Abstract
Background and aims: Short chain fatty acids (SCFA) exert profound effects on the colonic mucosa. In particular, SCFA modulate mucosal immune functions. The antimicrobial cathelicidin LL-37 is expressed by colon epithelial cells. In the present study the effect of SCFA on LL-37 expression was investigated.
Methods: LL-37 expression in vivo was assessed by immunohistochemistry. Real time quantitative reverse transcription-polymerase chain reaction was employed to determine LL-37 expression in colonocytes in vitro after treatment with various cytokines, SCFA, or flavone. LL-37 levels were correlated to cell differentiation which was determined by alkaline phosphatase (AP) activity. In addition, intracellular signalling pathways such as MEK-ERK (mitogen/extracellular signal protein kinase (MEK)/extracellular signal regulated protein kinase (ERK)) and p38/mitogen activated protein (MAP) kinase were explored.
Results: In vivo, LL-37 expression in healthy mucosa was restricted to differentiated epithelial cells in human colon and ileum. In colonocytes, increased LL-37 expression associated with cell differentiation was detected in vitro following treatment with butyrate, isobutyrate, propionate, and trichostatin A. Flavone induced LL-37 transcription but did not affect AP activity while cytokines had no effect. To dissect pathways mediating differentiation and LL-37 expression, specific inhibitors were applied. Inhibition of the protein kinase MEK enhanced butyrate induced AP activity while LL-37 expression in colon epithelial cells was blocked. In contrast, inhibition of p38/MAP kinase blocked cell differentiation without inhibiting LL-37 expression.
Conclusions: Expression of the cathelicidin LL-37 in colonocytes and cellular differentiation are separately modulated by SCFA via distinct signalling pathways. These data may provide a rationale for dietary modulation of mucosal defence mechanisms.
Keywords: cathelicidin LL-37, short chain fatty acids, colon, innate immunity, MEK-ERK
The single cell layer of the colonic epithelium is an active barrier against the external environment and the enormous load of intestinal bacteria. In addition to forming a physical barrier, the epithelium is armed with an array of effector molecules, including antimicrobial peptides.1,2 These peptides can be considered as endogenous antibiotics and are widespread in nature as immediate defence effectors. They have been found in invertebrates, vertebrates, and plants, as well as in bacteria, and several human antimicrobial peptides have been characterised.3–5 They are mainly stored in vacuoles of granulocytes ready for activation upon stimulation or secreted directly onto mucosal surfaces by epithelial cells.
The cathelicidins constitute a family of precursor proteins with a well conserved cathelin pro-region, followed by a highly variable C terminal antimicrobial domain. The only human cathelicidin gives rise to LL-37, a 37 residue mature antimicrobial peptide, after cleavage from the cathelin propart.6–8 LL-37 is present in neutrophils and lymphocytes.7,9 In addition, LL-37 is synthesised by bone marrow,6 keratinocytes of inflamed skin,10 lung epithelium,11 and squamous epithelia of human mouth, tongue, oesophagus, cervix, and vagina.12 Both purified and chemically synthesised LL-37 peptides exhibit potent and comparable antimicrobial activities in vitro.5,13
Antimicrobial peptides are active effector molecules in intestinal mucosal defence and constitute an integral part of immediate responses at epithelial barriers.10,14,15 Mice deficient in the metalloproteinase matrilysin, which is responsible for processing epithelial α-defensins (cryptdins) of the small intestine, are more sensitive to orally administered bacteria.16 Furthermore, certain bacteria have evolved mechanisms to overcome the antimicrobial peptide barrier in human colon; Shigella bacteria downregulate LL-37 and HBD-1 expression in the colon epithelium, as a potential invading mechanism.17 In contrast, transgenic mice, expressing additional human defensin in their Paneth cells, demonstrate increased survival after challenge with Salmonella typhimurium.18 Taken together, these results emphasise the importance of the peptides in the barrier function of gut epithelia.
Alterations of the colonic epithelial barrier may occur in response to dietary changes, medical treatment, or disease. Lack of dietary fibre can facilitate bacterial translocation from the gut.19 Short chain fatty acids (SCFA)—namely, acetate, propionate, and butyrate—are derived from bacterial fermentation of undigested dietary fibre in the colon.20 Butyrate and other SCFA exert profound effects on colonic physiology as they affect fluid absorption, colonocyte metabolism, proliferation and differentiation, gut motility, and mucosal inflammation.20,21
Spontaneously differentiating colon epithelial cells and cells treated with the differentiation inducing agent butyrate expressed more LL-37 than untreated controls in a recently published study.22 LL-37 was not upregulated in response to stimulation with various proinflammatory mediators. Therefore, the authors concluded that cell differentiation is the key determinant of LL-37 expression in colon epithelial cells.22 In the present study, regulation of LL-37 expression in human intestinal epithelial cells was further characterised. We report that the effect of SCFA on LL-37 expression is not strictly coupled to the stage of cellular differentiation. Intracellular signalling pathways are critical for SCFA modulated LL-37 expression in colon epithelial cells.
