Abstract
Background: Integrins mediate cell matrix adhesion and regulate cell growth and survival. In colonic epithelial cells, α2β1 integrin controls glandular differentiation and proliferation. Butyrate stimulates differentiation and induces apoptosis in vitro.
Aims: We investigated whether butyrate induction of apoptosis was associated with perturbation of integrin mediated cell matrix adhesion.
Methods: Three colonic cancer cell lines (SW1222, SW620, LS174T) were studied. Adhesion to extracellular matrix proteins, expression of α2β1 integrin, and apoptosis were studied in adherent cells after treatment with 4 mM butyrate.
Results: Butyrate decreased the attachment to type I collagen in SW620 cells and type I and IV collagen in LS174T cells. The decreased cell attachment was associated with downregulation of α2β1 integrin and increased apoptosis in adherent cells. No changes in α2β1 expression or matrix adhesion were seen in SW1222 cells, which were also found to be less sensitive to butyrate induction of apoptosis. Downregulation of α2β1 integrin preceded the detection of apoptosis.
Conclusion: Apoptosis induced by butyrate is associated with downregulation of expression and functional activity of α2β1 integrin. Perturbation of cell matrix adhesion may be a novel mechanism by which butyrate induces apoptosis in colorectal cancer cells.
Keywords: butyrate, α2β1 integrin, apoptosis, colorectal cancer
Survival of epithelial cells depends on signals generated by the interaction with components of the basement membrane. Integrins, transmembrane αβ heterodimers, link the extracellular matrix (ECM) to the intracellular cytoskeletal network and to multiple signalling pathways whose activation is important for cell differentiation, survival, and growth.1 In normal colonic epithelial cells, loss of integrin mediated anchorage to ECM induces a form of apoptosis called “anoikis”.2
Previous studies have shown a cell specific role of different integrins in the apoptotic signalling pathway. Downregulation of the αv subunit leads to apoptosis in human melanoma cells3 and overexpression of α5β1 provided protection against apoptosis induced by serum deprivation or other cytotoxic agents in a colonic carcinoma cell line.4 Expression of a number of different integrins acts in concert with changes in basement membrane composition to mediate differentiation along the crypt-villus axis in the human intestinal mucosa.5 The α2β1 integrin is the main collagen receptor and in vivo is predominantly expressed at the basolateral domain of intestinal cells towards the base of the crypt. Relatively high levels of α2β1 expression have also been found in undifferentiated colonic cells in vitro.6 The α2β1 integrin has been shown to be involved in intestinal cell adhesion to collagen types I and IV, and laminin,7 and its engagement is required for cell migration.8 We have previously shown that α2β1 mediates induction and maintenance of gland morphogenesis of colorectal epithelial cells.9 Butyrate, the 4 carbon fatty acid, is generated in the human colon from the anaerobic bacterial fermentation of dietary fibres.10 In vitro studies have indicated that butyrate modulates growth, differentiation, and survival of colon adenocarcinoma cell lines.11–13 However, the cellular and molecular mechanisms by which butyrate may induce apoptosis are unclear.14 In this study, we have investigated whether butyrate can affect integrin expression and whether butyrate induced apoptosis in colon cancer cells is mediated via perturbation of expression and functional activity of α2β1 integrin.
METHODS
Cell lines and culture conditions
Well differentiated (SW1222)15 and poorly differentiated (SW620, LS174T)16,17 human colon carcinoma cell lines were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, UK) containing 10% fetal bovine serum (Gibco, UK) at 37°C in 5% CO2 in air at 100% humidity. For all the experiments, 1×106 cells were seeded onto 100 mm tissue culture Petri dishes (Falcon, UK) precoated with collagen type I; the experiment commenced when the cells were judged to be 60–80% confluent by inverted light microscopy. Triplicate Petri dishes were exposed for 48 hours to 4 mM butyrate (Sigma, St Louis, Missouri, USA).
Cell matrix adhesion assay
Microtitre plates were coated with 50 μl/well of type I collagen (Cohesion, California, USA), type IV collagen (Sigma), laminin (Sigma), and bovine serum albumin (BSA) (Sigma) at a concentration of 20 μg/ml and left uncovered in a laminar flow hood overnight to allow normal evaporation. Trypsinised (trypsin-EDTA 0.05%/0.02%; Gibco) cells were washed three times in serum free DMEM, resuspended, and plated onto previously coated plates. After three hours of incubation, the supernatant was removed and unattached cells were washed away three times with phosphate buffered saline (PBS). The attached cells were fixed with 3% paraformaldehyde and stained with 0.5% toluidine blue in 3% paraformaldehyde. Cell attachment was estimated at 595 nm on a ELISA plate reader. We have previously shown that maximal attachment (60%) to ECM protein coated plates is reached after three hours of incubation.7
Integrin expression
Flow cytometry
Integrin expression was evaluated by fluorescence activated cell sorting (FACS). Cells were detached with trypsin/EDTA for six minutes, washed in PBS, and incubated with mouse antihuman α2 and β1 subunit monoclonal antibody (Chemicon, California, USA). After three washes cells were incubated with secondary fluorescein isothiocyanate (FITC) conjugated antibody (Dako, UK) in the dark. Cells were washed in PBS and analysed by flow cytometry using a FACSscan (Becton Dickinson, UK). Cells incubated with secondary antibody alone where used as negative controls. Debris and aggregates were excluded using dot plots of side scatter against forward scatter. At least 10 000 cells per sample were acquired and results were expressed as mean fluorescence intensity after subtraction of control fluorescence. To confirm that trypsinisation did not affect integrin expression, cells were incubated with trypsin/EDTA for five, 10, and 15 minutes, and analysed by FACS.
