Abstract
Background: Autoimmune pancreatitis is a unique clinical entity proposed recently, and is sometimes associated with inflammation of other organs.
Aims: To examine the pathophysiology of the pancreas and other organs in patients with autoimmune pancreatitis.
Patients and methods: We evaluated clinicopathological findings in six resected and one autopsied patient with autoimmune pancreatitis. The pancreas, peripancreatic tissue, bile duct, and gall bladder were examined histologically and immunohistochemically. Biopsied salivary gland and cervical lymph node of one patient were also examined. We also performed similar immunohistochemical examinations in pancreatectomy specimens from 10 patients with alcoholic chronic pancreatitis and biopsied salivary glands from five patients with Sjögren’s syndrome.
Results: Stenosis of the extrahepatic bile duct was detected in all patients. Histological findings were characterised by diffuse lymphoplasmacytic infiltration with marked interstitial fibrosis and acinar atrophy, obliterated phlebitis of the pancreatic veins, and involvement of the portal vein. Immunohistochemically, diffusely infiltrating cells consisted predominantly of CD4 or CD8 positive T lymphocytes and IgG4 positive plasma cells. Similar inflammatory processes also involved the peripancreatic tissue, extrahepatic bile duct, gall bladder, and salivary gland. Lymph nodes were swollen with infiltration of IgG4 positive plasma cells. None of these findings was seen in alcoholic chronic pancreatitis or Sjögren’s syndrome.
Conclusions: The development of the specific inflammations in extensive organs as well as the pancreas in patients with autoimmune pancreatitis strongly suggests a close relationship between autoimmune pancreatitis and multifocal fibrosclerosis.
Keywords: autoimmune pancreatitis, multifocal fibrosclerosis, chronic pancreatitis, IgG4, plasma cell
Since Sarles and colleagues1 reported a particular type of pancreatitis with hypergammaglobulinaemia, many investigators have suggested that an autoimmune mechanism may be involved in some patients with pancreatitis.2–4 Recently, the concept of autoimmune pancreatitis has been proposed in which several unique clinical and roentographic findings have been shown.5–8 In the TIGAR-O risk factor classification system of chronic pancreatitis proposed in 2001, autoimmunity was categorised as one of six risk factors.9
Autoimmune pancreatitis has been occasionally observed in association with Sjögren’s syndrome, primary sclerosing cholangitis, or primary biliary cirrhosis.10 However, the autoimmune mechanisms that contribute to the pathogenesis or physiology of pancreatitis remain poorly understood. We studied seven patients with autoimmune pancreatitis clinicopathologically, and addressed particularly the relationship between autoimmune pancreatitis and multifocal fibrosclerosis.
MATERIALS AND METHODS
Patients and materials
Between 1987 and 2002, 16 patients were diagnosed as having autoimmune pancreatitis at Tokyo Metropolitan Komagome Hospital. We diagnosed autoimmune pancreatitis according to the presence of the following criteria: (1) enlarged pancreas on ultrasonography or computed tomography; (2) irregular narrowing of the main pancreatic duct on endoscopic retrograde cholangiopancreatography (ERCP); (3) increased serum gammaglobulin level or presence of autoantibodies; and (4) clinical improvement with steroid therapy. We examined clinical and roentographic findings, and immunological results, including autoantibodies (antinuclear antibody, antimitochondrial antibody, and rheumatoid factor). Pancreatoduodenectomy was performed in six patients with suspicion of pancreatic tumour. Autopsy was performed in one patient who died of associated pulmonary carcinoma. We examined these seven cases histologically and immunohistochemically.
Immunohistochemistry
All specimens from the seven cases were fixed in 10% formaldehyde. Serial sections (4 μm thick) were cut from paraffin embedded tissue blocks. Immunohistochemical analysis was carried out on sequent sections using the avidin-biotin-peroxidase complex method. Sections of the pancreas, including peripancreatic tissue, extrahepatic bile duct, and gall bladder were stained with the primary antisera listed in table 1 ▶. Sections of biopsied salivary gland and cervical lymph node from case No 2 were also examined.
Table 1.