MATERIALS AND METHODS
Cells and stimulation experiments
The colon epithelial cell lines SW620 (ATCC CCL-227), SW480 (ATCC CCL-228), and HT-29 (ATCC HTB-38) were used for expression studies. SW620 and SW480 cells were grown in minimal essential medium with 10% fetal calf serum (FCS), 2 mmol/l l-glutamine, 100 U/ml penicillin, and 100 μg streptomycin (Life Technologies, Karlsruhe, Germany). HT-29 cells were cultured in RPMI medium (Life Technologies) supplemented with 5% FCS and 2 mmol/l l-glutamine. In addition, we used an epithelial cell line named Geki2 that was developed in our laboratory and derived from a colon adenoma of a 56 year old female. All cell lines were cultured in 75 cm2 flasks in a humidified atmosphere at 37°C in the presence of 5% CO2. Acetate (5 mmol/l), propionate (10 mmol/l), butyrate (2 mmol/l, 4 mmol/l), isobutyrate (0.2 mmol/l, 2 mmol/l), lactate (0.2 mmol/l, 2 mmol/l), flavone (25–150 μmol/l) (all from Sigma-Aldrich, Steinheim, Germany), or trichostatin A (400 ng/ml) (Calbiochem, California, USA) was added to serum free medium and cells were incubated for up to 48 hours.
Analyses of cell signalling pathways
To investigate the involvement of intracellular signalling pathways such as the MEK-ERK (mitogen/extracellular signal protein kinase (MEK)-extracellular signal regulated protein kinase (ERK)) pathway or the p38/mitogen activated protein (MAP) kinase pathway in butyrate induced cell differentiation and LL-37 expression, additional studies were performed. SW620 and HT-29 colon cells were incubated with or without the specific MEK inhibitor U0126 (20 μmol/l) (Cell Signalling, Massachusetts, USA) for 30 minutes before butyrate (2 mmol/l) or trichostatin A (400 ng/ml) addition to the culture medium, and cells were incubated for up to 48 hours. Furthermore, SW620 and HT-29 cells were treated with or without the p38/MAP kinase inhibitor SB203580 (10 μmol/l) (Calbiochem) for 30 minutes prior to butyrate treatment.
RT-PCR and TaqMan real time RT-PCR assay
At each time point, total RNA was prepared from the attached growing cell fraction using Trifast (Peqlab, Erlangen, Germany) according to the manufacturer’s instructions. An RNA clean up protocol was performed followed by treatment with RNAse free DNAse (RNeasy; Quiagen, Hilden, Germany). All RNA material was denatured at 94°C for five minutes and chilled to 4°C. A semiquantitative reverse transcription-polymerase chain reaction (RT-PCR) approach by PCR amplification with subsequent Southern blotting and hybridisation was performed, as described previously.17
For quantification, expression of LL-37 and the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were measured in triplicate from 2–5 independent RNA extractions by real time quantitative RT-PCR using a PE Applied Biosystems (ABI, Foster City, California, USA) PRISM model 7700 sequence detection instrument. GAPDH transcript numbers were measured by PCR reactions using the GAPDH-PDAR-Housekeeping Kit (ABI) which does not amplify GAPDH pseudogenes. The sequences of forward and reverse primers, as designed by Primer Express (ABI) for quantification of LL-37 mRNA were 5‘-ACC CAG CAG GGC AAA TCT C-3‘ and 5‘-GAA GGA CGG GCT GGT GAA G-3‘, respectively. The TaqMan fluorogenic probe used for LL-37 was 5‘-TGT TAT CCT TAT CAC AAC TGA T-3‘ FAM label. A standard curve was constructed from serial dilutions of cDNA synthesised from a known quantity of total RNA from U937 monocytic cells (ACC5, DSMZ Braunschweig, Germany). LL-37 and GAPDH starting transcript number values for the standard curve were set arbitrarily. LL-37 and GAPDH values in unknown samples were quantified by measuring Ct and reading the corresponding value off the standard curve. LL-37 expression was then normalised to GAPDH expression and LL-37 expression levels in medium treated control cells were considered to be “1” in LL-37 expressing cells (HT-29, Geki-2 cells). Relative expression of LL-37 was calculated by relating LL-37 expression in treated cells to control cells. SW620 and SW480 did not express LL-37 transcripts at baseline and when treated with the medium control. In order to quantify LL-37 expression in those cells, LL-37 expression after 24 h hours of treatment with butyrate 2 mmol/l was considered to be “1” and all other levels were correlated with this value.
