Abstract
Background and aims: The precise aetiology of achalasia is unknown although autoimmunity has been implicated and is supported by several studies. We screened sera from patients with achalasia or gastro-oesophageal reflux disease (GORD) to test for circulating antimyenteric neuronal antibodies.
Methods: Serum was obtained from 45 individuals with achalasia, 16 with GORD, and 22 normal controls. Serum was used in immunohistochemistry to label whole mount preparations of ileum and oesophagus of the guinea pig and mouse. Also, sections of superior cervical and dorsal root ganglia, and spinal cord were examined.
Results: Positive immunostaining of the myenteric plexus was detected in significantly more achalasia and GORD samples than control samples (achalasia, p<0.001; GORD, p<0.01), and immunoreactivity was significantly more intense with achalasia and GORD serum samples than controls (achalasia, p<0.01; GORD, p<0.05). There was no correlation between intensity of immunoreactivity and duration of achalasia symptoms. In most cases, achalasia and GORD sera stained all ileal submucosal and myenteric neurones, and oesophageal neurones. Immunostaining was not species specific; however, immunostaining was largely specific for enteric neurones. Western blot analysis failed to reveal specific myenteric neuronal proteins that were labelled by antibodies in achalasia or GORD serum.
Conclusions: These data suggest that antineuronal antibodies are generated in response to tissue damage or some other secondary phenomenon in achalasia and GORD. We conclude that antineuronal antibodies found in the serum of patients with achalasia represent an epiphenomenon and not a causative factor.
Keywords: autoantibodies, autoimmune, enteric nervous system, oesophageal motility, neurogastroenterology, gastro-oesophageal reflux disease, achalasia
Idiopathic achalasia is a common and well characterised primary motility disorder of the oesophageal body and lower oesophageal sphincter (LOS) involving absence of progressive peristalsis in the oesophageal body and abnormal relaxation of the LOS. Clinically, achalasia is manifested by slowly progressive dysphagia to solids and liquids. There is delayed passage of material from the oesophagus to the stomach, without evidence of an obstructing lesion. The disorder is observed in men and women primarily in the fifth and sixth decades of life, although achalasia can present at any age.1,2
While achalasia is a long recognised clinical entity, the underlying pathophysiology remains poorly understood.1,3 The primary motor disorder is failure of relaxation of the LOS and absence of peristalsis in the smooth muscle. As myenteric neurones synthesising nitric oxide are responsible for the inhibitory component of oesophageal peristalsis and LOS relaxation, it is considered likely that these neurones are involved in this disease. Data to support this come from histochemical, immunohistochemical, and biochemical studies.4,5 Although morphological studies demonstrate widespread infiltration and lesions in the myenteric plexus, careful evaluation of excitatory or other components of the human myenteric plexus has not been performed.
A histopathological hallmark of achalasia is degeneration of the neural elements that innervate the muscle in the oesophageal wall.6 This is associated with an inflammatory infiltrate (predominantly T lymphocytes) of the myenteric plexus—providing evidence of an immune mediated destruction of the myenteric plexus.7,8 While potential aetiologies proposed for achalasia include infection, genetic predisposition, spontaneous neurodegeneration, and others, autoimmune mediated ganglion destruction has gained support because serum from achalasia patients has been shown to contain antineuronal antibodies.9,10 These previous reports used immunohistochemistry to label sections of rat oesophagus, stomach, and ileum with sera from patients with achalasia, and reported that a higher proportion of sera from achalasic patients had autoantibodies that labelled myenteric neurones compared with controls. What has not been shown is whether antineuronal antibodies in the serum of subjects with achalasia specifically label myenteric neurones. To address this issue, serum was obtained from individuals with manometrically and endoscopically confirmed achalasia, as well as from individuals with signs and symptoms of gastro-oesophageal reflux disease (GORD) and from normal controls. We tested whether antineuronal antibodies in achalasia serum were specific for myenteric neurones, using the guinea pig ileum as a model. We also tested the regional specificity of antineuronal antibodies by examining labelling in the oesophageal myenteric plexus, and the ileal submucosal plexus. When antineuronal cytoplasmic labelling was observed in the myenteric plexus we sought to characterise the neuronal population that was labelled using immunohistochemistry and immunoblotting. To test the species specificity of these antibodies, we examined the murine myenteric plexus. Lastly, in selected samples, we examined whether antineuronal antibodies that label myenteric neurones also label other autonomic neurones (in the superior cervical ganglion), sensory neurones (in dorsal root ganglia), or spinal neurones.
METHODS
After approval by the institutional review boards at each participating institution, sera were obtained from 45 achalasia patients, 16 individuals with GORD, and from 22 normal individuals for the purposes of the study. All patients in the achalasia group had clinical symptoms of achalasia, as well as supporting oesophageal manometry findings and abnormal relaxation of the LOS. All individuals with GORD reported frequent symptoms suggestive of this diagnosis and required medical therapy with antacids or antisecretory agents. None of the patients in the GORD group had undergone surgical antireflux therapy, and all denied dysphagia.
