Abstract
Background: Impaired regeneration and dysfunction of the cirrhotic liver following partial hepatectomy (PHx) are the most serious risk factors for postoperative liver failure.
Aims: Using naked hepatocyte growth factor (HGF) plasmid by the electroporation (EP) in vivo method, we investigated HGF for its role and mechanism of proliferation and restoration of liver mass in cirrhotic mice following PHx.
Animals: Eight week old female mice were used.
Methods: HGF plasmid 50 μg was injected intramuscularly and transferred by EP in vivo once a week for three weeks. After establishment of carbon tetrachloride induced cirrhosis, mice underwent PHx. The HGF treated group was given naked HGF plasmid four days before PHx, and additional HGF was given once a week until they were killed, while a control group was given only empty plasmid. Mice were killed 2, 4, 10, and 14 days after PHx. Morphological and functional restoration of the liver were examined, as well as activation of mitogen activated protein kinase (MAPK) and mRNA levels of HGF activator (HGFA).
Results: The HGF treated group demonstrated a continuous threefold increase in HGF levels in plasma. Therapy with HGF in cirrhotic PHx resulted in effective liver regeneration via restoration of HGFA and activation of MAPK p44/p42, accelerated normalisation of liver function, and increased collagen degradation.
Conclusions: HGF gene therapy by in vivo EP may be useful for hepatic resection in cirrhotic livers by stimulating liver proliferative and collagenolytic capacities, as well as accelerating functional recovery.
Keywords: electroporation, hepatocyte growth factor, cirrhosis, gene therapy
Cirrhosis is the end stage of chronic liver injury caused by viral hepatitis or alcohol intake, and effective treatment capable of reversing cirrhosis has not been developed. Most malignant neoplasms of the liver in Japan arise from chronically damaged livers, typically a cirrhotic liver.1 Two main factors are responsible for the irreversibility of cirrhosis. One involves the increased and continuous deposition of extracellular matrix (ECM), the result of increased collagen synthesis accompanied by insufficient breakdown of collagen.2 The other factor is the impaired capability of liver regeneration, which predisposes to postoperative dysfunction or liver failure.3
Previous studies have shown that a fibrotic liver following hepatic resection regenerates very slowly in comparison with the normal liver.3–5 However, little is known of the mechanism of retarded liver growth; furthermore, growth factors can promote cirrhotic regeneration but this mechanism is still under investigation.
Hepatic regeneration following partial hepatectomy (PHx) is a complicated process in which liver cells switch from a quiescent state to a proliferative state and re-enter the cell cycle.6,7 Many growth factors regulate this process by providing both stimulatory and inhibitory signals for hepatocyte proliferation.8,9 Tumour necrosis factor α and interleukin 6 are implicated in the initial step by which hepatocytes enter replicative competence.10,11 Hepatocyte growth factor (HGF) and transforming growth factor α (TGF-α) stimulate further replication,12,13 and finally, TGF-β and activin suppress cell growth and terminate liver regeneration at a set point.14,15
In addition, mitogen activated protein kinases (MAPKs) are activated in response to growth factors and cytokines, and play a central role in many cellular responses, including proliferation, migration, and differentiation.16,17 MAPKs p44/p42, also called extracellular signal regulated kinase (Erk) 1 and 2, are activated by phosphorylation of threonine and tyrosine residues. They form a key signalling pathway involved in the regulation of G1 phase progression in proliferating hepatocytes18 but little is known about activation of this signal pathway in the cirrhotic liver.
In the cirrhotic liver, therapeutic strategies must rely on achieving liver regeneration and increased ECM degradation.19 HGF is a pluripotent growth factor displaying a remarkable ability to promote tissue repair and organ regeneration after injury.20 HGF is a potent agent for acceleration of tissue regeneration following an acute insult, as well as amelioration of tissue fibrosis and dysfunction in chronic conditions.21–23 Despite this, the long term effects of exogenous HGF remain poorly understood. As it is rapidly cleared by the liver in vivo, exogenous HGF is extremely unstable in the blood circulation with a half life of only 3–5 minutes.24 This makes it almost impossible to sustain a constantly high level of exogenous HGF in the circulation, even with repeated injections of HGF protein at short intervals. One way to overcome this problem is to develop a gene transfer strategy allowing persistent expression of HGF protein in vivo, an essential step towards evaluating its therapeutic potential in whole animals.
We have previously reported on electroporation (EP) mediated HGF gene therapy in acute liver injury, and have achieved high HGF expression in vivo temporarily.25 Like other non-viral methods, this approach has obvious advantages, including easy preparation of a large amount of plasmid without immunogenicity and with proven safety in vivo.26 Unlike the other naked DNA method in vivo, which rapidly injects a large volume of physiological saline as vehicle,27–29 our method is much simpler. From a clinical point of view, our method also avoids using damaged liver as a target organ to express exogenous HGF.
Therefore, the aims of the present study were to assess the efficacy of a continuous HGF supply by an in vivo EP method, to evaluate its beneficial action in cirrhotic liver following hepatic resection, and to assess the proliferative mechanism in cirrhotic liver.
MATERIALS AND METHODS
Plasmid DNA
Plasmid pKSCX-HGF was modified and constructed by inserting the full length cDNA of rat HGF into the Xba I and BamH I sites, as described previously25; the plasmid was prepared and dissolved in Endofree TE buffer.
Animals
Induction of cirrhosis
Eight week old female C57BL/6 mice were used throughout this experiment (Sankyo Lab, Osaka, Japan). Mice were maintained under specific pathogen free conditions in the animal centre at Toyama Medical and Pharmaceutical University according to animal care guidelines. Cirrhosis was induced by intragastric administration at a dose of 25 ml/kg body weight of 5% carbon tetrachloride (CCl4) (Wako, Tokyo, Japan) dissolved in olive oil, three times a week for seven weeks.