Immunohistochemistry
Healthy colorectal mucosa and biopsy material of the distal ileum obtained from patients during another study23 and from patients undergoing routine colonoscopy were analysed. The study was approved by the ethics committee at the Faculty of Medicine, University of Würzburg. Biopsies were taken with a standard tong and staining with a specific LL-37 polyclonal rabbit antiserum was performed as described previously.17 Briefly, biopsies were fixed in 5% formaldehyde and embedded in paraffin. Serial sections were stained with a 1/4000 dilution of the specific LL-37 antiserum (2.5 μg/μl) according to a standard procedure using a biotin-streptavidin detection system (Multilink; BioGenex, California, USA). As a negative control, the primary antibody was preabsorbed with excess amounts of the synthetic LL-37 peptide, as previously described.17
Apoptosis assays
Attached growing SW620 and HT-29 cells were treated with butyrate or flavone, as described above, harvested and dispensed to 106 cells/ml in cold binding buffer (CBB; 10 mmol/l Hepes, 150 mmol/l NaCl, 2.5 mmol/l CaCl2, 10 mmol/l MgCl2, 20% bovine serum albumin). The cell suspension (495 μl) was placed into an Eppendorf microfuge tube and 5 μl of annexin-V-fluoroisothiocyanate (Sigma-Aldrich) were added, stored on ice for 13 minutes, and centrifuged (900 rpm, 4°C, 10 minutes). The pellet was diluted in 500 μl of CBB, and 5 μl of propidium iodine (50 μg/ml; Sigma) were added to indicate the integrity of the cell membrane and to differentiate early (annexin V+/propidium iodine−) and late (annexin V+/propidium iodine+) apoptotic cells. Cells were washed and resuspended in 500 μl of CBB for analysis by FACScan (Becton Dickinson, Mannheim, Germany). After excitation at 488 nm, emission of FITC and propidium iodine was recorded through specific band pass filters: 530 (30) nm for FITC (FL-1), 675 (22) nm for propidium iodine (FL-3), and appropriate electronic compensations were adjusted. Analyses was performed by WIN.MDI 2.8 (Joseph Trotter, © 1993–1998). For further measurement of late apoptosis, cells (250 000 cells/slide) were cultured on chamber slides and treated with butyrate as described above. After fixation, apoptosis was quantified using a commercial kit by counts of cells stained positive after the TUNEL reaction (Roche, Mannheim, Germany).
Differentiation assay
Alkaline phosphatase (AP) activity was used to measure differentiation of the investigated cells. AP activity is one of a number of accepted surrogate markers of colon cell differentiation.22,24–27 For the assay, attached growing cells were washed with cold phosphate buffered saline, scraped, sonicated (2×20 seconds), and centrifuged at 5000 rpm for 10 minutes at 4°C. AP activity in the supernatant was measured by hydrolysis of p-nitrophenyl phosphate at pH 9.8 and 25°C (Modular; Roche Diagnostics). Cellular protein was determined by a modified Lowry assay using a commercially available kit (Biorad, Hercules, California, USA). Enzyme activity was expressed as mU per mg of protein, where one unit represents the enzyme activity hydrolysing 1 μmol of substrate per minute.
Statistical analyses
All statistical analyses were performed using SigmaStat 2.03 (SPSS Inc., San Rafael, California, USA). The Komolgorov-Smirnov test was used to test the data for normal distribution. The Student’s t test was used to calculate statistical differences.
RESULTS
Butyrate induces LL-37 expression in colon epithelial cells but also AP activity, a marker of colon cell differentiation
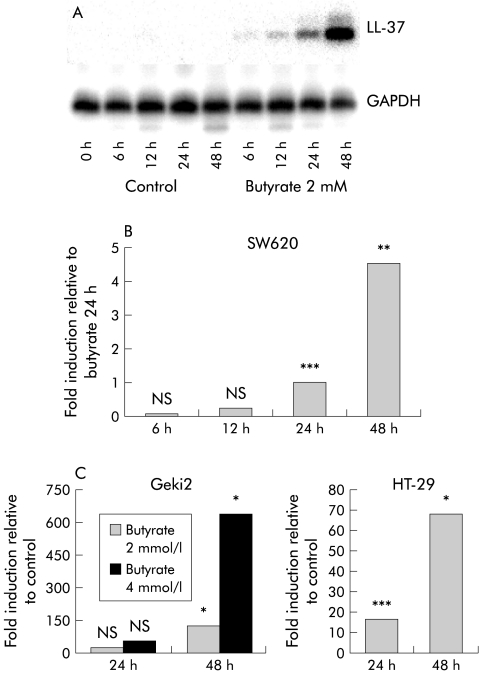
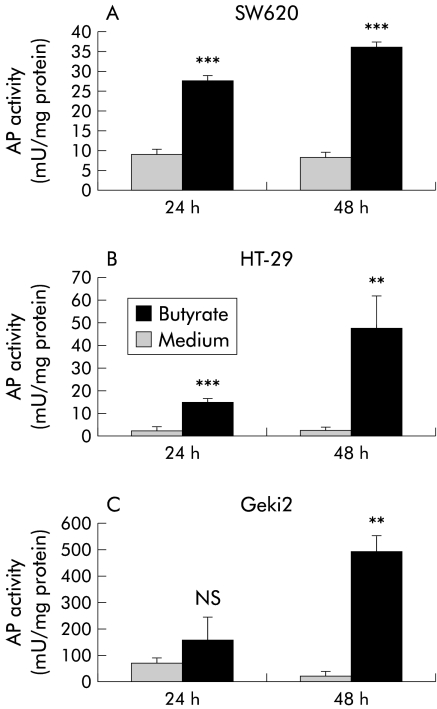
RT-PCR analyses of SW620 or SW480 colon cells showed no expression of LL-37 while Geki2 and HT-29 colon epithelial cells showed a basal level of LL-37 expression. Following incubation with butyrate, LL-37 transcripts were detected after six hours in SW620 and SW480 cells and prolonged incubation with butyrate resulted in a time and dose dependent induction of LL-37 expression (fig 1 ▶, fig 3A ▶). Geki2 colon cells incubated with butyrate (2 mmol/l) showed 126-fold (mean) induction of LL-37 expression after 48 hours which was further enhanced with increasing butyrate concentrations (fig 1 ▶, fig 3A ▶). In butyrate treated HT-29 cells, similar results were observed (fig 1 ▶). In parallel, induction of LL-37 expression was detected in butyrate treated short term cultures of freshly isolated colorectal epithelial cells (data not shown). Simultaneously, butyrate induced differentiation in the investigated cells as measured by AP activity in HT-29, SW620, SW480, and Geki2 colon cells. In untreated Geki2 cells, AP activity was higher than in the other investigated cell lines and increased significantly after 48 hours of incubation with butyrate (fig 2 ▶). Stimulation with inflammation mediators, including tumour necrosis factor α, interleukin 1α, interleukin 6, interferon γ, interferon α, and lipopolysaccharide had no effect on LL-37 expression in untreated or butyrate pretreated colon epithelial cells (data not shown).