Immunofluorescence
Cells were seeded onto glass coverslips precoated with collagen (20 μg/ml), cultured for two days, and then treated with butyrate (4 mM) for 48 hours. Coverslips were then washed by dipping in PBS prior to fixation in 4% (w/v) paraformaldehyde and permeabilisation with Triton X 100 (0.05%) (Sigma). The α2β1 integrin was immunofluorescently labelled with mouse monoclonal antibodies against the α2 and β1 subunit (Chemicon) followed by FITC conjugated goat antimouse F(ab‘)2 fragments (Dako, UK). Coverslips were mounted onto glass slides with Vectashield containing 4‘,6-diamino-2-phenylindole dihydrochloride (DAPI) from Vector (Burlington, California, USA) before viewing on a Leica TCS-NT confocal laser scanning microscope attached to a Leica DM IRBE inverted epifluorescence microscope (Leica Microsystems, Mannheim, Germany). An oil immersion objective lens ×63, NA 1.32, was used and imaging parameters were selected to optimise resolution. In order to analyse the localisation of α2 and β1 integrin in the entire cell, 18–23 horizontal sections were obtained at 1 μm intervals from the apical to the basal compartment.
Western blotting
Whole cell lysates from 1×106 cells were prepared for western blotting, as described by Palmer and colleagues.18 Briefly, proteins were resolved on 10% polyacrylamide gels and transferred onto Immobilon P polyvinylidene difluoride membranes (Millipore, Massachusetts, USA). Rabbit polyclonal anti-α2 and mouse monoclonal anti-β1 integrin subunit antibodies were used (Chemicon). These antibodies detected single bands of 160 kDa and 130 kDa, respectively. Blots were subsequently probed with anti α-tubulin (Sigma) as a loading control. Detection was achieved using a horseradish peroxidase conjugated antimouse second antibody and visualised using Lumi-Glo chemiluminescent substrate (KPL, USA) following the manufacturer’s instructions.
Assessment of apoptosis
Detection of apoptosis by annexin V/7-AAD staining
Annexin V detects phosphatidylserine on the outer plasma membrane of apoptotic cells.19 Translocation of phosphatidylserine has been shown to occur at an early stage of apoptosis and can be detected in adherent cells in culture.20,21 Progressive loss of membrane permeability was evaluated by 7-amino-actinomycin (7AA-D) incorporation and used to discriminate apoptotic from necrotic cells.19 Unstained cells, cells stained with annexin V phycoerythrin (PE) conjugated (PharMingen, California, USA) with 7-AAD (PharMingen) alone were used as controls to set up compensations and quadrants. After incubation with annexin V-PE and 7-AAD in annexin V binding buffer (PharMingen), trypsinised cells were collected, washed in PBS, and analysed by FACSscan. Apoptosis and α2 and β1 integrin expression were concomitantly evaluated by triple labelling analysis.
Western blot analysis of poly(ADP-ribose) polymerase (PARP)
Induction of apoptosis was also determined by poly(ADP-ribose) polymerase (PARP) cleavage. In previous studies we and others11,13 have shown that butyrate induced apoptosis leads to an increased proportion of floating cells. These floating cells have the morphological characteristics of apoptotic cells and produce a DNA ladder on gel electrophoresis.13 Whole cell lysates from 1×106 cells were prepared from both floating and attached colon cancer cells. PARP protein was detected by a mouse antihuman monoclonal antibody (Alexis, San Diego, USA) and a horseradish peroxidase conjugated goat antimouse secondary antibody. The antibody recognised both the full length (116 kDa) and cleaved (85 kDa) fragments.
Statistical analyses
Data were expressed as the means (SEM) of three separate experiments. Data were compared by analysis of variance (ANOVA). A p value <0.05 was considered statistically significant in all cases. All statistics were performed using the SPSS (version 9.0, SPSS Inc., Chicago, Illinois, USA) for Windows package.