After establishment of cirrhosis, six mice that were given CCl4 throughout the study were subjected to repeated HGF gene transfer by EP weekly, on three occasions, to monitor the HGF expression pattern in vivo. Blood samples were collected weekly and detected by ELISA for rat HGF (Institute of Immunology, Tokyo, Japan). Values are expressed as ng/ml. The remainder of the mice were subjected to hepatectomy with HGF or empty plasmid transferred by in vivo EP.
HGF gene in vivo electroporation
Four days before hepatectomy, HGF gene or empty plasmid was transferred into muscle by EP, according to our previous procedure.25 Briefly, 50 μg of plasmid DNA were injected into the tibialis anterior muscle while the muscle was held by an electrode (a pair of stainless steel pincers 10 mm in diameter), and electric pulses were delivered to the muscle by an electric pulse generator (CUY 21; Tokiwa Science, Tokyo, Japan). The square shaped pulse had a voltage of 25 during pulse duration. Three pulses of the indicated voltage, followed by three of opposite polarity, were administered at each injection site at a rate of 1 pulse/s, 100 ms duration. Additional HGF gene was transferred once a week until they were killed.
Partial hepatectomy
Three days after finial CCl4 administration, all mice underwent 70% PHx by removal of the anterior two lobes and posterior left lobe,30 and were sacrificed at 2, 4, 10, and 14 days after hepatectomy. Blood and liver samples were stored at −80°C.
Histological examination
After routine processing of paraffin sections, 3 μm thick hepatectomised and remnant liver sections were made. Hepatectomised sections were stained with Sirius red F3BA for confirming cirrhosis, while remnant sections were either stained with Sirius red F3BA for monitoring ECM changes or for immunohistochemical study of proliferating cell nuclear antigen (PCNA) and Ki67
For Sirius red F3BA staining, sections were stained with 0.1% (w/v) Sirius red F3BA (Sigma, St Louis, Missouri, USA) for one hour in a saturated aqueous solution (1.2% w/v) of picric acid (Wako); the final pH of the solution was 2.0. After staining, slides were rinsed for two minutes in 0.01 N HCl solution to remove unbound dye; following dehydration in alcohol, slides were mounted for observation.
Immunohistochemistry
After deparaffinisation and rehydration, sections were inactivated in endogenous peroxidase in 3% H2O2 for 10 minutes followed by incubation with normal sera. The sections were then incubation with polyclonal PCNA antibody (Dako, Copenhagen, Denmark; diluted 1:1000) or ready to use polyclonal Ki67 antibody (Ylem, Italy) at 4°C overnight, respectively. After washing with phosphate buffered saline, sections were treated with EnVision+ Peroxidase, Rabbit (Dako) for 30 minutes. Peroxidase activity was determined with diaminobenzidine solution. For quantitative analysis, the proliferation index (PI) (percentage of stained cells) was calculated at three random fields of each slide. The number of hepatocytes per individual section was 2072 (255) in the HGF treated group and 2088 (271) in controls.
Measurement of alanine aminotransferase, albumin, and total bilirubin
To evaluate remnant liver function in both HGF treated and non-treated groups after PHx, plasma alanine aminotransferase (ALT) and total bilirubin levels were assayed by test paper using the Reflotron-checker (Reflotron; Roche Ltd, Basel, Switzerland). Normal values for ALT and total bilirubin were less than 40 U/l and 1 mg/dl, respectively.
Western blot analysis
Western blot analysis was performed as previously described.31 Briefly, frozen tissue was homogenised in 0.01 M TNE buffer (pH 7.8) (1 ml/0.1 g tissue) containing 5% protease inhibitor cocktail (Sigma), followed by centrifugation in a microfuge at top speed for 20 minutes. Protein concentrations were assayed using the Bio-Rad Protein Assay (Bio-Rad, Hercules, California, USA).
Samples were separated by electrophoresis on 10% polyacrylamide gels and transferred to Immobilon-PVDF (Millipore, Bedford, Massachusetts, USA). After brief incubation with 5% non-fat milk to block non-specific binding, the membrane was exposed overnight to specific phospho p44/ p42 MAPK antibodies (New England Biolabs, Beverly, Massachusetts, USA; 1:1000) at 4°C. The membrane was washed and exposed to alkaline phosphatase conjugated secondary antibodies and visualised by incubation in CDP star assay buffer (New England Biolabs) according to the protocol provided by the company. Phosphorylated p44/ p42 MAPK activity was quantified by laser densitometric analysis of the radiographic film using NIH Image software. Values were means (SD) of triplicate experiments.
Probes
Complementary DNA (cDNA) for hepatocyte growth factor activator (HGFA)32 was kindly given by Dr Okajima, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control.
mRNA extraction and northern blot analysis
Remnant liver tissue was homogenised and poly(A+)RNA was obtained using FastTrack 2.0 mRNA Isolation Kits (Invitrogen, Carlsbad, California, USA). Northern blot was performed using 5 μg of poly(A+)RNA and hybridisation was performed using [α-32P]-labelled cDNA probes, as previously described.31 After hybridisation, membranes were washed with sodium saline citrate containing sodium dodecyl sulphate at 60°C. Relative intensity of the signal was determined by laser densitometric analysis of the radiographic film. Values were means (SD) of triplicate experiments corrected for loading differences using GAPDH as an internal control.
Statistical analysis
Data are expressed as mean (SD). Differences between groups were evaluated by Student’s t test. A p value of <0.05 was considered statistically significant.