Figure 1.
Expression of LL-37 in SW620 colon cells detected by reverse transcription-polymerase chain reaction (RT-PCR) and Southern hybridisation during butyrate (2 mmol/l) treatment (A). Quantitative real time RT-PCR analyses of LL-37 expression in SW620 colon cells (B). Cells were incubated with butyrate (2 mmol/l) and real time RT-PCR analyses using a TaqMan system were performed. No LL-37 transcripts were detected in medium control treated SW620 cells at any time point. LL-37 expression levels are therefore displayed as “fold induction” relative to induction after incubation with butyrate 2 mmol/l for 24 hours. **p<0.01; ***p<0.001. Similar results were obtained in SW480 cells (data not shown). Induction of LL-37 transcription in Geki2 (left) and HT-29 (right) colon cells incubated with butyrate 2 or 4 mmol/l is displayed as “fold induction” relative to medium treated control Geki2 cells and HT-29 cells, respectively (C). *p<0.05; ***p<0.001.
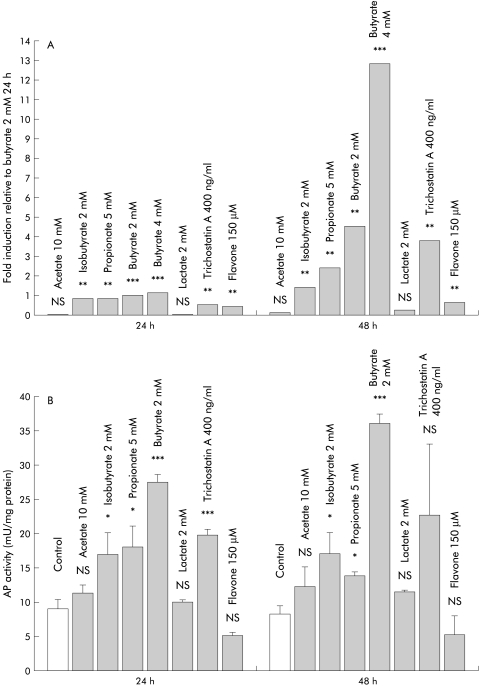
Figure 3.
(A) Comparison of LL-37 induction by different short chain fatty acids, lactate, flavone, and trichostatin A in SW620 colon cells. LL-37 expression was analysed by quantitative real time reverse transcription-polymerase chain reaction analyses, as described. **p<0.01; ***p<0.001. (B) Effect of assorted luminal factors on alkaline phosphatase (AP) activity as a surrogate marker of colon epithelial cell differentiation. SW620 cells were incubated with the indicated substrates and AP activity was measured. Values are expressed as mU of AP activity per mg cellular protein and are means (SD). *p<0.05; ***p<0.001.
Figure 2.
Effect of butyrate on alkaline phosphatase (AP) activity of SW620, HT-29, and Geki2 colon epithelial cells. Cells were incubated with butyrate 2 mmol/l or medium alone and AP activity was measured. Values are expressed as mU of AP activity per mg of cellular protein and are means (SD). **p<0.01; ***p<0.001. Similar results were obtained in SW480 cells (data not shown).
Induction of LL-37 transcription in colon epithelial cells by additional dietary compounds
To evaluate if induction of LL-37 transcription in colon epithelial cells is specific for butyrate, the effects of other SCFA and distinct colon luminal factors were investigated. Incubation of SW620 cells with isobutyrate and propionate resulted in a significant induction of LL-37 transcription after 24 hours, while 48 hours of incubation did not result in further induction. Equivalent doses (2 mmol/l) of butyrate and isobutyrate had similar effects (fig 3A ▶) and again, LL-37 induction was paralleled by increased AP activity (fig 3B ▶). The effect of isobutyrate and propionate on cell differentiation however was significantly lower than the effect of butyrate (p<0.001). In contrast, incubation with acetate or lactate for 48 hours did not affect LL-37 expression or AP activity in the investigated colon cells (fig 3 ▶).
Flavone, another dietary compound which regulates colon cell differentiation,28 induced LL-37 expression after 24 hours but no further induction was observed after 48 hours of incubation (fig 3A ▶). Despite this significant effect on LL-37 expression, incubation with flavone did not result in a significant increase in AP activity in SW620 and HT-29 cells (fig 3B ▶).
LL-37 regulation by SCFA might involve core protein modulation
Many of the effects of butyrate are attributed to reversible inhibition of histone deacetylases resulting in modulation of core histone and non-histone proteins and subsequent gene activation or suppression.29 In our studies, trichostatin A, a synthetic inhibitor of histone deacetylases, also induced LL-37 expression in HT-29 and SW620 cells, suggesting involvement of this mechanism (fig 3 ▶).