RESULTS
Butyrate reduces cell matrix adhesion
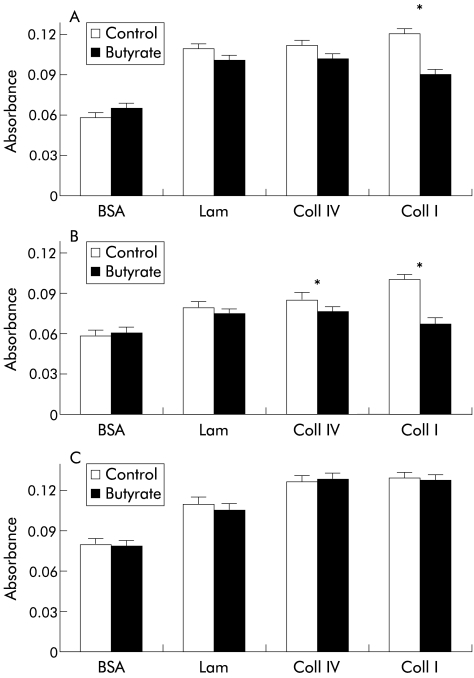
The cell matrix adhesion assay was used to investigate whether butyrate could affect the ability of colorectal cell lines to bind different matrices. After 48 hours of incubation with 4 mM butyrate, cells were trypsinised and allowed to attach to precoated microtitre plates. Preliminary experiments showed that trypsinisation did not affect α2 and β1 integrin expression as measured by FACS (data not shown). Butyrate treatment significantly decreased the attachment of SW620 (p=0.003) (fig 1A ▶) cells to type I collagen coated plates. Type I (p=0.01) and type IV (p=0.03) collagen binding was also decreased in LS174T cells (fig 1B ▶) whereas no difference in cell adhesion was found in the SW1222 cell line (fig 1C ▶) for all matrices tested. Adhesion to laminin was not affected by butyrate in all cell lines and no difference in adhesion to BSA (negative control) between treated and untreated cells was found.
Figure 1.
Effect of butyrate (4 nmM) on adhesion of SW620 (A), LS174T (B), and SW1222 (C) cells to different extracellular matrices and bovine serum albumin (BSA) (negative control). Butyrate treatment decreased the attachment to collagen type I (Coll I) in SW620 cells and to collagen types I and IV (Coll IV) in LS174T cells. No changes in cell matrix adhesion were found in SW1222 cells. Values are mean (SEM) of three different experiments performed in triplicate. *p<0.05 compared with control. Lam, laminin.
Butyrate reduces α2β1 integrin expression in adherent cells
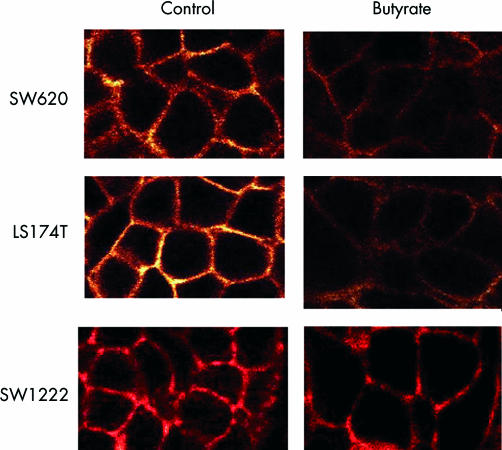
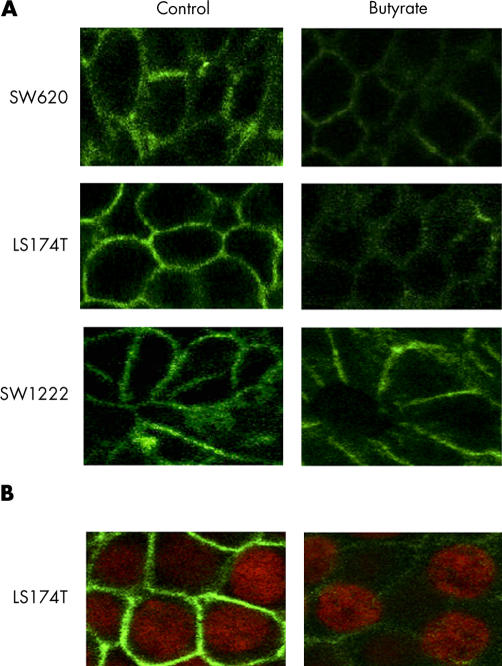
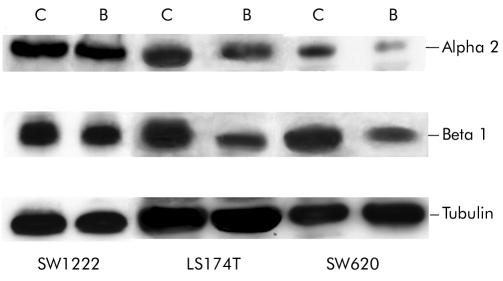
As α2β1 integrin is the prime mediator of collagen binding in colonic epithelial cells, we investigated the effect of butyrate on α2β1 expression and cellular localisation. Treatment with butyrate (4 mM) for 48 hours decreased expression of the α2 and β1 integrin subunit in SW620 and LS174T but not in SW1222 cell lines (table 1 ▶). Immunofluorescence staining of α2 and β1 subunits was also analysed by confocal microscopy. Cross sectional images demonstrated that α2β1 expression was predominantly basolateral in all cells and was significantly reduced by butyrate treatment in LS174T and SW620 but not in SW1222 cell line (figs 2–3 ▶ ▶). In agreement with the FACS data, the decrease in α2β1 membranous expression was observed in the majority of cells. Analysis of the series of horizontal sections taken from the apical to the basal compartment showed that the reduction in α2β1 membranous expression occurred consistently throughout the cell. In order to confirm that butyrate decreased integrin expression, total cellular levels were determined by western blotting. Immunoblot analysis confirmed that α2β1 protein levels were reduced after treatment with butyrate in LS174T and SW620 but not in SW1222 cell lines (fig 4 ▶).