RESULTS
Continuous supply of rat HGF by in vivo electroporation
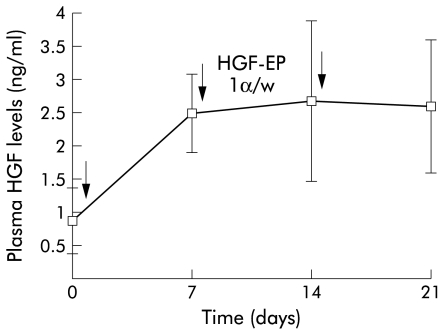
Before HGF gene treatment, plasma HGF levels were 0.88 (0.49) ng/ml in cirrhotic mice. With repeated weekly injection and EP of naked HGF plasmid into muscle, a substantial increase in HGF by ELISA was seen, as shown in fig 1 ▶. Plasma concentrations of HGF increased to 2.48 ng/ml, even after the first administration, which were almost threefold baseline values, and levels were sustained for at least three weeks. This expression pattern was quite different from our previous results of a single HGF gene transfer, where levels had already decreased to baseline by three weeks.25
Figure 1.
Continuous supply of hepatocyte growth factor by in vivo electroporation (HGF-EP). Plasmid (50 μg) was injected into the tibialis anterior muscle and transferred weekly by EP, as indicated by the arrows. Plasma samples from mice following repetitive EP were collected weekly. HGF levels were determined by a specific ELISA for rat HGF protein. Data are presented as mean (SD) (n=6).
Histological study of the liver
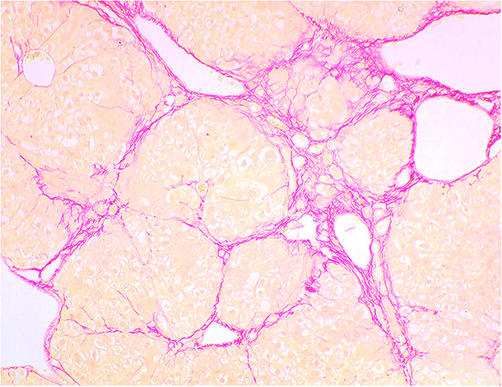
Liver specimens showed formation of regenerative nodules separated by fibrous septa. The formation of thin fibrotic septa at the portal and central areas was observed, and nodule formation was evident in all mice before PHx (fig 2 ▶). There were no marked differences in ECM content between HGF treated and control groups before PHx.
Figure 2.
Histological changes in the liver in carbon tetrachloride treated mice before partial hepatectomy. The liver showed extensive fibrotic development and formation of regenerative nodules. There were no differences between hepatocyte growth factor and control groups (Sirius red staining, original magnification ×40).
Morphological regeneration of the liver following 70% PHx
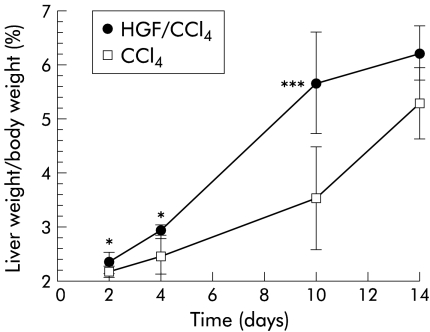
The ratio of liver weight/body weight (%) at various intervals in HGF and control groups is shown in fig 3 ▶. After PHx, body weight in both groups was slightly decreased. However, remnant liver weight as well as body weight recovered more quickly in the HGF group. Significant differences in the ratio between groups were found as early as day 2, peaking on day 10 after PHx (5.66 (0.94)% v 3.52 (0.97)%, respectively; p<0.001), when body weight in the HGF group had already recovered, suggesting that sustained expression of HGF gene in vivo markedly promotes remnant liver as well as body weight growth, even in cirrhotic mice.
Figure 3.
Effect of hepatocyte growth factor (HGF) on liver regeneration in mice with carbon tetrachloride (CCl4) induced cirrhosis after 70% hepatectomy. Mice were killed and livers were harvested at different time points, as described in materials and methods. Result are presented as percentage of remnant liver weight/body weight. Significant differences were shown from day 2 and peaked on day 10, when the HGF group (HGF/CCl4) had already recovered to normal. Data are mean (SD). *p< 0.05, ***p<0.001 versus the control group (CCl4) (n=6).
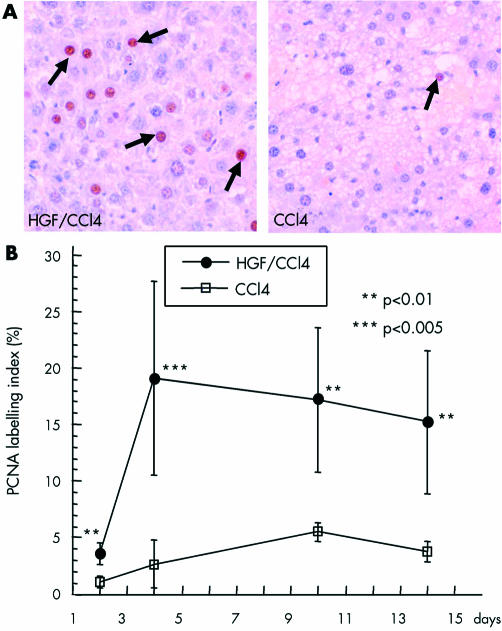
We also examined its effect on hepatocyte proliferation after PHx. Immunostaining of PCNA is illustrated in fig 4 ▶. PI began to increase significantly by day 2, peaked at 19 (4.9)% on day 4 (6.3-fold higher than controls), and was sustained at high levels (over 13%) for at least two weeks. In controls, PI reached a maximum of 5.52 (0.78)% on day 10 and began to decrease. In addition, immunostaining of the other proliferating marker Ki67 was also found to be significantly increased in the HGF group, confirming that exogenous HGF strongly promotes hepatic proliferation. PI values of both Ki67 and PCNA are summarised in table 1 ▶. There was a significant correlation between these two proliferating markers (r=0.77), despite the fact that they did not correspond to each other.
Figure 4.
Effect of hepatocyte growth factor (HGF) on hepatic proliferation after partial hepatectomy. (A) Immunostaining of proliferating cell nuclear antigen (PCNA) was performed, as described in materials and methods. PCNA positive nuclei were stained dark brown, as shown by the arrows, and expressed as a labelling index—percentage of the total number of hepatocyte nuclei. (B) The HGF carbon tetrachloride (HGF/CCl4) group showed a significantly higher labelling index which peaked on day 4 and was sustained at high levels to day 14. Data are mean (SD). **p<0.01, ***p<0.005 versus the control group (CCl4) (n=6).