Butyrate induced LL-37 expression does not correlate with increased cell death
As butyrate and flavone are potent inducers of apoptosis, we wanted to exclude the fact that induction of LL-37 expression is associated with increased cell death. Therefore, analyses were performed to detect apoptotic cells among the attached growing cell fraction which was used in our expression studies. FACS analyses of positive stained cells for propidium iodine and annexin V revealed no differences when comparing butyrate treated and control cells. After 24 hours of butyrate treatment, 0.1% (mean) of HT-29 cells stained positive for annexin V and negative for propidium iodine as a sign of early apoptosis compared with 0.3% in controls. Positive stained cells for propidium iodine and annexin V (late apoptosis) were shown for 0.2% of butyrate treated cells and 1.5% of controls. After 48 hours, no differences between butyrate treated and control cells were detected for early (0.1% v 0.1%) or late (0.8% v 0.9%) apoptosis. Similar results were observed for SW620 colon cells (data not shown). TUNEL assays for the detection of late apoptotic cells confirmed these results and revealed no significant difference between butyrate treated and untreated cells (data not shown). No difference in the number of early or late apoptotic cells was observed when comparing flavone treated SW620 and control cells (data not shown). Incubation with flavone 25–100 μmol/l did not result in significant apoptosis induction in HT-29 cells. However, in attached growing HT-29 cells treated with flavone 150 μmol/l, early apoptosis was detected in 9.1% (mean) after 24 hours and in 5.5% after 48 hours, which was a significant (p<0.05) increase compared with control cells.
The presence of LL-37 in biopsies of the intestinal mucosa is restricted to differentiated epithelial cells
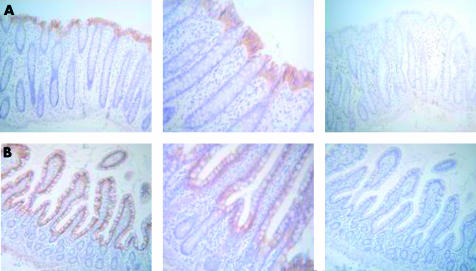
To determine whether expression of LL-37 in differentiating colon epithelial cell lines and short term cultures represents the expression pattern in vivo, sections of adult human colon and human ileum were immunostained with the LL-37 specific polyclonal antibody. A gradient of the LL-37 peptide was detected along the colonic crypt with the most prominent staining in the differentiated colonocytes at the epithelial surface while no sign of LL-37 was observed at the base of the crypt (fig 4 ▶). Immunohistochemical analyses of distal small intestinum revealed a similar staining pattern; LL-37 peptide was predominantly expressed in the surface epithelium of the villus and only weak or no staining was detected in the deeper crypt epithelium (fig 4 ▶).
Figure 4.
Expression of LL-37 peptide in epithelial cells in human colon and ileum. LL-37 expression was detected in colorectal (A) and ileal (B) biopsy specimens from healthy individuals by staining serial sections with the polyclonal antibody for LL-37. Sections are shown at 100× and 250× magnifications. As a negative control, the primary antibody was preabsorbed with the synthetic LL-37 peptide (displayed on the right panel).
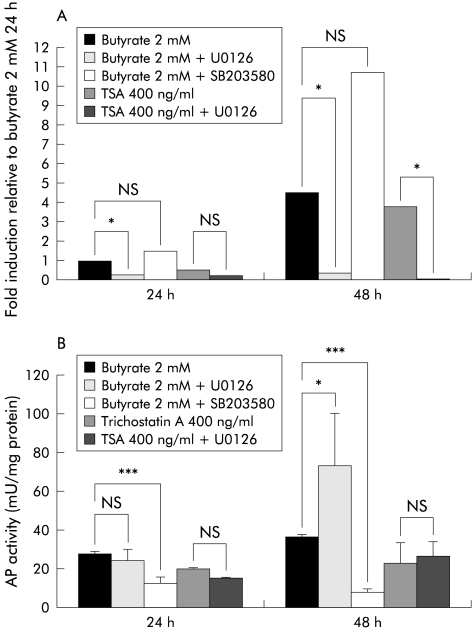
Influence of MEK/ERK and p38/MAP kinase signalling pathways on modulation of butyrate/trichostatin A induced AP activity and LL-37 expression in colon epithelial cells
To determine if butyrate regulated LL-37 expression and AP activity share common signal transduction pathways, we utilised the specific MEK inihibitor U0126 and the p38/MAP kinase inhibitor SB203580. In SW620 cells, butyrate induced LL-37 transcription was blocked by U0126, suggesting involvement of the MEK-ERK pathway (fig 5 ▶). In addition, trichostatin A induction of LL-37 expression was blocked by inhibition of the MEK-ERK pathway. In contrast, AP activity was clearly enhanced in SW620 cells when U0126 was added prior to butyrate treatment. However, incubation with U0126 prior to trichostatin A treatment did not affect AP activity in SW620 cells (fig 5 ▶).
Figure 5.
Effect of specific kinase inhibitors on butyrate induced alkaline phosphatase (AP) activity and LL-37 expression. SW620 colon cells were incubated with butyrate or trichostatin A (TSA) after preincubation with or without the MEK inhibitor U0126 or the p38/MAP kinase inhibitor SB203580, and LL-37 expression was analysed by quantitative reverse transcription-polymerase chain reaction (A). (B) Influence of the inhibitors on butyrate or trichostatin A induced AP activity as a surrogate marker for differentiation. *p<0.05; ***p<0.001.