Monoclonal antibodies used to investigate the inflammatory infiltrate in the organs of patients with autoimmune pancreatitis, alcoholic chronic pancreatitis, and Sjögren’s syndrome
| Antigen | Source | Pretreatment | Dilution |
| CD4 T cell subset (clone 1F6) | Novocastra | Autoclave | 1:20 |
| CD8 T cell subset (clone C8/144B) | Dako | Autoclave | 1:100 |
| CD20 B cells (clone L26) | Dako | Not done | 1:200 |
| HLA-DR (clone TAL. 1B5.) | Dako | Autoclave | 1:30 |
| IgG | MBL | Pronase | 1:750 |
| IgG4 | The Binding Site | Pronase | 1:500 |
| IgA | Dako | Pronase | 1:5000 |
| IgM | Dako | Pronase | 1:5000 |
The number of immunohistochemically identified CD4, CD8 positive T, or CD20 positive B lymphocytes, and IgG, IgA, IgM, or IgG4 positive plasma cells was counted in the periductal lesion of the pancreas, peripancreatic lesions, lymph nodes, bile duct, gall bladder wall, and salivary gland per high power field (hpf). Four fields were analysed per case. Degree of infiltrated inflammatory cells was classified as:
++ (severe: ≥100/hpf);
+ (moderate: <100, ≥30/hpf);
± (slight: <30, ≥10/hpf);
− (negative: <10/hpf).
We also performed similar immunohistochemical examinations in pancreatectomy specimens from 10 patients with alcoholic chronic pancreatitis and in biopsied salivary glands from five patients with Sjögren’s syndrome.
RESULTS
Clinical and roentographic findings
There were seven patients (six males and one female), aged 65–79 years (mean 71.9). The initial symptom was icterus in six patients. Marked stenosis of the bile duct was detected in all patients. The lower bile duct was affected in six cases and the upper and middle bile duct in one. Swelling of the pancreas and narrowing of the main pancreatic duct were detected in all patients. Hypergammaglobulinaemia (≥2.0 g/dl) was observed in four patients who also showed elevated levels of serum IgG (≥1800 mg/dl). Autoantibodies were positive in five patients. Two patients had other autoimmune diseases (table 2 ▶).
Table 2.
Clinical features of patients with autoimmune pancreatitis
| Autoimmune antibodies | |||||||||||
| Case No | Age (y) | Sex | Initial symptom | Stenosis of bile duct | Swelling of pancreas | Gamma globulin (g/dl) | ANA | AMA | RF | Associated diseases | Treatment |
| 1 | 69 | M | Icterus | Lower | Diffuse | 3.01 | + | + | − | Resection | |
| 2 | 65 | F | Icterus | Lower | Diffuse | 4.64 | + | − | + | RA, Sjogren’s syndrome | Resection |
| 3 | 77 | M | Icterus | Lower | Head | 1.77 | + | − | - | Resection | |
| 4 | 71 | M | Epigastralgia | Lower | Head | 3.63 | + | ND | − | Resection | |
| 5 | 79 | M | Icterus | Upper/middle | Diffuse | 0.81 | + | − | − | Sclerosing cholangitis | Resection |
| 6 | 65 | M | Icterus | Lower | Head | 1.70 | − | − | + | Resection | |
| 7 | 77 | M | Icterus | Lower | Diffuse | 3.87 | ND | ND | ND | PTBD | |
ANA, antinuclear antigen; AMA, antimitochondrial antigen; RF, rheumatoid factor; RA, rheumatoid arthritis;ND, not done; PTBD, percutaneous transhepatic biliary drainage.