Table 1.
Proliferation index of proliferating cell nuclear antigen (PCNA) and Ki67 in both hepatocyte growth factor (HGF) and control groups, 2, 4, 10, and 14 days after partial hepatectomy
| Day 2 | Day 4 | Day 10 | Day 14 | ||||||||
| HGF | − | + | − | + | − | + | − | + | |||
| PCNA | 1.10 (0.49) | 3.59 (0.94)** | 2.67 (2.15) | 19.06 (8.54)*** | 5.52 (0.78) | 17.34 (6.35)** | 3.74 (0.91) | 13.14 (3.36)** | |||
| Ki67 | 2.41 (0.88) | 5.15 (2.76) | 4.71 (1.95) | 19.10 (8.04)*** | 6.81 (1.50) | 22.46 (2.65)*** | 2.88(0.44) | 13.63 (4.63)** | |||
Value are mean (SD) positive percentage; n=6 in each group.
**p<0.01, ***p<0.005.
Liver functional restoration after 70% PHx
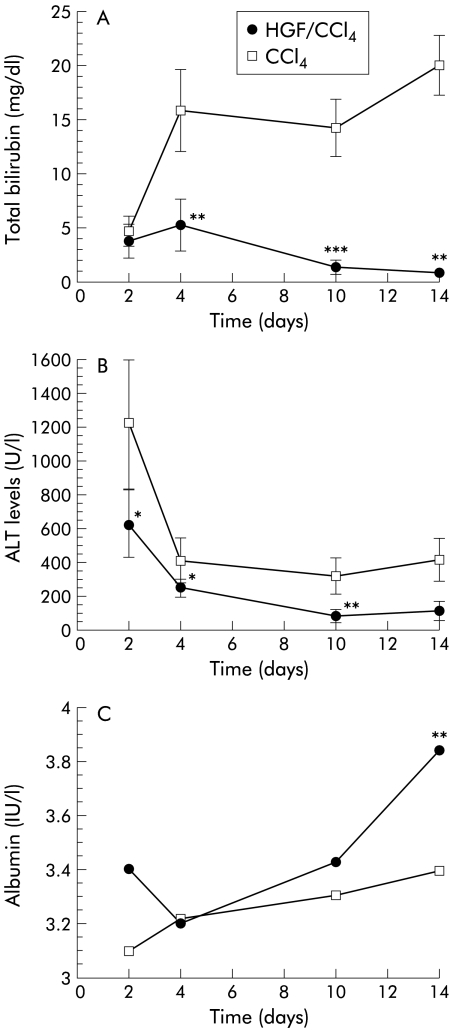
Total bilirubin peaked four days after PHx in both groups, but levels in the HGF group were significantly lower, with a maximum of 6.24 mg/dl, which decreased to normal levels by day 14. Levels in the control group peaked at 15.86 mg/dl (p<0.001) and were sustained at higher levels over two weeks (fig 5A ▶). ALT values peaked two days after PHx and decreased thereafter. ALT levels in the HGF group were relatively low and recovered more quickly compared with controls (fig 5B ▶). Albumin restoration was much slower. In the HGF group, albumin reached 3.85 g on day 14 after PHx while controls only reached 3.4 g of albumin (p<0.05) (fig 5C ▶).
Figure 5.
Effect of hepatocyte growth factor (HGF) on total bilirubin, alanine aminotransferase (ALT), and albumin after partial hepatectomy. (A) Changes in total bilirubin in HGF carbon tetrachloride (HGF/CCl4) and control (CCl4) groups. Total bilirubin was significantly suppressed in the HGF group and recovered to normal by day 14 while controls sustained high levels. (B) Changes in ALT. In the HGF group, ALT levels were significantly lower and normalised quickly. (C) Changes in albumin. At day 14, albumin levels were significantly higher in the HGF group. Data are mean (SD). *p<0.01, **p<0.005, ***p<0.001 versus the control group (n=6).
Changes in ECM content after PHx
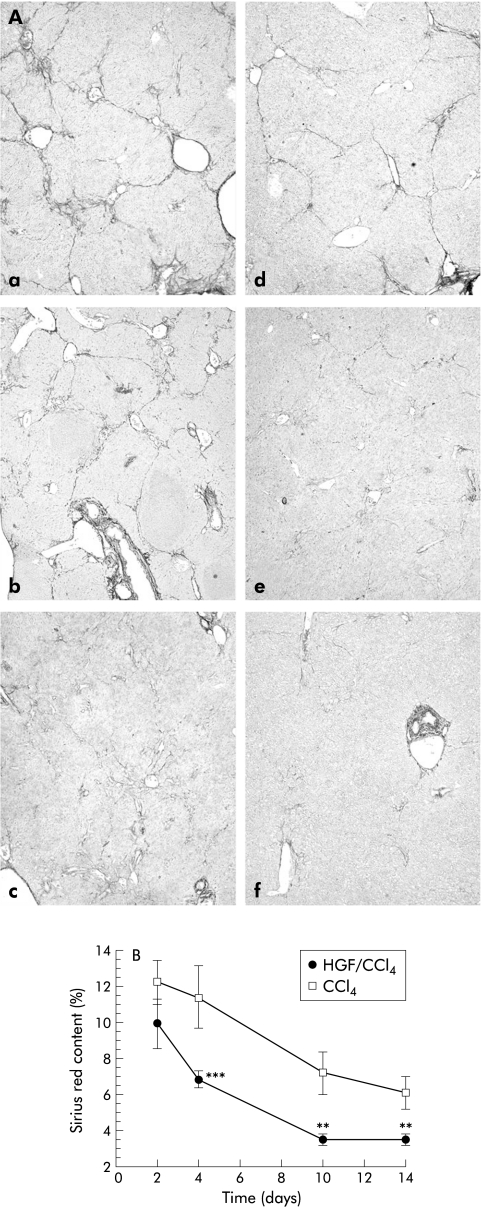
Figure 6 ▶ shows sequential staining of ECM in the liver after PHx in both HGF and control groups. At day 4, both showed clear regenerative nodules separated by fibrous septa; however, the HGF group was thinner with an ECM content of 6.79 (0.48)% while the non-treated group had 11.38 (1.74)% (p<0.001). On day 10, ECM decreased with HGF to 3.43 (0.31)% and on day 14 was almost unmeasurable, similar to normal liver; in the non-treated group, ECM content was 6.07 (0.89)% (p<0.005) on day 14.