In HT-29 colon cells, butyrate incubation for 24 hours (48 hours) resulted in a 20-fold (65-fold) increase in LL-37 transcripts relative to control HT-29 cells. U0126 blocked this induction after 24 hours (p=0.002) and 48 hours (p=0.004) of incubation (data not shown). Again, MEK-ERK blockage enhanced butyrate induced AP activity fivefold in HT-29 cells after 24 hours (p=0.017) and approximately sevenfold after 48 hours (p=0.002) in HT-29 colon cells (data not shown).
Blocking the p38/MAP kinase pathway with SB203580 had divergent effects on butyrate induced LL-37 transcription in SW620 cells; LL-37 induction was not affected after 24 hours, however after 48 hours a rather supportive effect was observed. In contrast, butyrate induced AP activity in SW620 cells was totally blocked by SB203580 after 24 and 48 hours (fig 5 ▶).
DISCUSSION
Epithelium derived antimicrobial peptides (peptide antibiotics) are important components of host defence at mucosal surfaces.1,2 Several antimicrobial peptides have been characterised in humans, including the defensins and one cathelicidin that liberates the LL-37 peptide.5,30 Peptide antibiotics have been investigated in distinct pathological conditions and the results emphasise their importance in innate defence.10,17,31–35 However, comprehensive knowledge of their expression and regulation is still lacking. In the present study, expression of the cathelicidin LL-37 in human colon epithelium was investigated to elucidate the regulatory pathways of antimicrobial defences in gut epithelia.
The human colon harbours a total population of approximately 1014 bacteria from up to 400 different species.36 A sufficient bactericidal defence barrier is therefore essential for host defence. The single cell layer of the colon epithelium maintains this barrier and produces an array of immune mediators, including antimicrobial peptides. Indeed, these peptides were prophetically suggested to be key regulators of the natural flora in the gut over a decade ago37 and current research data support the validity of this suggestion. Consequently, human β-defensin-2 has been shown to be upregulated in response to bacterial presence and proinflammatory mediators in colon epithelium.15 In contrast, the antimicrobial peptides LL-37 and β-defensin-1 are constitutively expressed in colon epithelial cells and a recent report claimed cell differentiation to be the key determinant for LL-37 expression in the colon.22
The results of our study confirm certain observations of this earlier work. In addition, various stimulators and inhibitors were used in order to evaluate regulatory pathways and to discriminate between differentiation and LL-37 transcription. Prominent epithelial staining was detected at the top of the colonic crypt which is in accordance with the recycling model of the colon epithelial cells; stem cells at the base of the colonic crypts divide and migrate along the crypt to the epithelial surface and undergo differentiation at the same time.38 In addition, we detected a similar staining pattern of LL-37 expression in the human distal small intestine. Again, differentiated enterocytes at the top of the villus showed prominent intracellular staining for LL-37, while in the cells of the deeper crypt epithelium LL-37 was not detected (fig 5 ▶).
SCFA serve as primary substrates for colonocyte metabolism and are well known inducers of differentiation in colorectal cancer cells.24,28 Butyrate, isobutyrate, and propionate induced differentiation, as measured by AP activity in the investigated cells, and simultaneously LL-37 expression increased in a time and dose dependent manner. This observation was further complemented with results obtained in a more differentiated adenoma derived cell line, Geki2, which showed approximately 10-fold higher basal AP activity than the other colon cell lines examined. However, in Geki2 colon cells the more differentiated status at baseline was not associated with higher LL-37 expression. Furthermore, in contrast with SCFA, stimulation with flavone had no effect on differentiation but induced LL-37 expression significantly. These results suggest distinct pathways for induction of the genes involved in differentiation on the one hand and expression of the gene encoding LL-37 on the other. The expression pattern of LL-37 in the colon crypt implies that these pathways are activated simultaneously in vivo.
Butyrate induced differentiation in human colon cells is associated with alterations of the MEK-ERK signal transduction pathway.25,26 Inhibition of MEK-ERK activation has been shown to enhance the differentiating effect of butyrate on colon epithelial cells.25 Accordingly, in our study, the MEK inhibitor U0126 enhanced butyrate induced AP activity in the investigated colon cells but blocked butyrate induced LL-37 expression. In contrast, blocking of the MAP kinase p38 resulted in inhibition of butyrate induced differentiation while LL-37 expression was not affected. Thus our data suggest that SCFA induced differentiation and the effect on LL-37 expression in colon epithelial cells are dependent on distinct intracellular pathways. Butyrate stimulation seems to activate different MAP kinases; while ERK enhances LL-37 transcription via activation of MEK, p38 activates induction of differentiation. Interestingly, the MEK-ERK pathway is also critical for LL-37 regulation in leucocytes such as monocytic cells (Schauber et al, unpublished results). These data underscore the importance of signalling pathways in the regulation of distinct effectors in innate immunity.