Table 1.
Effect of butyrate on α2β1 integrin expression in three colorectal cancer cell lines
| Control α2 | Butyrate α2 | Control β1 | Butyrate β1 | |
| SW1222 | 466.3 (15) | 450 (18.3) | 524.4 (18.6) | 514 (8.3) |
| SW620 | 289.6 (10.5) | 132.6 (11.6)* | 356 (15.1) | 216.6 (14)* |
| LS174T | 330.3 (12) | 220.6 (13)* | 352.7 (18) | 201.4 (20.1)* |
Data are mean fluorescence intensity (MFI) and values are expressed as mean (SEM) of three different experiments. *p<0.001.
Figure 2.
Expression and localisation of the α2 integrin subunit in colorectal cancer cells by confocal microscopy. Treatment with butyrate (4 mM) for 48 hours decreased α2 subunit expression in SW620 and LS174T but not in SW1222 cell lines.
Figure 3.
(A) Expression and localisation of β1 integrin subunit (green) in colorectal cancer cells by confocal microscopy. Treatment with butyrate (4 mM) for 48 hours decreased β1 subunit expression in SW620 and LS174T but not in SW1222 cell lines. (B) Expression of β1 integrin subunit (green) and nuclei stained with 4‘,6-diamino-2-phenylindole dihydrochloride (DAPI) (red) in the SW620 cell line. The reduction in β1 subunit expression was evident in cells showing a normal nuclear morphology. Images are representative of four separate experiments.
Figure 4.
Butyrate (4 mM) treatment for 48 hours decreased α2β1 integrin expression in LS174T and SW620 cell lines. Lysates of 106 cells from untreated (C) and butyrate treated (B) cultures were resolved by sodium dodecyl sulphate-polyacrylamide gel electrophoresis and α2 (160 kDa) and β1 (130 kDa) protein levels determined by western immunoblotting. Equal loading and transfer were shown by repeat probing with anti-α tubulin.
Butyrate induction of apoptosis is associated with downregulation of α2β1 expression in viable adherent cells
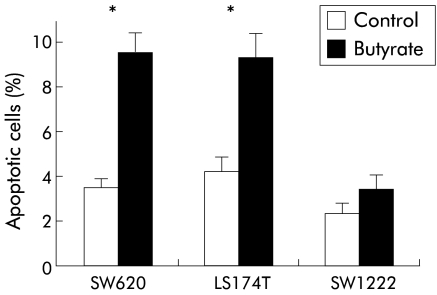
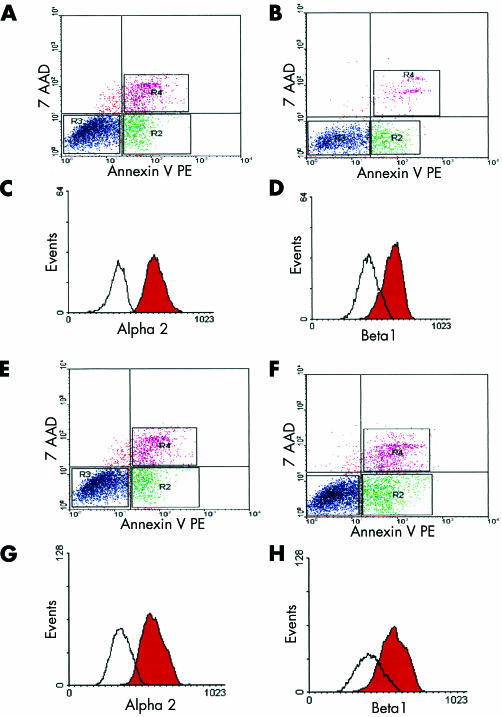
Apoptosis in adherent cells was determined by annexin V binding, as previously described.21 Butyrate treatment for 48 hours significantly increased the percentage of apoptotic cells compared with controls in SW620 (9.6 (0.5) v 3.5 (0.2); p<0.01) and LS174T (9.3 (0.6) v 4.3 (0.3); p<0.01) cell lines, as shown in fig 5 ▶. The SW1222 cell line was less sensitive to butyrate treatment and no significant increase in apoptosis was detected (2.4 (0.2) v 3.3 (0.4); p=0.123). Annexin V binding in combination with 7-AAD uptake has been used previously to distinguish viable, apoptotic, and necrotic populations among adherent colonic carcinoma cells.22 In all experiments the percentage of necrotic cells was not different when treated and untreated cells were compared. PARP is an early target of active caspase 3 during apoptosis and the resulting 85 kDa cleavage product has now been recognised as a sensitive marker of this process. An increase in cleaved PARP was detected in adherent cells from butyrate treated samples in SW620 and LS174T cell lines but not in the SW1222 cell line (fig 6 ▶). This is consistent with early induction of apoptosis in adherent cells exposed to butyrate. As expected,23 no full length PARP was detected in floating apoptotic cells (fig 6 ▶). In our study, when apoptosis and integrin expression were measured simultaneously by triple labelling analysis, decreased expression of α2β1 integrin was detected in adherent viable treated cells compared with controls (fig 7C ▶, D, G, H). Indeed, by confocal microscopy, reduced α2β1 expression seen in butyrate treated adherent cells was evident in cells exhibiting normal nuclear morphology (fig 4B ▶) as well as in those showing signs of apoptosis, suggesting that the decrease in α2β1 integrin expression preceded induction of apoptosis by butyrate.