Serum antibodies were evaluated by standard indirect immunofluorescence techniques, as previously described.11 All procedures involving the use of animals were reviewed and approved by the Office of Animal Care Management at the University of Vermont and the Animal Care Committee of the University of Calgary.
For studies of whole mount preparations, healthy adult guinea pigs and mice were deeply anaesthetised and then killed by exsanguination, and the oesophagus and ileum (guinea pigs) or ileum (mice) were removed, and pinned out flat in a sylgard coated Petri dish in a normal Krebs solution. The tissue was fixed in 2% paraformaldehyde with 0.2% picric acid. After rinsing in 0.1 M phosphate buffered saline (PBS), the intestine was dissected into layers, exposing the myenteric plexus and longitudinal muscle. The tissue was then incubated for 24 hours at ∼23°C or for 48 hours at 4°C in human serum (1:100–1:800), thoroughly rinsed, and exposed to goat antihuman IgG labelled with the fluorophore Cy3 (Jackson ImmunoResearch Laboratories, Inc., West Grove, Pennsylvania, USA). When positive immunoreactivity was detected with a given human serum, preparations were double stained with antisera directed against mouse anti-Hu (Molecular Probes, Eugene, Oregon, USA), which is present in all enteric neurones.11 In additional studies, antibodies directed against nitric oxide synthase (NOS) (Santa Cruz Biotechnology, Santa Cruz, California, USA), calbindin (Swant, Bellinzona, Switzerland), or calretinin (Chemicon International, Inc., Temecula, California, USA) were used to determine whether antineuronal antibodies were targeting distinct subsets of guinea pig myenteric neurones. NOS positive neurones are inhibitory motor neurones or descending interneurones, calbindin positive neurones are intrinsic primary afferent neurones, and calretinin positive neurones are cholinergic motor neurones that project to the longitudinal muscle and interneurones.12 Following a final set of rinses, tissues were mounted on APTEX coated (Sigma Aldrich, St Louis, Missouri, USA) glass slides and coverslipped in Citifluor (Citifluor Ltd, London, UK).
When double labelling was conducted, the human antibodies were visualised with Cy3 labelled secondary antisera, and the other primary antibodies were visualised with fluorescein isothiocyanate (FITC) labelled secondary antisera. Control experiments were conducted to test for cross reactivity of secondary antisera with each of the primary antisera used in double label experiments. For example, secondary antisera raised against rabbit and mouse were used in control tissue that had been exposed to human raised primary antisera, but not rabbit or mouse raised primary antisera, to verify that no non-specific immunostaining was detected. Additional control studies involved preabsorption of serum samples from each group with a liver substrate (Sigma-Aldrich) at concentrations of 100 mg/ml13 to verify that antibodies that labelled enteric neurones were organ and tissue specific. In these studies, no difference was detected in any of the groups between immunostaining resulting from serum that was or was not exposed to liver substrate. Furthermore, immunostaining was not detected when serum samples that yielded intense neuronal staining were applied to sections of liver or kidney.
For studies of other neural tissues, guinea pigs were deeply anaesthetised with sodium pentobarbital (60 mg/kg intraperitoneally; MTC Pharmaceuticals, Cambridge Ontario, Canada) and perfused via the ascending aorta with physiological saline (150 ml) followed by cold 4% paraformaldehyde (300 ml). After perfusion, the spinal cord, thoracic dorsal root ganglia, and superior cervical ganglia were removed, and fixed by immersion in the same fixative overnight at 4°C. Tissues were washed in PBS, then immersed overnight at 4°C in PBS containing 20% sucrose. Sections were cut on a cryostat (12 μm) and mounted onto glass slides coated with poly-d-lysine (Sigma). Sections were incubated for 48 hours at 4°C in human sera (1:100–1:800).
Slides were examined on an Olympus AX70 or a Zeiss Axioplan fluorescence photomicroscope. Filter sets included the following: for Cy3, excitation 510–550 nm, emission, 590 nm; for FITC, 470 nm excitation, 525 nm emission. Images were captured with an Optronics MagnaFire CCD camera, attached to the Olympus AX70 microscope or a Sensys Camera (Photometrics, Tucson Arizona, USA) attached to the Zeiss Axioplan microscope. Images were cropped in Adobe Photoshop with minimal alteration (minor adjustments to brightness and contrast), and transferred to Microsoft PowerPoint for construction of figure sets.
Immunoblotting methods
Freshly dissected guinea pig longitudinal muscle-myenteric plexus (LMMP) preparations were homogenised in the presence of a protease inhibitor cocktail consisting of leupeptin (2 μg/ml), phenylmethylsulphonyl fluoride (40 μg/ml), and benzamide (1.2 μg/ml). Membrane bound and cytoplasmic proteins were separated by centrifugation at 10 000 g for 20 minutes. Protein concentration was measured with a Bio-Rad protein assay reagent using bovine serum albumin as a standard. Electrophoresis and transfer protocols were run according to the manufacturer’s instructions (Invitrogen Living Science, Carlsbad, California, USA). Protein (20 μg) in 20 μl of buffer (2% β-mercaptoethanol and 2× sodium dodecyl sulphate loading buffer) was added to each well and gels were run at 125 V for 90–100 minutes and transferred onto nitrocellulose membranes at 25 V for 100–110 minutes.