Figure 6.
Changes in extracellular matrix (ECM) in liver sections following partial hepatectomy (PHx). (A) Control carbon tetrachloride (CCl4) group (a–c); hepatocyte growth factor/CCl4 (HGF/CCl4) group (d–f), four days after PHx (a, d), 10 days after PHx (b, e), and 14 days after PHx (c, f). Sections on day 4 after PHx showed extensive fibrotic development which was attenuated in the HGF group. ECM values in the HGF group degraded quickly and were normal by day 14 (original magnification ×40). (B) ECM content was assayed and expressed as a percentage of the total area of liver section. Data are mean (SD). **p<0.005, ***p<0.001 versus the control group (n=6).
Activation of mitogen activated protein kinase in vivo
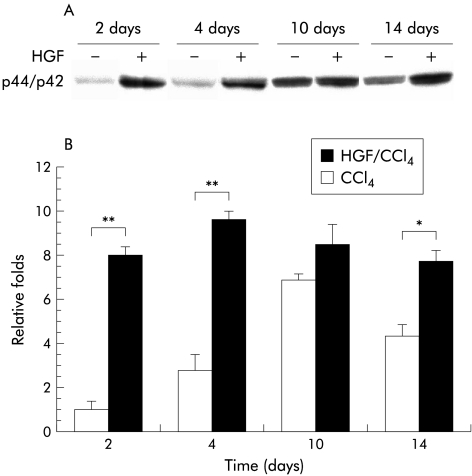
As shown in fig 7 ▶, phosphorylated p44-MAPK (Erk1) and p42-MAPK (Erk2) peaked on day 4 following PHx in the HGF group compared with day 10 in controls. Activation of Erk1/Erk2 was closely related to PCNA expression, confirming that it plays a role in hepatocyte proliferation. Persistent elevation of phosphorylated Erk1/Erk2 was observed through to day 14 in the HGF group while it was greatly decreased in controls by day 14. The results show that exogenous HGF stimulates activation of Erk1/Erk2 in the cirrhotic liver, promoting activation levels, as well as prolonging its duration of expression.
Figure 7.
(A) Western blot of phosphorylated mitogen activated protein kinases (MAPK) p44/p42 in liver homogenates. Mice were subjected to partial hepatectomy with or without hepatocyte growth factor (HGF). Tissue was harvested from the respective animals at the indicated times and prepared as described in materials and methods. Liver protein (30 μg) was analysed by 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis. MAPK p44/ p42 expression in controls (carbon tetrachloride (CCl4)) on day 2 was used as the standard for comparing other samples. (B) Following densitometric analysis of three separate samples in each group, relative changes in MAPK p44/ p42 expression were evaluated. The results revealed significantly higher and persistent activation of MAPK p44/ p42 in the HGF group (HGF/CCl4). Values are expressed as mean (SD). *p<0.01, **p<0.005 versus the control group (n=6).
Northern blot
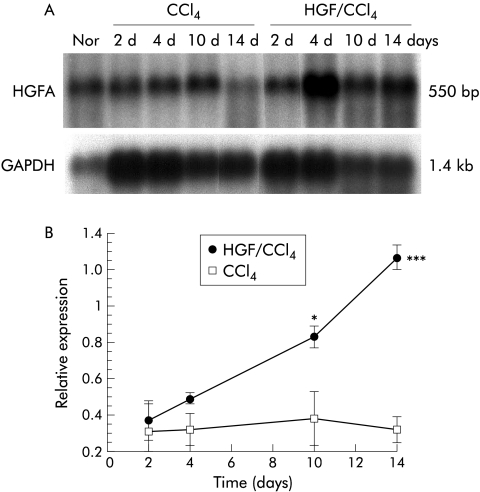
Expression of HGFA was markedly suppressed in cirrhotic liver (fig 8 ▶); levels almost recovered with HGF treatment by day 10. In contrast, controls maintained low levels, suggesting that a continuous supply of exogenous HGF accelerates HGFA recovery in the cirrhotic liver.
Figure 8.
Induction of hepatocyte growth factor activator (HGFA) gene after partial hepatectomy (PHx). (A) Poly (A+)RNA was isolated from HGF carbon tetrachloride (HGF/CCl4) and control (CCl4) groups at various time points (2, 4, 10, 14 days) after PHx. Changes in mRNA levels of HGFA were evaluated by northern blot. Each lane contained 5 μg of poly (A+)RNA. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression was evaluated concurrently in the same samples to show lane to lane equivalency of mRNA loading. Representative autoradiographs are shown. (B) The ratio of HGFA/GAPDH in normal livers at 10 hours after PHx was selected as a standard to which each ratio was compared for relative expression. Analysis of HGFA mRNA expression in three mice/group/time point showed a decrease in cirrhotic livers which was significantly increased by HGF by day 4 and recovered to normal by day 14. Values are mean (SD). *p<0.05, ***p<0.001 versus the control group.