Histological and immunohistochemical findings
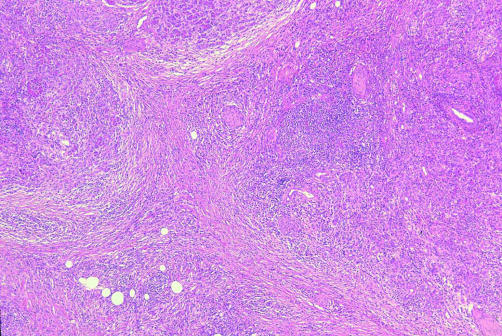
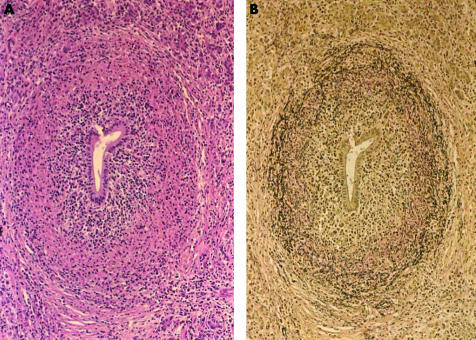
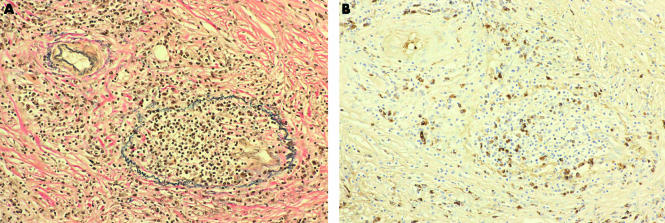
Histological findings in autoimmune pancreatitis can be summarised as follows: diffuse lymphoplasmacytic infiltration and marked interstitial fibrosis with acinar atrophy throughout the pancreas involving the retroperitoneal peripancreatic tissue (fig 1 ▶); narrowing of the pancreatic duct by periductal fibrosis with lymphoplasmacytic infiltration (fig 2A, B ▶); obliterated phlebitis of the variable sized pancreatic veins and involvement of the portal vein with lymphoplasmacytic infiltrate and proliferation of fibroblasts in and around the walls of the veins (fig 3A ▶); and diffuse thickening of the wall of the common bile duct and gall bladder with marked fibrosis and lymphoplasmacytic infiltration (table 3 ▶). Calcification or pseudocyst was not detected. Regional and cervical lymph nodes were swollen up to 2.0 cm in diameter and showed marked follicular hyperplasia and dense plasmacytic infiltration. Histological examination of the biopsied salivary gland showed diffuse acinar atrophy with interstitial fibrosis and lymphoplasmacytic infiltration.
Figure 1.
Characteristic low power view of the pancreas showing prominent interstitial fibrosis with marked atrophy of the exocrine pancreas and diffuse inflammatory cell infiltration throughout the pancreas (haematoxylin-eosin, ×8)
Figure 2.
Periductal non-occlusive fibrosis with lymphoplasmacytic infiltration and irregular condensation, and rupture of the elastic fibres of the pancreatic ductal wall. ((A) Haematoxylin-eosin, ×24; (B) Elastica Van Gienson, ×24.)
Figure 3.
Pancreatic vein showing complete luminal obliteration with prominent cellular infiltrates and fibrosis ((A) Elastica Van Gienson, ×50). Infiltration of IgG4 positive plasma cells was detected in the obliterated vein and in the interstitium around it ((B) immunostaining of IgG4, ×50).
Table 3.
Histological and immunohistochemical findings of the pancreas, peripancreatic tissue, lymph nodes, biliary tract, and salivary gland in patients with autoimmune pancreatitis, alcoholic chronic pancreatitis, and Sjögren’s syndrome
| Cellular infiltration | ||||||||
| Lymphocyte | Plasma cell | |||||||
| Fibrosis | CD4+ T cell | CD8+ T cell | CD20+ B cell | IgG+ | IgG4+ | IgA+ | IgM+ | |
| Autoimmune pancreatitis | ||||||||
| Pancreas | ++ | ++ | ++ | +/− | + | + | − | − |
| Peripancreatic tissue | +/− | ++ | ++ | +/− | + | + | − | − |
| Peripancreatic lymph nodes | − | ++ | ++ | ++ | + | + | − | − |
| Cervical lymph node | − | ++ | ++ | ++ | + | + | − | − |
| Upper bile duct wall | + | + | + | +/− | +/− | +/− | − | − |
| Lower bile duct wall | ++ | ++ | ++ | +/− | + | + | − | − |
| Gall bladder wall | + | + | + | − | +/− | +/− | − | − |
| Salivary gland | + | + | + | + | + | + | − | − |
| Alcoholic chronic pancreatitis | ||||||||
| Pancreas | ++ | +/− | +/− | +/− | − | − | − | − |
| Peripancreatic lymph nodes | − | ++ | ++ | ++ | − | − | − | − |
| Lower bile duct wall | + | +/− | +/− | +/− | − | − | − | − |