For immunodetection, the nitrocellulose membranes were blocked in a 5% solution of non-fat dried milk in Tris buffered saline with 0.05% Tween-20 (TBS-T) at 4°C for 2–24 hours. Membranes were then incubated for two hours in human serum, diluted to 1:200 in a milk/TBS-T solution. Membranes were thoroughly rinsed 3× five minutes in TBS-T and then incubated for one hour in an affinity purified horseradish peroxidase coupled goat antihuman secondary antibody (Jackson ImmunoResearch), diluted in 5% milk/TBS-T solution at 1:5000. The membrane was rinsed again for 3× five minutes in TBS-T. Immunostaining was visualised by soaking the membranes in Renaissance Chemiluminscence Reagent (NEN Life Science Products, Boston, Massachusetts, USA) for one minute and exposing them to autoradiography film (NEN Life Science Products) for 3–5 minutes. Control experiments were run by exposing the nitrocellulose membranes to the secondary antibody without prior exposure to the human serum; no bands were detected with this procedure.
Data analysis
All serum samples were initially screened at a dilution of 1:100. If immunoreactivity was observed, stepwise doubling dilutions were performed until optimal immunoreactivity (highest signal to lowest background) was achieved. This was usually at a dilution of ∼1:400. Samples were blindly scored by two experienced immunohistochemists (GMM and LME in Vermont; WH and KAS in Calgary) using a semiquantitative rating scale. A scale of 0–2 was used where: 0=no neuronal staining above background; 1=moderate staining of neurones in the myenteric plexus; and 2=intense staining of neurones in the myenteric plexus. Background staining was defined as that produced when the secondary antibody alone was applied to the tissues. Data between groups were compared with χ2 analysis or Fisher’s exact test. In the achalasia group, duration of symptoms and duration since diagnosis were compared with intensity of immunoreactivity using linear regression analysis. This information was not available for the GORD population. For all tests, p<0.05 was considered statistically significant.
Age and sex distribution of study population
Achalasia was defined by accepted clinical criteria and confirmed by oesophageal manometry testing which demonstrated the absence of peristaltic contractions in the oesophageal body and absent or incomplete relaxation of the LOS. Additional manometric findings may have included elevated LOS pressures and resting oesophageal pressures.14 In addition, most patients also had supporting radiological and endoscopic findings consistent with the diagnosis of achalasia. Study subjects may have been untreated or have received treatment with endoscopic balloon dilation, operative myotomy, or endoscopically directed injections of botulinum toxin.
GORD was defined by accepted clinical criteria, as described by Voutilainen and colleagues.15 Patients described chronic (>6 months) pyrosis and/or regurgitation with or without endoscopic evidence of erosive oesophagitis, Barrett’s oesophagus, oesophageal ulcer, or stricture.
The sex distributions and mean ages of individuals in the populations used in this study were as follows: control, 68% female, 40.4 years; achalasia, 46% female, 53.9 years; GORD, 56.2% female, 40.9 years. Achalasia and GORD patients were identified by database and contacted by telephone. A single 10 ml sample of whole blood was drawn from study patients and controls after informed consent was obtained. All patients were asked to complete a questionnaire designed to exclude connective tissue and autoimmune disease, other gastrointestinal motility disorders, diabetes mellitus, use of immunosuppressive medications, chronic viral illness, and also a list of regular medications. To avoid subjects with other disorders where the presence of confounding autoantibodies may be demonstrated, we excluded patients with connective tissue diseases, small cell lung cancer, diabetes mellitus, and poorly described gastrointestinal motility disorders defined by the answers from the questionnaire. Participation in this study was purely voluntary.
RESULTS
Sera from achalasia and GORD patients contain antibodies that bind to myenteric neurones
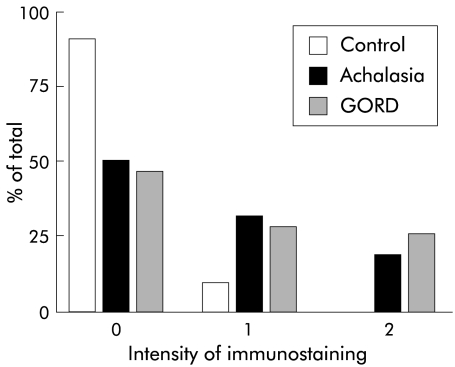
Sera from controls, subjects with achalasia, and those with GORD were applied to whole mount preparations of the guinea pig LMMP. As reported by others,9,10 a significantly higher proportion of achalasia serum samples labelled neurones in the myenteric plexus than in control subjects. In the achalasia group, 51% of serum samples (23/45 samples) labelled the myenteric plexus whereas in the control group only 9% (2/22 samples) of preparations were positively stained (defined as an intensity score of 1 or 2; p<0.001, Fisher’s exact test). When evaluated on a scale of 0–2, intensity of immunoreactivity was significantly greater with achalasia serum than with normal control serum (p<0.01, χ2 test) (figs 1, 2 ▶ ▶). There was no correlation, by linear regression analysis, between the intensity of staining observed with achalasia serum and the duration of symptoms (p=0.7) or time since diagnosis (p=0.3).