DISCUSSION
In this study, we achieved a continuous supply of HGF by electroporation (EP), a non-viral gene transfer method in vivo which has been widely used to introduce DNA into various types of cells, both in vitro and in vivo.33,34 In common with other non-viral methods, it has various advantages over viral vectors where all of the tissues and cells are theoretical targets. In addition, it is easy and quick to perform; repeated administration of DNA is possible, no specialised processing for DNA construction is required, and severe tissue damage is not observed. In this study, with repeated delivery of naked plasmid, threefold levels of HGF were sustained. The expression pattern was markedly better than with HGF gene delivery via high doses of adenoviral vector35 in which it was extremely difficult to sustain HGF levels due to adverse immune responses.
The hepatic resection model can transiently induce a high concentration of endogenous HGF36 but an impaired regenerative and functional capacity of the cirrhotic liver counters the response to endogenous HGF changes. In this study, quantitative analysis with PCNA and Ki67 staining also yielded information on such retarded restoration. Both Ki67 and PCNA are regarded as proliferating markers but there are important differences in expression of these two proteins. Ki67 starts to be expressed in mid G1, through S and G2, and reaches its peak in M phase. In contrast, PCNA can be detectable from the M phase to G0 and/or G1, with a much longer half life. Previous studies showed that PCNA had a higher positive rate in comparison with that of Ki67.37 Our results showed that the PI of these two markers was similar, which may be due in part to the Ki67 antibody, as we used a polyclonal antibody in our study. In addition, PCNA and Ki67 were significant correlated in this study.
HGF, secreted as an inactive single chain protein (scHGF), is proteolytically cleaved at the Arg-Val-Val (aa494–495) site to form an active two chain HGF (tcHGF). Although the HGF receptor (c-Met) is capable of binding both inactive scHGF and active tcHGF with similar avidity, only tcHGF can activate the c-Met tyrosine kinase domain.38 Recent studies also showed that increased plasma HGF does not simply correlate with biologically active HGF,36 which is 1.9-fold higher than scHGF in stimulating hepatic regeneration.39 We hypothesised that the mechanism of retarded regeneration in cirrhotic liver is due to blockade of scHGF activation. Proteases are known to cleave scHGF to active tcHGF: urokinase plasminogen activator (uPA), tissue-type plasminogen activator, blood coagulation factor XIIa, and HGFA. Among them, HGFA is suggested to be the major activator of hepatic regeneration40 although uPA is also important in the early stages of hepatic regeneration.41 Our northern blots showed that expression of HGFA was obviously suppressed in the cirrhotic liver but could be enhanced by exogenous HGF such that levels recovered at day 10, suggesting that accumulation of HGF in vivo might restore HGFA and promote HGF maturation through a positive feedback manner. mRNA levels of uPA were not different in the two groups (data not shown).
When bound to tcHGF, the Met receptor dimerises and becomes phosphorylated on tyrosine residues to stimulate several signal pathways.42 The most known pathway is the Ras→Raf→Mek→Erk-MAPK cascade, which then leads to cellular responses, including proliferation.43,44 Western blot showed that MAPK p44/p42 was strongly activated by exogenous HGF, unlike the ordinary activation pattern in normal liver following Phx18; persistent elevation was observed in the HGF gene treated group, closely related to high level expression of PCNA. We believe that exogenous HGF in vivo accelerates HGF maturation via HGFA restoration in cirrhosis. After its maturation, the MAPK pathway plays a role in mediating HGF effects on hepatocyte proliferation. Thus we suggest that pretreatment and a long term supply of high level HGF is required to promote cirrhotic regeneration. In support of this view, another report showed that HGF administration following liver injury was less effective than pretreatment due to delayed active HGF emergence.45 However, to date, we have not detected an active form of HGF at the protein level. Further study is necessary to confirm the relation between expression of HGFA and active HGF.
Concerning the antifibrotic effects of HGF, two different mechanisms are proposed. One is an increase in hepatic collagenase activity which promotes degradation of the ECM components.46 Our data extended this result by demonstrating more rapid degradation of ECM with exogenous HGF after PHx. In the HGF group, ECM values decreased to normal levels on day 14 while controls sustained elevated levels. Another pathway is reduction of mRNA levels of procollagens and TGF-β1.47 TGF-β1 is a crucial factor in liver fibrosis and a potent growth inhibitor of hepatocytes. Ueki and colleagues21 demonstrated that HGF could antagonise TGF-β1 directly so as to improve liver regeneration by inhibiting TGF-β1 expression.
Liver function after PHx is crucial in relation to postoperative liver insufficiency.39 In this study, we assessed parameters indicative of liver function and their sequential changes during liver regeneration. The results showed that exogenous HGF prevented hepatocytes from severe damage after PHx and accelerated normalisation, especially for recovery of total bilirubin and ALT levels. The mechanism responsible for improved functional capacity after HGF remains unclear. Our previous data showed that HGF inhibits DNA fragmentation and apoptosis in acute liver injury, which is one mechanism underlying its cytoprotective effects in vivo,25 but such effects were not confirmed in our cirrhotic model. Ogura and colleagues48 reported that recombinant HGF accelerates serum albumin recovery quickly after cirrhotic resection due to its stimulation of albumin synthesis; however, our results showed that albumin was restored more slowly in comparison with ALT and total bilirubin. HGF can restore functional capacity after PHx but this is delayed in contrast with its effect on morphological restoration.
In summary, electroporation in vivo is a convenient, safe, and efficient method of delivering and expressing high levels of exogenous HGF in vivo. By repeated administration, we achieved sustained long term expression of the HGF gene. HGF in vivo restored HGFA for HGF maturation, and promoted effective proliferation in cirrhotic liver via growth related signal transduction MAPK p44/p42. HGF also increased removal of deposited collagen and accelerated normalisation of liver function. Thus HGF gene delivery by EP in vivo is a potential therapeutic approach for hepatic resection with liver cirrhosis.