| Sjögren’s syndrome | ||||||||
| Salivary gland | + | + | + | + | − | − | − | − |
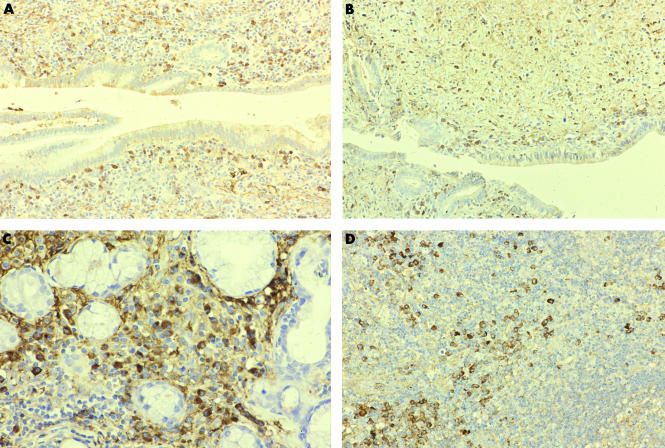
Immunohistochemically, diffuse infiltrates in the pancreas consisted predominantly of CD4 or CD8 positive T lymphocytes, and IgG4 positive plasma cells rather than CD20 positive B lymphocytes (fig 4A ▶). CD8 positive T lymphocytes were occasionally found in the pancreatic duct epithelium itself. Infiltration of CD4 or CD8 positive T lymphocytes and IgG4 positive plasma cells were detected in the obliterated veins (fig 3B ▶). The epithelium of small sized pancreatic ducts and the bile duct focally expressed HLA-DR, which were surrounded by HLA-DR positive T lymphocytes. This pattern of cellular infiltration was the same as that of the peripancreatic tissue, upper and lower bile duct (fig 4B ▶), gall bladder, and salivary gland (fig 4C ▶). Plasma cells infiltrated in the regional or cervical lymph nodes were also positive for IgG4 (fig 4D ▶). Marked infiltration of CD4 or CD8 positive T lymphocytes or IgG4 positive plasma cells was not observed in the pancreas or biliary tract of patients with alcoholic chronic pancreatitis. Infiltration of IgG4 positive plasma cells was not detected in lymph nodes of patients with alcoholic chronic pancreatitis or salivary glands of those with Sjögren’s syndrome.
Figure 4.
Many IgG4 positive plasma cells infiltrated around the pancreatic duct ((A) immunostaining of IgG4, ×40) and the common bile duct ((B) immunostaining of IgG4, ×40). Infiltration of IgG4 positive plasma cells was detected in the salivary gland ((C) immunostaining of IgG4, ×100) and cervical lymph node ((D) immunostaining of IgG4, ×80).
DISCUSSION
Autoimmune pancreatitis is a clinical entity proposed recently in which several unique clinical findings have been shown,6–8 but the pathogenesis and pathophysiology are still unclear. The clinical characteristics of autoimmune pancreatitis in our series can be summarised as follows: (1) frequent involvement in relatively elderly men; (2) diffuse enlargement of the pancreas; (3) diffuse irregular narrowing of the main pancreatic duct on ERCP; (4) stenosis of the common bile duct; (5) increased levels of serum gammaglobulin or immunoglobulin G; (6) presence of autoantibodies; (7) occasional association with other autoimmune diseases; and (8) effective steroid therapy. Entities termed sclerosing pancreatitis11 or pancreatitis with narrowing pancreatic duct12 are similar and may overlap with autoimmune pancreatitis. Coexistence of pancreatitis with other systematic exocrinopathies also overlaps with autoimmune pancreatitis.
Histological and immunohistochemical findings in patients with autoimmune pancreatitis were characteristic. CD4 or CD8 positive T lymphocytes and IgG4 positive plasma cells infiltrated diffusely with marked interstitial fibrosis and obliterated phlebitis through the pancreas. Similar inflammatory processes involved the peripancreatic tissue, extrahepatic bile duct, gall bladder, and salivary gland. Lymph nodes were swollen with infiltration of IgG4 positive plasma cells.