Figure 1.
Comparison of antineuronal immunoreactivity evaluated using the blinded grading system. The intensity of immunoreactivity was significantly greater with serum from individuals with achalasia and gastro-oesophageal reflux disease (GORD) than with control serum. Only serum from achalasia and GORD patients demonstrated intense (grade 2) staining.
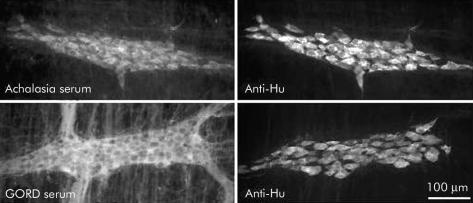
Figure 2.
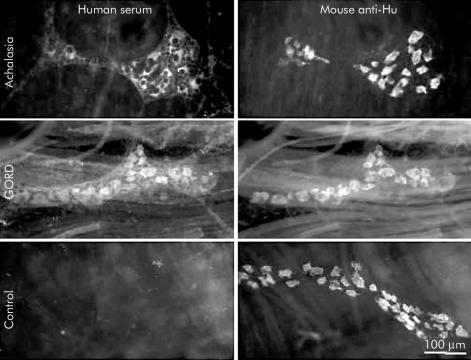
Photomicrographs of guinea pig myenteric ganglia that were double labelled with antiserum directed against the pan-neuronal protein Hu (anti-Hu), as well as serum from individuals with achalasia (top panels) or gastro-oesophageal reflux disease (GORD) (bottom panels). Note that all neurones that were immunostained with anti-Hu serum were also immunostained with serum from this achalasia serum sample and this GORD serum sample.
Previous reports indicated that immunoreactivity was not detected in the myenteric plexus when it was immunostained with serum from patients with GORD.9,10 In contrast with these reports, we detected neuronal cytoplasmic immunoreactivity with 8/16 (50%) GORD serum samples, a value significantly greater than that detected with control serum (p<0.01), but not significantly different from the achalasia group (fig 1 ▶). Furthermore, the intensity of staining with GORD serum was significantly greater than that detected with normal serum (p<0.05, χ2 test).
In most cases, all myenteric neurones were immunostained with achalasia or GORD serum
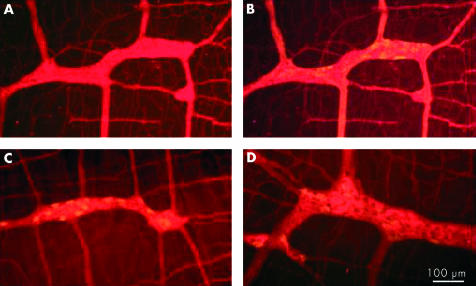
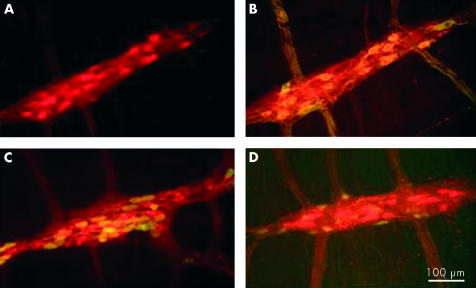
In most achalasia and GORD serum samples that were evaluated with specific neuronal labelling, all of the neurones in the myenteric plexus were immunostained, as demonstrated by double labelling with antiserum directed against the cytoplasmic pan-neuronal marker Hu (fig 2 ▶). This was further supported by immunostaining preparations with antisera directed against NOS, calbindin, and calretinin (fig 3 ▶). In many cases, neural processes in the interganglionic fibre bundles were also stained. Only two of the samples from the achalasia group (4.4%) specifically labelled myenteric neurones that were also NOS but not calbindin or calretinin positive (fig 4 ▶), indicating a possible specificity for inhibitory neurones. There were no remarkable features of these cases, relative to the other achalasia cases, that could account for these differences.
Figure 3.
Examples of immunoreactivity with an achalasia serum sample that was typical in that it appeared to stain all neurones and nerve fibres in the preparation. (A) Staining with human serum only. (B) Staining with human serum (red) plus serum directed against nitric oxide synthase (green). (C) Double staining with human serum (red) plus serum directed against calbindin (green). (D) Staining with human serum (red) plus serum directed against calretinin (green).
Figure 4.
In two cases (4.4%), serum derived from achalasia patients selectively immunostained nitrergic neurones. (A) Staining with human serum alone (red). (B) Staining with human serum plus serum directed against nitric oxide synthase (green). Note that most neurones are yellow, indicating that they are double labelled. (C) Double staining with human serum (red) plus serum directed against calbindin (green). (D) Staining with human serum (red) plus serum directed against calretinin (green).