Abbreviations
PHx, partial hepatectomy
HGF, hepatocyte growth factor
scHGF, single chain hepatocyte growth factor
tcHGF, two chain hepatocyte growth factor
TGF, transforming growth factor
EP, electroporation
ECM, extracellular matrix
CCl4, carbon tetrachloride
PCNA, proliferating cell nuclear antigen
PI, proliferation index
ALT, alanine aminotransferase
HGFA, hepatocyte growth factor activator
MAPKs, mitogen activated protein kinases
ERK, extracellular signal regulated kinase
GAPDH, glyceraldehyde-3-phosphate dehydrogenase
uPA, urokinase plasminogen activator
REFERENCES
- 1.Shiratori Y, Shiina S, Imamura M, et al. Characteristic difference of hepatocellular carcinoma between hepatitis B- and C-viral infection in Japan. Hepatology 1995;22:1027–33. [DOI] [PubMed] [Google Scholar]
- 2.Bickel M, Baringhaus KH, Gerl M, et al. Selective inhibition of hepatic collagen accumulation in experimental liver fibrosis in rats by a new prolyl 4-hydroxylase inhibitor. Hepatology 1998;28:404–11. [DOI] [PubMed] [Google Scholar]
- 3.Andiran F, Ayhan A, Tanyel FC, et al. Regenerative capacities of normal and cirrhotic livers following 70% hepatectomy in rats and the effect of α-tocopherol on cirrhotic regeneration. J Surg Res 2000;89:184–8. [DOI] [PubMed] [Google Scholar]
- 4.Kawasaki S, Imamura H, Bandai Y, et al. Direct evidence for the intact hepatocyte theory in patients with liver cirrhosis. Gastroenterology 1992;102:1351–5. [PubMed] [Google Scholar]
- 5.Hashimoto M, Watanabe G. Functional capacity of the cirrhotic liver after partial hepatectomy in the rat. Surgery 1999;126:541–7. [PubMed] [Google Scholar]
- 6.Li W, Liang X, Leu JI, et al. Global changes in interleukin-6-dependant gene expression patterns in mouse livers after partial hepatectomy. Hepatology 2001;33:1377–86. [DOI] [PubMed] [Google Scholar]
- 7.Francavilla A, Panella C, Polimeno L, et al. Hormonal and enzymatic parameters of hepatic regeneration in patients undergoing major liver resections. Hepatology 1990;12:1134–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michalopoulos GK, DeFrances MC. Liver regeneration. Science 1997;276:60–6. [DOI] [PubMed] [Google Scholar]
- 9.Fausto N, Laird AD, Webber EM. Liver regeneration. 2. Role of growth factors and cytokines in hepatic regeneration. FASEB J 1995;9:1527–36. [DOI] [PubMed] [Google Scholar]
- 10.Cressman DE, Greenbaum LE, DeAngelis RA, et al. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science 1996;274:1379–83. [DOI] [PubMed] [Google Scholar]
- 11.Yamada Y, Kirillova I, Peschon JJ, et al. Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proc Natl Acad Sci U S A 1997;94:1441–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogura Y, Hamanoue M, Tanabe G, et al. Hepatocyte growth factor promotes liver regeneration and protein synthesis after hepatectomy in cirrhotic rats. Hepatogastroenterology 2001;48:545–9. [PubMed] [Google Scholar]
- 13.Block GD, Locker J, Bowen WC, et al. Population expansion, clonal growth, and specific differentiation patterns in primary cultures of hepatocytes induced by HGF/ SF, EGF and TGFα in a chemically defined (HGM) medium. J Cell Biol 1996;132:1133–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kogure K, Omata W, Kanzaki M, et al. A single intraportal administration of follistatin accelerates liver regeneration in partially hepatectomized rats. Gastroenterology 1995;108:1136–42. [DOI] [PubMed] [Google Scholar]
- 15.Moriuchi A, Hirono S, Ido A, et al. Additive and inhibitory effects of simultaneous treatment with growth factors on DNA synthesis through MAPK pathway and G1 cyclins in rat hepatocytes. Biochem Biophys Res Commun 2001;280:368–73. [DOI] [PubMed] [Google Scholar]
- 16.Cowley S, Paterson H, Kemp P, et al. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH3T3 cells. Cell 1994;77:841–52. [DOI] [PubMed] [Google Scholar]
- 17.Pagés G, Lenormand P, L’Allemain G, et al. Mitogen-activated protein kinases p42mapk and p44mapk are required for fibroblast proliferation. Proc Natl Acad Sci U S A 1993;90:8319–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Talarmin H, Rescan C, Cariou S, et al. The mitogen-activated protein kinase kinase/extracellular signal-regulated kinase cascade activation is a key signaling pathway involved in the regulation of G1 phase progression in proliferating hepatocytes. Mol Cell Biol 1999;19:6003–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernández-Muñoz R, Diaz-Muñoz M, Suárez-Cuenca S, et al. Adenosine reverses a preestablished CCl4-induced micronodular cirrhosis through enchancing collagenolytic activity and stimulating hepatocyte cell proliferation in rats. Hepatology 2001;34:677–87. [DOI] [PubMed] [Google Scholar]
- 20.Ishiki Y, Ohnishi H, Muto Y, et al. Direct evidence that hepatocyte growth factor is a hepatotrophic factor for liver regeneration and has a potent antihepatitis effect in vivo. Hepatology 1992;16:1227–35. [PubMed] [Google Scholar]
- 21.Ueki T, Kaneda Y, Tsutsui H, et al. Hepatocyte growth factor gene therapy of liver cirrhosis in rat. Nat Med 1999;5:226–30. [DOI] [PubMed] [Google Scholar]
- 22.Fujimoto J. Reversing liver cirrhosis: impact of gene therapy for liver cirrhosis. Gene Ther 1999;6:305–6. [DOI] [PubMed] [Google Scholar]
- 23.Shiota G, Kunisada T, Oyama K, et al. In vivo transfer of hepatocyte growth factor gene accelerate proliferation of hepatic oval cells in a 2-acetylaminofluorene/partial hepatectomy model in rats. FEBS Lett 2000;470:325–30. [DOI] [PubMed] [Google Scholar]