Hamano and colleagues11 reported that high serum IgG4 concentrations were observed in patients with autoimmune pancreatitis but not in other diseases of the pancreas or biliary tract, and suggested that the pathogenesis of autoimmune pancreatitis is closely related to the IgG4 subclass of immune complexes. In our immunohistochemical study, infiltration of IgG4 positive plasma cells was detected in extensive organs as well as in the pancreas of patients with autoimmune pancreatitis, but was not observed in the pancreas, biliary tract, or lymph nodes of patients with alcoholic chronic pancreatitis, or the salivary glands of those with Sjögren’s syndrome. This phenomenon seems to be a specific change in autoimmune pancreatitis.
Ectors and colleagues13 reported that the specific pathological change of non-alcoholic chronic pancreatitis was a conspicuous periductal inflammation consisting mainly of CD4 or CD8 positive T lymphocytes that apparently caused duct obstruction, and proposed the term chronic duct obstructive pancreatitis. These morphological findings of the pancreas were similar to those of our cases of autoimmune pancreatitis.
Multifocal fibrosclerosis is an uncommon fibroproliferative systemic disorder with multiple manifestations, including retroperitoneal fibrosis, sclerosing cholangitis, Riedel’s thyroiditis, fibrotic pseudotumour of the orbit and fibrosis of the salivary glands. The microscopic pathology of these disorders is similar, showing a fibrotic process infiltrated with blood vessels, lymphocytes, and plasma cells. It is suggested that they are all interrelated and probably different manifestations of a common disorder of fibroblastic proliferation.14 One of the characteristic pathological findings of multifocal fibrosclerosis with retroperitoneal fibrosis is a phlebitis which begins with inflammation of the vessel wall. The elastic lamellae are spilt up and the lumen is filled with connective tissue that contains capillaries and lymphocytes.15 These findings are the same as obliterated phlebitis extensively detected in autoimmune pancreatitis, and the process is similar to narrowing of the pancreatic duct induced by rupture of the elastic fibres of ductal wall. Nine cases of pancreatic pseudotumour or chronic pancreatitis associated with multifocal fibrosclerosis have been reported in the literature.16–23 They were seven males and two females, and the age range was 31–63 (mean 48.9). All five patients treated with steroid improved. In retroperitoneal fibrosis, males outnumber females by 3 to 1, usual time of onset is in middle age, and steroid therapy is sometimes effective.24,25
The development of specific inflammation in extensive organs as well as in the pancreas, and frequent involvement of relatively elderly men with autoimmune pancreatitis, strongly suggest a close relationship between autoimmune pancreatitis and multifocal fibrosclerosis. So-called autoimmune pancreatitis may not be a simple pancreatitis but one lesion of systemic disease of multifocal fibrosclerosis.
Abbreviations
ERCP, endoscopic retrograde cholangiopancreatography
hpf, high power field
REFERENCES
- 1.Sarles H, Sarles JC, Muratore R, et al. Chronic inflammatory sclerosis of the pancreas—An autonomous pancreatic disease? Am J Dig Dis 1961;6:688–98. [DOI] [PubMed] [Google Scholar]
- 2.Sarles H, Muratore R, Sarles JC, et al. Aetiology and pathology of chronic pancreatitis. Bibl Gastroenterol 1965;7:75–120. [Google Scholar]
- 3.Nakano S, Takeda I, Kitamura K, et al.Vanishing tumor of the abdomen in patient with Sjögren’s syndrome. Am J Dis 1978;23:75–9S. [Google Scholar]