At present, only one pathogenic condition involving the human colon has been studied with respect to LL-37 expression.17 During Shigella infection, LL-37 transcription is turned off in the colonic epithelium. The LL-37 peptide cannot be detected in colon epithelial cells during active disease in vivo. Interestingly, Rabbani et al observed clinical improvement in rabbits with experimental shigellosis when the animals were treated with SCFA.39 Whether this observation can be linked to enhanced expression of epithelial antimicrobial peptides remains to be determined. From this angle, Shigella might be able to promote inhibition of the MEK kinase. Indeed, MAP kinases have recently entered the spotlight as targets for viral and bacterial pathogens.40,41
Notably, nothing is known of the consequences of increased antimicrobial peptide expression on the commensal intestinal flora, which is critical for protection of the mucosa against enteropathogenic microbes. A pathological increase in the activity of endogenous antibiotics would not then be beneficial to the host but might have deleterious consequences.
In summary, this study gives new insights into the regulation of the antimicrobial cathelicidin LL-37 in human colon mucosa and may provide the basis for therapeutic manipulation of LL-37 expression. However, it remains to be elucidated if butyrate and other dietary substrates can strengthen the epithelial defence barrier by upregulating LL-37 and other effectors of innate immunity in vivo.
Acknowledgments
The technical assistance of Kerstin Backhaus, Donata Kuhn, Elisabeth Kelber, and Gerda Dusel is acknowledged. We wish to thank Dr Kerstin Falk (Swedish Institute for Infectious Disease Control, Karolinska Institute) for invaluable help in setting up the real time RT-PCR assay and Dr Matthias Eck and Dr Bernd Schmausser (Department of Pathology, University of Würzburg) for help with the immunohistochemistry. This study was supported by grants from the Swedish Medical Research Council, the Swedish Foundation for Strategic Research, Petrus and Augusta Hedlund’s Foundation, Ruth and Richard Julin’s Foundation, Professor Nanna Svartz’ Foundation, Boehringer Ingelheim Fonds, and Deutsche Forschungsgemeinschaft.
Abbreviations
MEK, mitogen/extracellular signal protein kinase
ERK, extracellular signal regulated protein kinase
MAP, mitogen activated protein
SCFA, short chain fatty acids
AP, alkaline phosphatase
RT-PCR, reverse transcription-polymerase chain reaction
FCS, fetal calf serum
GAPDH, glyceraldehyde-3-phosphate dehydrogenase
CBB, cold binding buffer
REFERENCES
- 1.Gudmundsson GH, Agerberth B. Neutrophil antibacterial peptides, multifunctional effector molecules in the mammalian immune system. J Immunol Methods 1999;232:45–54. [DOI] [PubMed] [Google Scholar]
- 2.Bevins CL, Martin-Porter E, Ganz T. Defensins and innate host defence of the gastrointestinal tract. Gut 1999;45:911–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia JR, Krause A, Schulz S, et al. Human beta-defensin 4: a novel inducible peptide with a specific salt-sensitive spectrum of antimicrobial activity. FASEB J 2001;15:1819–21. [PubMed] [Google Scholar]
- 4.Harder J, Bartels J, Christophers E, et al. A peptide antibiotic from human skin. Nature 1997;387:861. [DOI] [PubMed] [Google Scholar]
- 5.Gudmundsson GH, Agerberth B, Odeberg J, et al. The human gene FALL39 and processing of the cathelin precursor to the antibacterial peptide LL-37 in granulocytes. Eur J Biochem 1996;238:325–32. [DOI] [PubMed] [Google Scholar]
- 6.Agerberth B, Gunne H, Odeberg J, et al. FALL-39, a putative human peptide antibiotic, is cysteine-free and expressed in bone marrow and testis. Proc Natl Acad Sci U S A 1995;92:195–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cowland JB, Johnsen AH, Borregaard N. hCAP-18, a cathelin/pro-bactenecin-like protein of human neutrophil specific granules. FEBS Lett 1995;368:173–6. [DOI] [PubMed] [Google Scholar]
- 8.Larrick JW, Hirata M, Balint RF, et al. Human CAP18: a novel antimicrobial lipopolysaccharide-binding protein. Infect Immun 1995;63:1291–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agerberth B, Charo J, Werr J, et al. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood 2000;96:3086–93. [PubMed] [Google Scholar]
- 10.Frohm M, Agerberth B, Ahangari G, et al. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem 1997;272:15258–63. [DOI] [PubMed] [Google Scholar]
- 11.Bals R, Wang X, Zasloff M, et al. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc Natl Acad Sci U S A 1998;95:9541–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frohm Nilsson M, Sandstedt B, Sorensen O, et al. The human cationic antimicrobial protein (hCAP18), a peptide antibiotic, is widely expressed in human squamous epithelia and colocalizes with interleukin-6. Infect Immun 1999;67:2561–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johansson J, Gudmundsson GH, Rottenberg ME, et al. Conformation-dependent antibacterial activity of the naturally occurring human peptide LL-37. J Biol Chem 1998;273:3718–24. [DOI] [PubMed] [Google Scholar]