Figure 5.
Effect of butyrate (4 mM) on apoptosis in different colorectal cancer cell lines. Results are expressed as percentage of apoptotic cells (annexin V positive/7-amino-actinomycin negative). Butyrate treatment for 48 hours significantly increased the number of apoptotic cells in SW620 and LS174T cell lines. Values are expressed as mean (SEM) from three different experiments. *p<0.01 compared with control.
Figure 6.
Effect of butyrate (4 mM) on poly(ADP-ribose) polymerase (PARP) cleavage. Both adherent and floating cells were collected for protein extraction. Lysates of 106 cells from untreated (C) and butyrate treated (B) (48 hours) cultures were resolved by sodium dodecyl sulphate-polyacrylamide gel electrophoresis and probed with anti-PARP monoclonal antibody. Protein levels were determined by western immunoblotting. The position of the full length (116 kDa) and proteolytic fragment (86 kDa) of PARP is indicated. Loading is shown by repeat probing with anti-α tubulin.
Figure 7.
Butyrate (4 mM) treatment decreased α2β1 integrin expression in live adherent cells. Dot plot analysis of annexin V-phycoerythrin (PE) binding and 7-amino-actinomycin (7-AAD) uptake in SW620 (A, B) and LS174T (E, F) cell lines. Adherent cells were analysed from untreated (A, E) and treated (48 hours) (B, F) samples. The lower left quadrant of each panel shows viable cells (annexin V negative/7-AAD negative), the lower right represents early apoptotic cells (annexin V positive/7-AAD negative), and the upper right contains non-viable necrotic cells (annexin V positive/7-AAD positive). Histograms show α2 and β1 integrin expression in SW620 (C, D) and LS174T (G, H) adherent viable cell populations identified and gated in R3 using a triple labelling analysis. The red peak represents the untreated sample, and the white peak the treated (48 hours) sample. The y axis represents cell number and the x axis fluorescence intensity. Representative results from three different experiments.
DISCUSSION
In this study we have shown that butyrate induced apoptosis was associated with downregulation of membranous expression and functional activity of the α2β1 integrin molecule in two of three colon carcinoma cell lines tested. Integrin α and β chains are synthesised at different rates in the endoplasmic reticulum and associated in dimers before leaving the Golgi apparatus.24 Only functional dimers are transported to the plasma membrane whereas isolated chains are rapidly degraded. Our finding of decreased expression, as shown by both FACS and western blotting, suggests an action of butyrate on integrin expression rather than targeting. Butyrate has been shown to affect gene expression in a variety of cultured cells.25–27 Different molecular mechanisms are involved, including hyperacetylation of histones, histone phosphorylation, and DNA methylation.28–30
In our study, downregulation of α2β1 was accompanied by reduced functional ability of cells to adhere to extracellular matrix proteins. In particular, treatment with butyrate significantly decreased cell adhesion to collagen type I in SW620 and collagen types I and IV in LS174T cells. Cell matrix adhesion was not affected by butyrate in SW1222, a cell line in which no changes in α2β1 integrin expression were detected. There is a wide body of evidence showing that decreased cell adhesion results in altered signalling from the cell surface to the nucleus. Strater and colleagues2 have demonstrated that in normal colonic epithelium, cell survival is dependent on adhesion to matrix proteins such as collagen type I via a β1 dependent pathway. Binding of the β1 integrin family to ECM components results in the clustering of integrins and recruitment of a wide variety of molecules, leading to the formation of focal adhesions.31 These link the ECM to the actin cytoskeleton and provide a structural basis for cell viability.32,33
In keeping with a previous study, we found a significant increase in apoptosis in butyrate treated cells as early as 48 hours.13 In our study, apoptosis was detected at an early stage by annexin V binding and PARP cleavage, in cells still adherent, prior to complete cell detachment from the substratum. Having shown that butyrate at a concentration known to induce apoptosis13 downregulates α2β1 integrin expression it was of interest to determine whether this downregulation preceded induction of apoptosis. When apoptosis and integrin expression were evaluated simultaneously by triple labelling, α2β1 integrin levels were reduced before the appearance of apoptosis.
