PubMed] [Google Scholar]
- 28.Holzer P, Gamse R, Lembeck F. Distribution of substance P in rat gastrointestinal tract-lack of effect of capsaicin pretreatment. Eur J Pharmacol 1980;61:303–7. [DOI] [PubMed] [Google Scholar]
- 29.Szolcsanyi J, Bartho L. Capsaicin-sensitive afferents and their role in gastroprotection:an update. J Physiol Paris 2001;95:181–8. [DOI] [PubMed] [Google Scholar]
- 30.Okayasu I, Hatakeyama S, Yamada M, et al. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 1990;98:694–702. [DOI] [PubMed] [Google Scholar]
- 31.Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev 1991;43:143–201. [PubMed] [Google Scholar]
- 32.Joos GF, Pauwels RA. Pro-inflammatory effects of substance P:new perspectives for the treatment of airway disease? Trends Pharmacol Sci 2000;21:131–3. [DOI] [PubMed] [Google Scholar]
- 33.Okabe T, Hide M, Koro O, et al. Substance P induces tumor necrosis factor-alpha release from human skin via mitogen-activated protein kinase. Eur J Pharm 2000;398:309–15. [DOI] [PubMed] [Google Scholar]
- 34.Neubert JK, Maidment NT, Matsuka Y, et al. Inflammation-induced changes in primary afferent-evoked release of substance P within trigeminal ganglia in vivo. Brain Res 2000;871:181–91. [DOI] [PubMed] [Google Scholar]
- 35.Saban R, Saban MR, Nguyen NB, et al. Neurokinin-1 (NK-1) receptor is required in antigen-induced cystitis. Am J Pathol 2000;156:775–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nathan JD, Patel AA, McVey DC, et al. Capsaicin vanilloid receptor-1 mediates substance P release in experimental pancreatitis. Am J Physiol Gastrointestinal Liver Physiol 2001;281:G1322–8. [DOI] [PubMed] [Google Scholar]
- 37.Holzer P. Implications of tachykinins and calcitonin gene-related peptide in inflammatory bowel disease. Digestion 1998;59:269–83. [DOI] [PubMed] [Google Scholar]