- 14.Stolzenberg ED, Anderson GM, Ackermann MR, et al. Epithelial antibiotic induced in states of disease. Proc Natl Acad Sci U S A 1997;94:8686–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Neil DA, Porter EM, Elewaut D, et al. Expression and regulation of the human beta-defensins hBD-1 and hBD-2 in intestinal epithelium. J Immunol 1999;163:6718–24. [PubMed] [Google Scholar]
- 16.Wilson CL, Ouellette AJ, Satchell DP, et al. Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science 1999;286:113–17. [DOI] [PubMed] [Google Scholar]
- 17.Islam D, Bandholtz L, Nilsson J, et al. Downregulation of bactericidal peptides in enteric infections: a novel immune escape mechanism with bacterial DNA as a potential regulator. Nat Med 2001;7:180–5. [DOI] [PubMed] [Google Scholar]
- 18.Porter EM, Bevins CL, Ghosh D, et al. The multifaceted Paneth cell. Cell Mol Life Sci 2002;59:156–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spaeth G, Berg RD, Specian RD, et al. Food without fiber promotes bacterial translocation from the gut. Surgery 1990;108:240–6. [PubMed] [Google Scholar]
- 20.Scheppach W. Effects of short chain fatty acids on gut morphology and function. Gut 1994;35(Suppl 1):S35–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scheppach W, Sommer H, Kirchner T, et al. Effect of butyrate enemas on the colonic mucosa in distal ulcerative colitis. Gastroenterology 1992;103:51–6. [DOI] [PubMed] [Google Scholar]
- 22.Hase K, Eckmann L, Leopard JD, et al. Cell differentiation is a key determinant of cathelicidin LL-37/human cationic antimicrobial protein 18 expression by human colon epithelium. Infect Immun 2002;70:953–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hylla S, Gostner A, Dusel G, et al. Effects of resistant starch on the colon in healthy volunteers: possible implications for cancer prevention. Am J Clin Nutr 1998;67:136–42. [DOI] [PubMed] [Google Scholar]
- 24.Heerdt BG, Houston MA, Augenlicht LH. Potentiation by specific short-chain fatty acids of differentiation and apoptosis in human colonic carcinoma cell lines. Cancer Res 1994;54:3288–93. [PubMed] [Google Scholar]
- 25.Ding Q, Wang Q, Evers BM. Alterations of MAPK activities associated with intestinal cell differentiation. Biochem Biophys Res Commun 2001;284:282–8. [DOI] [PubMed] [Google Scholar]
- 26.Davido DJ, Richter F, Boxberger F, et al. Butyrate and propionate downregulate ERK phosphorylation in HT-29 colon carcinoma cells prior to differentiation. Eur J Cancer Prev 2001;10:313–21. [DOI] [PubMed] [Google Scholar]
- 27.Witt O, Schulze S, Kanbach K, et al. Tumor cell differentiation by butyrate and environmental stress. Cancer Lett 2001;171:173–82. [DOI] [PubMed] [Google Scholar]
- 28.Wenzel U, Kuntz S, Brendel MD, et al. Dietary flavone is a potent apoptosis inducer in human colon carcinoma cells. Cancer Res 2000;60:3823–31. [PubMed] [Google Scholar]
- 29.McKnight GS, Hager L, Palmiter RD. Butyrate and related inhibitors of histone deacetylation block the induction of egg white genes by steroid hormones. Cell 1980;22(2 Pt 2):469–77. [DOI] [PubMed] [Google Scholar]
- 30.Ganz T. Defensins and host defense. Science 1999;286:420–1. [DOI] [PubMed] [Google Scholar]
- 31.Agerberth B, Grunewald J, Castanos-Velez E, et al. Antibacterial components in bronchoalveolar lavage fluid from healthy individuals and sarcoidosis patients. Am J Respir Crit Care Med 1999;160:283–90. [DOI] [PubMed] [Google Scholar]
- 32.Goldman MJ, Anderson GM, Stolzenberg ED, et al. Human beta-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell 1997;88:553–60. [DOI] [PubMed] [Google Scholar]
- 33.Nizet V, Ohtake T, Lauth X, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature 2001;414:454–7. [DOI] [PubMed] [Google Scholar]
- 34.Frohm M, Gunne H, Bergman AC, et al. Biochemical and antibacterial analysis of human wound and blister fluid. Eur J Biochem 1996;237:86–92. [DOI] [PubMed] [Google Scholar]
- 35.O’Neil DA, Cole SP, Martin-Porter E, et al. Regulation of human beta-defensins by gastric epithelial cells in response to infection with Helicobacter pylori or stimulation with interleukin-1. Infect Immun 2000;68:5412–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gustafsson BE. The physiological importance of the colonic microflora. Scand J Gastroenterol Suppl 1982;77:117–31. [PubMed] [Google Scholar]
- 37.Boman HG. Antibacterial peptides: key components needed in immunity. Cell 1991;65:205–7. [DOI] [PubMed] [Google Scholar]
- 38.Barkla DH, Gibson PR. The fate of epithelial cells in the human large intestine. Pathology 1999;31:230–8. [DOI] [PubMed] [Google Scholar]
- 39.Rabbani GH, Albert MJ, Hamidur Rahman AS, et al. Short-chain fatty acids improve clinical, pathologic, and microbiologic features of experimental shigellosis. J Infect Dis 1999;179:390–7. [DOI] [PubMed] [Google Scholar]
- 40.Orth K, Palmer LE, Bao ZQ, et al. Inhibition of the mitogen-activated protein kinase kinase superfamily by a Yersinia effector. Science 1999;285:1920–3. [DOI] [PubMed] [Google Scholar]
- 41.Pleschka S, Wolff T, Ehrhardt C, et al. Influenza virus propagation is impaired by inhibition of the Raf/MEK/ERK signalling cascade. Nat Cell Biol 2001;3:301–5. [DOI] [PubMed] [Google Scholar]