Achalasia serum also labels ileal submucosal neurones and oesophageal myenteric neurones
Serum from achalasia and GORD subjects that specifically labelled myenteric neurones was also tested on whole mount preparations of the ileal submucosal plexus and the ganglionated plexus of the oesophagus. In both of these regions of the gastrointestinal tract, neurones were labelled in an identical manner to that seen in the myenteric plexus (figs 5, 6 ▶ ▶). These findings demonstrate a lack of specificity of antineuronal antibodies for neurones within certain regions of the gut.
Figure 5.
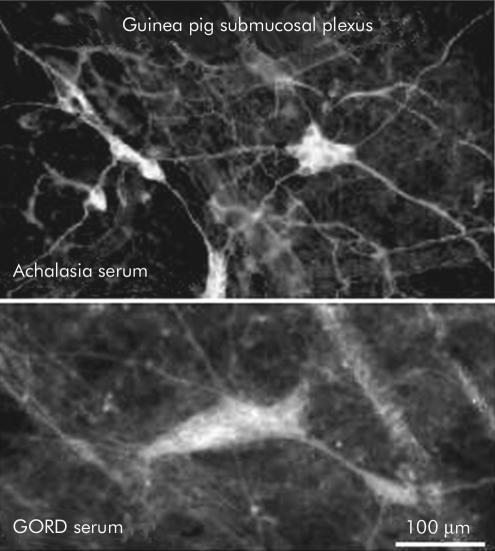
Neurones in the submucosal plexus of the guinea pig displayed immunoreactivity with serum from individuals with both achalasia (top) and gastro- oesophageal reflux disease (GORD) (bottom).
Figure 6.
Neurones in the myenteric plexus of the guinea pig oesophagus were stained by serum from individuals with achalasia or gastro-oesophageal reflux disease (GORD). Matching fields demonstrating immunoreactivity with human serum (left) and a monoclonal antiserum directed against the pan-neuronal protein Hu (right). Note that immunoreactivity is readily visible in oesophageal ganglia staining with sera from individuals with achalasia and GORD but not in the preparation exposed to control serum.
Immunostaining of enteric neurones with achalasia serum was not species specific
To test whether labelling of enteric neurones with achalasia and/or GORD serum was specific to guinea pig, we applied these sera to LMMP preparations of murine ileum. In the mouse, the ganglionated plexus of the ileum was positively labelled with achalasia and GORD serum, and the general appearance of the labelling was similar to that seen in guinea pig tissue (fig 7 ▶). These findings demonstrate a lack of species specificity of the antineuronal antibodies that we detected in achalasia and GORD sera.
Figure 7.
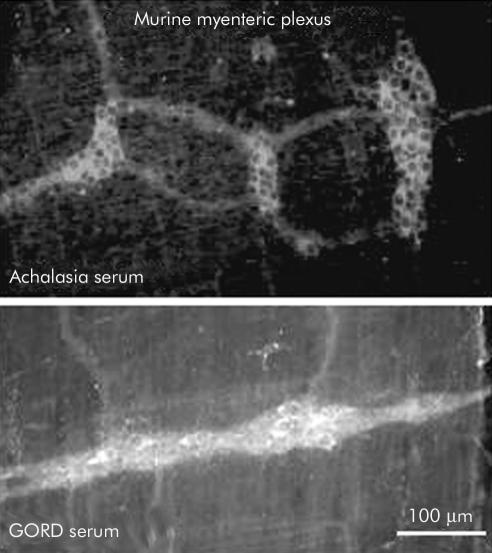
Neurones in the myenteric plexus of the mouse were immunostained with sera from individuals with achalasia or gastro-oesophageal reflux disease (GORD) in a manner similar to that of the guinea pig.
Immunostaining with achalasia and GORD serum was largely specific to the enteric nervous system
Sections from dorsal root ganglia, superior cervical ganglia, and the spinal cord were exposed to sera from 10 selected achalasia patients, seven GORD patients, and three controls. In these regions, immunoreactivity was not detected with serum samples from the control or GORD groups. When sera from the achalasia group were tested, immunoreactivity was detected in only two of 10 samples (both scored 2 on the rating scale for immunostaining in the ileum). Both of these serum samples resulted in intense (2 on a scale of 0–2) immunoreactivity in neurones of sensory and sympathetic ganglia, and in the spinal cord (fig 8 ▶). These findings demonstrate a specificity of these antibodies for the enteric nervous system.
Figure 8.