SW1222 cells were found to be less sensitive to induction of apoptosis by butyrate. Interestingly, this cell line also did not show modification in integrin expression or in functional adhesion. SW1222 is a very well differentiated cell line and it has been recently shown by Mariadason and colleagues34 that the degree of differentiation can affect sensitivity to butyrate induced apoptosis. Differentiated cells can use butyrate more rapidly, leading to lower intracellular concentrations not sufficient to induce significant effects on intracellular targets.34 Butyrate is of relevance in the colon since it is produced by bacterial metabolism of dietary fibres and is generally considered to be protective against colorectal cancer. It has been suggested that in colonic carcinoma cells butyrate induced apoptosis occurs via a terminal differentiation pathway, mimicking the apoptosis seen at the top of the colonic crypt where the senescent more differentiated cells are shed from the mucosal surface.35,36 In previous studies we have shown that alkaline phosphatase and E-cadherin, markers of enterocytic differentiation, are elevated in butyrate treated cells.37,38 In normal colon, the α2 chain is expressed mainly in epithelial cells lining the base of the crypts whereas it is markedly decreased in cells lining the surface epithelium.39,40 Moreover, it has been shown that during human colonic organogenesis there is a gradient in α2 expression which decreases from the crypt to the surface.41 It has been suggested that decreased adhesion secondary to reduced α2 expression may promote cell surface shedding.40 Although the β1 integrin subunit is equally distributed throughout the colonic epithelium, the degree of differentiation still influences its expression.42 It is of interest that downregulation of α2β1 integrin expression found in our in vitro model may reflect an effect of butyrate on differentiation similar to the maturation process described in the cells migrating up the crypt in vivo. Other integrin subunits, such as the α6, have been found to be expressed along all the crypt-villus axis and are not involved in the terminal differentiation of epithelial cells.43 Indeed, we found that treatment for 48 hours with 4 mM of sodium butyrate did not affect α6 integrin expression (Buda and Pignatelli, unpublished results). There is evidence that modulation of integrin expression and function is accompanied by changes in morphology and growth pattern in vitro.44 Loss of integrin matrix interaction triggers a physiological form of apoptosis called anoikis.45 We have shown that glandular morphogenesis of SW1222 cells grown in three dimensional collagen gel was reduced by addition of anti-β1 neutralising antibody.4 Furthermore, β1 integrin neutralising antibody induces anoikis in intestinal epithelial cells.46 In line with previous studies,3,4 our findings confirm that changes in expression of a single integrin receptor are associated with the apoptotic process.
In summary, in this study we have demonstrated that physiological concentrations of butyrate decrease the expression and functional activity of α2β1 integrin, associated with induction of apoptosis. We suggest that perturbation of cell matrix adhesion may be a potential mechanism by which butyrate induces apoptosis in human colorectal cancer cells. In addition, our results may have important implications for the understanding of the role played by butyrate in the regulation of homeostasis in the normal colonic mucosa.
Acknowledgments
We acknowledge the Medical Research Council for funding the School of Medical Sciences Cell Imaging Facility. We wish to thank Mr Peter Summers for excellent technical assistance.
Abbreviations
ECM, extracellular matrix proteins
DMEM, Dulbecco’s modified Eagle’s medium
BSA, bovine serum albumin
PBS, phosphate buffered saline
FACS, fluorescence activated cell sorting
FITC, fluorescein isothiocyanate
DAPI, 4‘,6-diamino-2-phenylindole dihydrochloride
7-AAD, 7-amino-actinomycin
PE, phycoerythrin
PARP, poly(ADP-ribose) polymerase
REFERENCES
- 1.Giancotti FG, Ruoslahti E. Integrin signaling. Science 1999;285:1028–32. [DOI] [PubMed] [Google Scholar]
- 2.Strater J, Wedding U, Barth TF, et al. Rapid onset of apoptosis in vitro follows disruption of beta 1-integrin/matrix interactions in human colonic crypt cells. Gastroenterology 1996;110:1776–84. [DOI] [PubMed] [Google Scholar]
- 3.Montgomery AM, Reisfeld RA, Cheresh DA. Integrin alpha v beta 3 rescues melanoma cells from apoptosis in three-dimensional dermal collagen. Proc Natl Acad Sci U S A 1994;91:8856–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Brien V, Frisch SM, Juliano RL. Expression of the integrin alpha 5 subunit in HT29 colon carcinoma cells suppresses apoptosis triggered by serum deprivation. Exp Cell Res 1996;224:208–13. [DOI] [PubMed] [Google Scholar]
- 5.Lussier C, Basora N, Bouatrouss Y, et al. Integrins as mediators of epithelial cell-matrix interactions in the human small intestinal mucosa. Microsc Res Tech 2000;51:169–78. [DOI] [PubMed] [Google Scholar]
- 6.Basora N, Vachon PH, Herring-Gillam FE, et al. Relation between integrin alpha7Bbeta1 expression in human intestinal cells and enterocytic differentiation. Gastroenterology 1997;113:1510–21. [DOI] [PubMed] [Google Scholar]
- 7.Pignatelli M, Bodmer WF. Integrin cell adhesion molecules and colorectal cancer. J Pathol 1990;162:95–7. [DOI] [PubMed] [Google Scholar]
- 8.Basson MD, Modlin IM, Madri JA. Human enterocyte (Caco-2) migration is modulated in vitro by extracellular matrix composition and epidermal growth factor. J Clin Invest 1992;90:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pignatelli M, Liu D, Nasim MM, et al. Morphoregulatory activities of E-cadherin and beta-1 integrins in colorectal tumour cells. Br J Cancer 1992;66:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rombeau J, Kripke SA, Settle RG. Short fatty acid; production, absorption, metabolism and intestinal effects. In: Vahouny GB, Kritchevsky D, eds.Dietary Fibers: Basic and Clinical Aspects. New York: Plenum Press, 1990:317–39.