- 24.Kawaida K, Matsumoto K, Shimazu H, et al. Hepatocyte growth factor prevents acute renal failure and accelerates renal regeneration in mice. Proc Natl Acad Sci U S A 1994;91:4357–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xue F, Takahara T, Yata Y, et al. Attenuated acute liver injury in mice by naked HGF gene transfer into skeletal muscle with electroporation. Gut 2002;50:558–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aihara H, Miyazaki J. Gene transfer into muscle by electroporation in vivo. Nat Biotech 1998;16:867–70. [DOI] [PubMed] [Google Scholar]
- 27.Liu F, Song YK, Liu D. Hydrodynamic-based transfection in animals by systemic administration of plasmid DNA. Gene Ther 1999;6:1258–66. [DOI] [PubMed] [Google Scholar]
- 28.Zhang G, Budker V, Wolff JA. High levels of foreign gene expression in hepatocytes after tail vein injections of naked plasmid DNA. Hum Gene Ther 1999;10:1735–7. [DOI] [PubMed] [Google Scholar]
- 29.Yang J, Chen S, Huang L, et al. Sustained expression of naked plasmid DNA encoding hepatocyte growth factor in mice promotes liver and overall body growth. Hepatology 2001;33:848–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Higgins GM, Anderson RM. Experimental pathology of the liver. I. Restoration of the liver of the white rat following partial surgical removal. Arch Pathol 1931;12:186. [Google Scholar]
- 31.Zhang LP, Takahara T, Yata Y, et al. Increased expression of plasminogen activator and plasminogen activator inhibitor during liver fibrosis of rats: role of stellate cells. J Hepatol 1999;31:703–11. [DOI] [PubMed] [Google Scholar]
- 32.Okajima A, Miyazawa K, Naitoh Y, et al. Induction of hepatocyte growth factor activator messenger RNA in the liver following tissue injury and acute inflammation. Hepatology 1997;25:97–102. [DOI] [PubMed] [Google Scholar]
- 33.Rols MP, Delteil C, Golzio M, et al. In vivo electrically mediated protein and gene transfer in murine melanoma. Nat Biotechnol 1998;16:168–71 [DOI] [PubMed] [Google Scholar]
- 34.Yamashita Y, Shinada M, Hasegawa H, et al. Electroporation-mediated interleukin 12 gene therapy for hepatocellular carcinoma in the mice model. Cancer Res 2001;61:1005–12. [PubMed] [Google Scholar]
- 35.Gao C, Jokerst R, Gondipalli P, et al. Intramuscular injection of an adenoviral vector expressing hepatocyte growth factor facilitates hepatic transduction with a retroviral vector in mice. Hum Gene Ther 1999;10:911–22. [DOI] [PubMed] [Google Scholar]
- 36.Pediaditakis P, Lopez-Talavera JC, Petersen B, et al. The processing and utilization of hepatocyte growth factor/scatter factor following partial hepatectomy in the rat. Hepatology 2001;34:688–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nguyen VN, Mirejovsky P, Mirejovsky T, et al. Expression of cyclin D1, Ki67 and PCNA in non-small cell lung cancer: prognostic significance and comparison with p53 and bcl-2. Acta Histochemica 2000;102:323–38. [DOI] [PubMed] [Google Scholar]
- 38. Lokker NA, Mark MR, Luis EA, et al. Structure-function analysis of hepatocyte growth factor: identification of variants that lack mitogenic activity yet retain high affinity receptor binding. EMBO J 1992;11:2503–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shima N, Tsuda E, Goto M, et al. Hepatocyte growth factor and its variant with a deletion of five amino acids are distinguishable in their biological activity and tertiary structure. Biochem Biophys Res Commun 1994;200:808–15. [DOI] [PubMed] [Google Scholar]
- 40.Miyazawa K, Shimomura T, Kitamura N. Activation of hepatocyte growth factor in the injured tissues is mediated by hepatocyte growth factor activator. J Biol Chem 1996;271:3615–8. [DOI] [PubMed] [Google Scholar]
- 41.Shimizu M, Hara A, Okuno M, et al. Mechanism of retarded liver regeneration in plasminogen activator-deficient mice: Impaired activation of hepatocyte growth factor after Fas-mediated massive hepatic apoptosis. Hepatology 2001;33:569–76. [DOI] [PubMed] [Google Scholar]
- 42.Hunter T. Oncoprotein networks. Cell 1997;88:333–46. [DOI] [PubMed] [Google Scholar]
- 43.Ried S, Jager C, Jeffers M, et al. Activation mechanisms of the urokinase-type plasminogen activator promoter by hepatocyte growth factor/scatter factor. J Biol Chem 1999;274:16377–86. [DOI] [PubMed] [Google Scholar]
- 44.Renshaw MW, Ren XD, Schwartz MA. Growth factor activation of MAP kinase require cell adhesion. EMBO J 1997;16:5592–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kosai K, Matsumoto K, Nagata S, et al. Abrogation of Fas-induced fulminant hepatic failure in mice by hepatocyte growth factor. Biochem Biophys Res Commun 1998;244:683–90. [DOI] [PubMed] [Google Scholar]
- 46.Matsuda Y, Matsumoto K, Ichida T, et al. Hepatocyte growth factor suppresses the onset of liver cirrhosis and abrogates lethal hepatic dysfunction in rats. J Biochem Tokyo 1995;118:643–9. [DOI] [PubMed] [Google Scholar]
- 47.Yasuda H, Imai E, Shiota A, et al. Antifibrotic effect of a deletion variant of hepatocyte growth factor on liver fibrosis in rats. Hepatology 1996;24:636–42. [DOI] [PubMed] [Google Scholar]
- 48.Ogura Y, Hamanoue M, Tanabe G, et al. Hepatocyte growth factor promotes liver regeneration and protein synthesis after hepatectomy in cirrhotic rats. Hepatogastroenterology 2001;48:545–9. [PubMed] [Google Scholar]