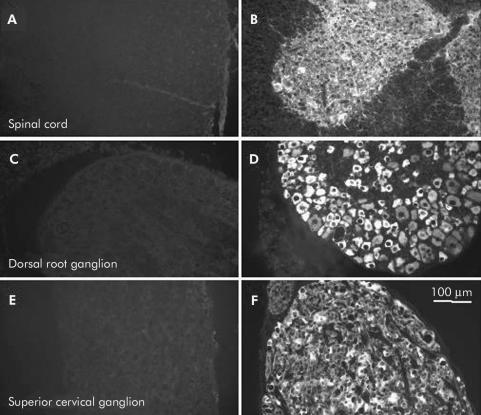
Neuronal immunostaining with achalasia serum was largely specific to the enteric nervous system. This figure demonstrates immunohistochemical results obtained with sera from two different achalasia patients, both of which gave intense immunoreactivity (grade 2) in myenteric neurones. When these sera were applied to sections of guinea pig spinal cord (A, B), dorsal root ganglion (C, D), and superior cervical ganglion (E, F), one of the serum samples did not result in detectable neuronal immunoreactivity in these regions (A, C, E), whereas intense immunoreactivity was detected with the other sample (B, D, F). In the current study, immunoreactivity was not detected in these regions with control serum, with serum from individuals with gastro-oesophageal reflux disease (GORD) serum, or in 8/10 achalasia samples that were tested.
Immunoreactivity was detected in neuronal nuclei with control, achalasia, and GORD serum samples
Another feature of immunostaining that was observed with sera from all three groups was neuronal nuclear immunoreactivity. In the GORD group, neuronal nuclear immunoreactivity was detected in 50% of samples whereas nuclear staining was detected in only 27.3% of control samples and 24.4% of achalasia samples (data not shown). There was no significant difference in the incidence of nuclear immunostaining among these groups.
Immunoblot analysis revealed multiple protein bands with serum samples from all three groups
Immunoblot analysis was used to determine if sera from the achalasia or GORD groups revealed specific protein bands that could be used to characterise the target(s) of neuronal immunoreactivity. Despite specific immunohistochemical labelling of myenteric neurones with achalasia and GORD samples, as described above, immunoblots involving protein purified from guinea pig LMMP preparations did not reveal protein bands that were consistently and specifically detected in serum samples from either group (data not shown). Control immunoblots using a rabbit polyclonal antiserum directed against calbindin D28, which is expressed by a subset of guinea pig myenteric neurones,16 revealed a single 28 kDa band confirming the integrity of proteins in the sample (data not shown). The lack of immunoblotting specificity supports our view that there is probably not one particular protein that is targeted by antineuronal antibodies in the serum of achalasia patients.
DISCUSSION
The aim of the current study was to test the hypothesis that antineuronal antibodies observed in the serum of patients with idiopathic achalasia exhibit the qualitative properties sufficient to support autoimmunity in the pathophysiology of the disorder. The concept that autoimmune mechanisms contribute to the clinical manifestations of idiopathic motility disorders is supported by the detection of circulating antibodies that target enteric neurones in patients with these disorders.9,10,17,18 This theory is supported in idiopathic achalasia by the findings that antineuronal antibodies exist in serum from achalasia patients,9,10 and loss of nitrergic neurones in the oesophagus, consistent with an autoimmune process.6
In order to implicate autoimmunity in the pathophysiology of achalasia, antibodies should demonstrate the following properties: (1) disease specificity; (2) regional target specificity; and (3) cellular target specificity. In the current study, we immunostained tissue samples with serum from normal controls, patients with achalasia, and individuals with GORD in order to test whether antibodies in achalasia serum exhibit these properties. Our findings support previous observations that the serum of achalasia patients contains antineuronal antibodies; in addition, we made the novel observation that serum from individuals with GORD also contains antineuronal antibodies that exhibit similar properties. Sera from both the achalasia and GORD groups that contain antineuronal antibodies typically label all neurones in the myenteric plexuses of the ileum and oesophagus, are largely specific for enteric neurones, and are not species specific. These results bring into question the cause and effect relationship of circulating antineuronal antibodies and the clinical manifestations of clinical achalasia. In other words, it is not clear whether antibodies that target neuronal proteins contribute to neuromuscular dysfunction, or whether the presence of these antibodies is an epiphenomenon.
Circulating antineuronal antibodies have been described in a variety of clinical syndromes in addition to idiopathic achalasia, including those that are associated with primary motility disorders of the gut. For example, in Chagas’ disease, autoantibodies that selectively recognise the M2 muscarinic receptor subtype have been demonstrated. These antibodies are detected significantly more often in Chagasic patients than in normal controls or patients with non-Chagasic achalasia.17,18 Also, there are recognised paraneoplastic phenomena that are associated with antineuronal autoantibodies. These include Lambert-Eaton myasthenic syndrome, in which antibodies directed against the P/Q subtype of the voltage activated calcium channel are present,19 and small cell lung carcinoma, where anti-Hu antibodies are detected and intestinal dysmotility is a common feature.20,21 In a recent study of serum from patients with autoimmune autonomic neuropathy, antibodies directed against neuronal nicotinic receptors were detected and there was a significant correlation between levels of the antinicotinic receptor antibodies and the severity of autonomic dysfunction.22
The specific protein target(s) of serum antibodies that results in neuronal immunoreactivity with sera from individuals with idiopathic achalasia or GORD has not been identified. In an attempt to resolve this issue, we used western blot analysis with proteins obtained from the myenteric plexus to establish whether achalasia and/or GORD sera specifically labelled proteins of a given molecular weight. These experiments were not conclusive because serum from achalasia and GORD patients, as well as control serum, demonstrated binding at numerous molecular weights, without discernable differences. Clarification of this issue may require functional studies to elucidate whether the antineuronal antibodies that were observed in this study actually have a specific effect on neuromuscular signalling and function in the gut. However, this may not be warranted given the properties of the immunohistochemical staining reported here.