- 11.Heerdt BG, Houston MA, Augenlicht LH. Potentiation by specific short-chain fatty acids of differentiation and apoptosis in human colonic carcinoma cell lines. Cancer Res 1994;54:3288–93. [PubMed] [Google Scholar]
- 12.Hague A, Elder DJ, Hicks DJ, et al. Apoptosis in colorectal tumour cells: induction by the short chain fatty acids butyrate, propionate and acetate and by the bile salt deoxycholate. Int J Cancer 1995;60:400–6. [DOI] [PubMed] [Google Scholar]
- 13.Hague A, Manning AM, Hanlon KA, et al. Sodium butyrate induces apoptosis in human colonic tumour cell lines in a p53-independent pathway: implications for the possible role of dietary fibre in the prevention of large-bowel cancer. Int J Cancer 1993;55:498–505. [DOI] [PubMed] [Google Scholar]
- 14.Williams AC, Collard TJ, Paraskeva C. An acidic environment leads to p53 dependent induction of apoptosis in human adenoma and carcinoma cell lines: implications for clonal selection during colorectal carcinogenesis. Oncogene 1999;18:3199–204. [DOI] [PubMed] [Google Scholar]
- 15.Leibovitz A, Stinson JC, McCombs WB, et al. Classification of human colorectal adenocarcinoma cell lines. Cancer Res 1976;36:4562–9. [PubMed] [Google Scholar]
- 16.Rutzky LP, Kaye CI, Siciliano MJ, et al. Longitudinal karyotype and genetic signature analysis of cultured human colon adenocarcinoma cell lines LS180 and LS174T. Cancer Res 1980;40:1443–8. [PubMed] [Google Scholar]
- 17.Fogh J, Trempe G. New human tumor cell lines. In: Fogh J, ed. Human Tumour Cells in Vitro. New York: Plenum Press, 1975:115–41.
- 18.Palmer DG, Paraskeva C, Williams AC. Modulation of p53 expression in cultured colonic adenoma cell lines by the naturally occurring lumenal factors butyrate and deoxycholate. Int J Cancer 1997;73:702–6. [DOI] [PubMed] [Google Scholar]
- 19.Vermes I, Haanen C, Steffens-Nakken H, et al. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods 1995;184:39–51. [DOI] [PubMed] [Google Scholar]
- 20.van Engeland M, Ramaekers FC, Schutte B, et al. A novel assay to measure loss of plasma membrane asymmetry during apoptosis of adherent cells in culture. Cytometry 1996;24:131–9. [DOI] [PubMed] [Google Scholar]
- 21.Clarke RG, Lund EK, Johnson IT, et al. Apoptosis can be detected in attached colonic adenocarcinoma HT29 cells using annexin V binding, but not by TUNEL assay or sub-G0 DNA content. Cytometry 2000;39:141–50. [PubMed] [Google Scholar]
- 22.Shiff SJ, Qiao L, Tsai LL, et al. Sulindac sulfide, an aspirin-like compound, inhibits proliferation, causes cell cycle quiescence, and induces apoptosis in HT-29 colon adenocarcinoma cells. J Clin Invest 1995;96:491–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hague A, Bracey TS, Hicks DJ, et al. Decreased levels of p26-Bcl-2, but not p30 phosphorylated Bcl-2, precede TGFbeta1-induced apoptosis in colorectal adenoma cells. Carcinogenesis 1998;19:1691–5. [DOI] [PubMed] [Google Scholar]
- 24.Heino J, Ignotz RA, Hemler ME, et al. Regulation of cell adhesion receptors by transforming growth factor-beta. Concomitant regulation of integrins that share a common beta 1 subunit. J Biol Chem 1989;264:380–8. [PubMed] [Google Scholar]
- 25.Davis T, Kennedy C, Chiew YE, et al. Histone deacetylase inhibitors decrease proliferation and modulate cell cycle gene expression in normal mammary epithelial cells. Clin Cancer Res 2000;6:4334–42. [PubMed] [Google Scholar]
- 26.Barshishat M, Polak-Charcon S, Schwartz B. Butyrate regulates E-cadherin transcription, isoform expression and intracellular position in colon cancer cells. Br J Cancer 2000;82:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maeda T, Towatari M, Kosugi H, et al. Up-regulation of costimulatory/adhesion molecules by histone deacetylase inhibitors in acute myeloid leukemia cells. Blood 2000;96:3847–56. [PubMed] [Google Scholar]
- 28.Sealy L, Chalkley R. The effect of sodium butyrate on histone modification. Cell 1978;14:115–21. [DOI] [PubMed] [Google Scholar]