- 4.Antal L, Kavai M, Szabo G, et al. Immunological investigations in acute chronic pancreatitis. Digestion 1980;20:100–5. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida K, Toki F, Takeuchi T, et al. Chronic pancreatitis caused by autoimmune abnormality. Proposal of concept of autoimmune pancreatitis. Dig Dis Sci 1995;40:1561–8. [DOI] [PubMed] [Google Scholar]
- 6.Horiuchi A, Kaneki T, Tamamura M, et al. Autoimmune chronic pancreatitis simulating pancreatic lymphoma. Am J Gastroenterol 1996;91:2607–9. [PubMed] [Google Scholar]
- 7.Ito T, Nakano I, Koyanagi S, et al. Autoimmune pancreatitis as a new clinical study. Three cases of autoimmune pancreatitis with effective steroid therapy. Dig Dis Sci 1997;42:1458–68. [DOI] [PubMed] [Google Scholar]
- 8.Okazaki K, Uchida K, Ohana M, et al. Autoimmune-related pancreatitis is associated with autoantibodies and a Th1/Th2-type cellular immune response. Gastroenterology 2000;118:573–81. [DOI] [PubMed] [Google Scholar]
- 9.Etemad B, Whitcomb DC. Chronic pancreatitis: diagnosis, classification, and new genetic developments. Gastroenterology 2001;120:682–707. [DOI] [PubMed] [Google Scholar]
- 10.Okazaki K, Uchida K, Chiba T. Recent concept of autoimmune-related pancreatitis. J Gastroenterol 2001;36:293–302. [DOI] [PubMed] [Google Scholar]
- 11.Hamano H, Kawa S, Horiuchi A, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med 2001;344: 732–8. [DOI] [PubMed] [Google Scholar]
- 12.Wakabatyashi T, Motoo Y, Kojima Y, et al. Chronic pancreatitis with diffuse irregular narrowing of the main pancreatic duct. Dig Dis Sci 1998;43:2415–25. [DOI] [PubMed] [Google Scholar]
- 13.Ectors N, Maillet B, Aerts R, et al. Non-alcoholic duct destructive chronic pancreatitis. Gut 1997;41:263–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Comings DE, Skubi KB, Eyes JV, et al. Familial multifocal fibrosclerosis. Ann Intern Med 1967;66:884–92. [DOI] [PubMed] [Google Scholar]
- 15.Meyer S, Hausman R. Occlusive phlebitis in multifocal fibrosclerosis. Am J Clin Pathol 1976;65:274–83. [DOI] [PubMed] [Google Scholar]
- 16.Clark A, Zeman RK, Choyke PL, et al. Pancreatic pseudotumors associated with multifocal idiopathic fibrosclerosis. Gastrointest Radiol 1988;13:30–2. [DOI] [PubMed] [Google Scholar]
- 17.Waldram R, Kopelman H, Tsarrtoulas D, et al. Chronic pancreatitis, sclerosing cholangitis, and sicca complex in two siblings. Lancet 1975;1:550–2. [DOI] [PubMed] [Google Scholar]
- 18.Sjögren I, Wengle B, Korsgren M. Primary sclerosing cholangitis associated with fibrosis of the submandibular glands and the pancreas. Acta Med Scand 1979;205:139–41. [DOI] [PubMed] [Google Scholar]
- 19.Case records of the Massachusetts General Hospital: case 6–1982. N Engl J Med 1982;306:349–58. [DOI] [PubMed] [Google Scholar]
- 20.Jafri SZH, Bree RL, Agha FP, et al. Inflammatory pseudotumor from sclerosing cholangitis. J Comput Assist Tomogr 1983;7:902–4. [DOI] [PubMed] [Google Scholar]
- 21.Laitt RD, Hubscher SG, Buckels JA, et al. Sclerosing cholangitis associated with multifocal fibrosis: a case report. Gut 1992;33:1430–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levey JM, Mathai J. Diffuse pancreatic fibrosis: an uncommon feature of multifocal idiopathic fibrosclerosis. Am J Gastroenterol 1998;93:640–2. [DOI] [PubMed] [Google Scholar]
- 23.Hastier P, Buckley M, Gall PL, et al. First report of association of chronic pancreatitis, primary biliary cirrhosis, and systemic sclerosis. Dig Dis Sci 1998;43:2426–8. [DOI] [PubMed] [Google Scholar]
- 24.Mitchinson MJ. Some clinical aspects of idiopathic retroperitoneal fibrosis. Br J Surg 1972;59:58–60. [DOI] [PubMed] [Google Scholar]
- 25.Baker LRI, Mallinson WJW, Gregory MC, et al. Idiopathic retroperitoneal fibrosis. A retrospective analysis of 60 cases. Br J Urol 1988;60:497–503. [DOI] [PubMed] [Google Scholar]