It is quite possible that the antineuronal antibodies in idiopathic achalasia and GORD observed in this study represent an epiphenomenon and do not contribute directly to these disorders. If these antibodies contributed directly to the clinical manifestations of achalasia and GORD, they would be expected to target proteins with a distribution that corresponds to the region that is affected; however, in the current study, we found that achalasia and GORD sera lacked this type of regional specificity. Serum from these patients labelled neurones in the ganglionated plexuses of the small intestine, as well as the oesophagus, and the immunoreactivity that we observed in the oesophagus was not qualitatively different from that seen in the small intestine. Motor abnormalities of the small intestine,23 gall bladder,24 and sphincter of Oddi25 have been reported in patients with achalasia, and the loss of myenteric ganglion cells6,26 and nitrergic inhibitory neurones4 extends to the stomach of some achalasia patients. These observations do not parallel the typical clinical course of idiopathic achalasia, which is normally limited to the oesophagus, and in which a global systemic destruction of myenteric nerves seems unlikely. In syndromes where specific antibodies are associated with gastrointestinal dysmotility, the involved regions of the bowel are more widespread, and autonomic dysfunction outside of the gut is also observed. As idiopathic achalasia is the one clearcut primary motility disorder in which the oesophagus is the focus of clinical symptoms, it is difficult to conceive that a circulating autoantibody is a principal aetiological agent.
In conclusion, the data reported here support the observation that antineuronal antibodies that target enteric neurones are present in the serum of achalasia patients. Antineuronal antibodies, which resulted in similar immunostaining of myenteric ganglia, were also present in the serum of patients with GORD. In general, the antibodies in achalasia serum were specific to enteric nerves, but were not specific to subpopulations of enteric neurones. These results bring into question the theory that antineuronal antibodies are a principal aetiological factor in the development of idiopathic achalasia. If this were the case, we would have expected specificity to nitrergic neurones located in the oesophagus and LOS, and we would not expect to detect comparable immunostaining with serum from GORD patients. We therefore conclude that antineuronal antibodies in serum from individuals from idiopathic achalasia, while being a common feature, do not contribute to clinical dysmotility of the oesophagus. Rather, the presence of antineuronal antibodies represents an epiphenomenon, and is unlikely to have a functional consequence. While we conclude that the circulating antineuronal antibodies probably do not contribute to the disease process, this does not preclude the likely possibility that autoimmunity contributes to the symptoms of achalasia. Because the production of antineuronal antibodies in achalasia and GORD may represent a response to tissue damage and a resulting degeneration of neural elements, it will be interesting in future studies to determine whether other disorders of the gastrointestinal tract, such as inflammatory bowel disease, are associated with the presence of autoantibodies. An important finding of this study is that antineuronal autoantibodies are not uncommon, and the existence of autoantibodies in a given individual’s serum is not necessarily a cause for alarm.
Acknowledgments
This work was supported by grants from the J Brooks Fisher Foundation, Pittsburgh, PA (PLM), the Canadian Institutes for Health Research (KAS), and NIH grants NS26995 and DK62267 (GMM). KAS is an Alberta Heritage Foundation for Medical Research Medical Scientist. The authors wish to thank Drs Steven Lidofsky and Brian Lacey for reviewing the manuscript, and Drs Richard Colletti and Lorne Price for aiding in the identification of appropriate subjects for this study. We also wish to thank MC Moses, RN, for acquiring serum samples and managing the database, and Dr J Silveira for assistance with the immunoblots.