- 29.Boffa LC, Gruss RJ, Allfrey VG. Manifold effects of sodium butyrate on nuclear function. Selective and reversible inhibition of phosphorylation of histones H1 and H2A and impaired methylation of lysine and arginine residues in nuclear protein fractions. J Biol Chem 1981;256:9612–21. [PubMed] [Google Scholar]
- 30.de Haan JB, Gevers W, Parker MI. Effects of sodium butyrate on the synthesis and methylation of DNA in normal cells and their transformed counterparts. Cancer Res 1986;46:713–16. [PubMed] [Google Scholar]
- 31.Giancotti FG. Integrin signaling: specificity and control of cell survival and cell cycle progression. Curr Opin Cell Biol 1997;9:691–700. [DOI] [PubMed] [Google Scholar]
- 32.Aplin AE, Howe AK, Juliano RL. Cell adhesion molecules, signal transduction and cell growth. Curr Opin Cell Biol 1999;11:737–44. [DOI] [PubMed] [Google Scholar]
- 33.Dedhar S, Williams B, Hannigan G. Integrin-linked kinase (ILK): a regulator of integrin and growth-factor signalling. Trends Cell Biol 1999;9:319–23. [DOI] [PubMed] [Google Scholar]
- 34.Mariadason JM, Velcich A, Wilson AJ, et al. Resistance to butyrate-induced cell differentiation and apoptosis during spontaneous Caco-2 cell differentiation. Gastroenterology 2001;120:889–99. [DOI] [PubMed] [Google Scholar]
- 35.Heerdt BG, Houston MA, Augenlicht LH. Potentiation by specific short-chain fatty acids of differentiation and apoptosis in human colonic carcinoma cell lines. Canc Res 1994;54:3288–94. [PubMed] [Google Scholar]
- 36.Mariadason JM, Rickard KL, Barkla DH, et al. Divergent phenotypic patterns and commitment to apoptosis of Caco-2 cells during spontaneous and butyrate-induced differentiation. J Cell Physiol 2000;183:347–54. [DOI] [PubMed] [Google Scholar]
- 37.Qualtrough D, Hinoi T, Fearon E, et al. Expression of CDX2 in normal and neoplastic human colon tissue and during differentiation of an in vitro model system. Gut 2002;51:184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Butt AJ, Hague A, Paraskeva C. Butyrate but not TGF-beta-induced apoptosis is associated with increased expression of the differentiation markers E-cadherin and alkaline phosphatase. Cell Death Diff 1997;4:725–32. [DOI] [PubMed] [Google Scholar]
- 39.Stallmach A, von Lampe B, Matthes H, et al. Diminished expression of integrin adhesion molecules on human colonic epithelial cells during the benign to malign tumour transformation. Gut 1992;33:342–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zutter MM, Santoro SA.Widespread histologic distribution of the alpha 2 beta 1 integrin cell-surface collagen receptor. Am J Pathol 1990;137:113–20. [PMC free article] [PubMed] [Google Scholar]
- 41.Dieckgraefe BK, Santoro SA, Alpers DH. Immunolocalisation of alpha-integrin subunits and extracellular matrix components during human colonic organogenesis. Gastroenterology 1996;110:58–71. [DOI] [PubMed] [Google Scholar]
- 42.Levy P, Robin H, Kornprobst M, et al. Enterocytic differentiation of the human Caco-2 cell line correlates with alterations in integrin signaling. J Cell Physiol 1998;177:618–27. [DOI] [PubMed] [Google Scholar]
- 43.Beaulieu JF. Differential expression of the VLA family of integrins along the crypt-villus axis in the human small intestine. J Cell Sci 1992;102(pt 3):427–36. [DOI] [PubMed] [Google Scholar]
- 44.Desloges N, Basora N, Perreault N, et al. Regulated expression of the integrin alpha9beta1 in the epithelium of the developing human gut and in intestinal cell lines: relation with cell proliferation. J Cell Biochem 1998;71:536–45. [PubMed] [Google Scholar]
- 45.Frisch SM, Ruoslahti E. Integrins and anoikis. Curr Opin Cell Biol 1997;9:701–6 [DOI] [PubMed] [Google Scholar]
- 46.Gauthier R, Harnois C, Drolet JF, et al. Human intestinal epithelial cell survival: differentiation state-specific control mechanisms. Am J Physiol Cell 2001;280:C1540–54. [DOI] [PubMed] [Google Scholar]