Abbreviations
FITC, fluorescein isothiocyanate
GORD, gastro-oesophageal reflux disease
LOS, lower oesophageal sphincter
LMMP, longitudinal muscle-myenteric plexus
NOS, nitric oxide synthase
PBS, phosphate buffered saline
TBS-T, Tris buffered saline with 0.05% Tween-20
REFERENCES
- 1.Paterson WG. Etiology and pathogenesis of achalasia. Gastrointest Endosc Clin N Am 2001;11:249–66. [PubMed] [Google Scholar]
- 2.Vaezi MF, Richter JE. Diagnosis and management of achalasia. American College of Gastroenterology Practice Parameter Committee. Am J Gastroenterol 1999;94:3406–12. [DOI] [PubMed] [Google Scholar]
- 3.Podas T, Eaden J, Mayberry M, et al. Achalasia: a critical review of epidemiological studies. Am J Gastroenterol 1998;93:2345–7. [DOI] [PubMed] [Google Scholar]
- 4.De Giorgio R, Di Simone MP, Stanghellini V, et al. Esophageal and gastric nitric oxide synthesizing innervation in primary achalasia. Am J Gastroenterol 1999;94:2357–62. [DOI] [PubMed] [Google Scholar]
- 5.Mearin F, Mourelle M, Guarner F, et al. Patients with achalasia lack nitric oxide synthase in the gastro- oesophageal junction. Eur J Clin Invest 1993;23:724–8. [DOI] [PubMed] [Google Scholar]
- 6.Csendes A, Smok G, Braghetto I, et al. Histological studies of Auerbach’s plexuses of the oesophagus, stomach, jejunum, and colon in patients with achalasia of the oesophagus: correlation with gastric acid secretion, presence of parietal cells and gastric emptying of solids. Gut 1992;33:150–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark SB, Rice TW, Tubbs RR, et al. The nature of the myenteric infiltrate in achalasia: an immunohistochemical analysis. Am J Surg Pathol 2000;24:1153–8. [DOI] [PubMed] [Google Scholar]
- 8.Raymond L, Lach B, Shamji FM. Inflammatory aetiology of primary oesophageal achalasia: an immunohistochemical and ultrastructural study of Auerbach’s plexus. Histopathology 1999;35:445–53. [DOI] [PubMed] [Google Scholar]
- 9.Verne GN, Sallustio JE, Eaker EY. Anti-myenteric neuronal antibodies in patients with achalasia: A prospective study. Dig Dis Sci 1997;42:307–13. [DOI] [PubMed] [Google Scholar]
- 10.Storch WB, Eckardt VF, Wienbeck M, et al. Autoantibodies to Auerbach’s plexus in achalasia. Cell Mol Biol (Noisy-le-grand) 1995;41:1033–8. [PubMed] [Google Scholar]
- 11.O’Donnell AM, Ellis LM, Riedl MS, et al. Distribution and chemical coding of orphanin FQ/nociceptin-immunoreactive neurons in the myenteric plexus of guinea pig intestines and sphincter of Oddi. J Comp Neurol 2001;430:1–11. [DOI] [PubMed] [Google Scholar]
- 12.Brookes SJ. Classes of enteric nerve cells in the guinea-pig small intestine. Anat Rec 2001;262:58–70. [DOI] [PubMed] [Google Scholar]
- 13.Galanis E, Frytak S, Rowland KM Jr, et al. Neuronal autoantibody titers in the course of small-cell lung carcinoma and platinum-associated neuropathy. Cancer Immunol Immunother 1999;48:85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castell DO, Castell JA. Esophageal Motility Testing. East Norwalk, CT: Appleton and Lange, 1994.
- 15.Voutilainen M, Sipponen P, Mecklin JP, et al. Gastroesophageal reflux disease: prevalence, clinical, endoscopic and histopathological findings in 1,128 consecutive patients referred for endoscopy due to dyspeptic and reflux symptoms. Digestion 2000;61:6–13. [DOI] [PubMed] [Google Scholar]
- 16.Furness JB, Trussell DC, Pompola S, et al. Calbindin neurons of the guinea-pig small intestine: quantitative analysis of their numbers and projections. Cell Tissue Res 1990;260:261–72. [DOI] [PubMed] [Google Scholar]
- 17.Goin JC, Borda ES, Auger S, et al. Cardiac M(2) muscarinic cholinoceptor activation by human chagasic autoantibodies: association with bradycardia. Heart 1999;82:273–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sterin-Borda L, Goin JC, Bilder CR, et al. Interaction of human chagasic IgG with human colon muscarinic acetylcholine receptor: molecular and functional evidence. Gut 2001;49:699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waterman SA. Autonomic dysfunction in Lambert-Eaton myasthenic syndrome. Clin Auton Res 2001;11:145–54. [DOI] [PubMed] [Google Scholar]
- 20.Lennon VA, Sas DF, Busk MF, et al. Enteric neuronal autoantibodies in pseudoobstruction with small-cell lung carcinoma. Gastroenterology 1991;100:137–42. [DOI] [PubMed] [Google Scholar]
- 21.Lee HR, Lennon VA, Camilleri M, et al. Paraneoplastic gastrointestinal motor dysfunction: clinical and laboratory characteristics. Am J Gastroenterol 2001;96:373–9. [DOI] [PubMed] [Google Scholar]
- 22.Vernino S, Low PA, Fealey RD, et al. Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies. N Engl J Med 2000;343:847–55. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt T, Pfeiffer A, Hackelsberger N, et al. Dysmotility of the small intestine in achalasia. Neurogastroenterol Motil 1999;11:11–17. [DOI] [PubMed] [Google Scholar]
- 24.Annese V, Caruso N, Accadia L, et al. Gallbladder function and gastric liquid emptying in achalasia. Dig Dis Sci 1991;36:1116–20. [DOI] [PubMed] [Google Scholar]
- 25.Hagenmuller F, Classen M. Motility of Oddi’s sphincter in Parkinson’s disease, progressive systemic sclerosis, and achalasia. Endoscopy 1988;20(suppl 1):189–92. [DOI] [PubMed] [Google Scholar]
- 26.Goldblum JR, Whyte RI, Orringer MB, et al. Achalasia. A morphologic study of 42 resected specimens. Am J Surg Pathol 1994;18:327–37. [PubMed] [Google Scholar]