Abstract
The two most common inherited forms of colorectal cancer are familial adenomatous polyposis and hereditary non-polyposis colorectal cancer. Simultaneous inheritance of both an APC gene mutation and a mismatch repair gene (for example, MLH1) mutation has never been described. In the present case report, we report rapidly progressive adenomatous polyposis in a 10 year old boy with a germline frame shift mutation in the APC gene and a germline splice site mutation in the MLH1 gene. Immunohistochemical investigations showed abnormal expression of β-catenin in early adenomas with low grade dysplasia, attributed to the APC gene mutation. Subsequent loss of function of the MLH1 gene, as shown by absent immunostaining of its protein in adenomas with high grade dysplasia, may well have caused the rapid progression to high grade dysplasia in many of the adenomas.
Keywords: familial adenomatous polyposis, adenomatous polyposis coli, hereditary non-polyposis colorectal cancer, adenomas, dysplasia
The two most common inherited conditions predisposing to colorectal cancer are familial adenomatous polyposis (FAP), caused by germline mutations in the APC (adenomatous polyposis coli) tumour suppressor gene, and hereditary non-polyposis colorectal cancer (HNPCC), caused by germline mutations in one of the DNA mismatch repair (MMR) genes. Together, FAP and HNPCC account for approximately 2% of colorectal cancer cases.1,2 In this report, a case is presented of a patient manifesting a severe phenotype who had inherited germline mutations in both the APC gene and the MMR gene MLH1.
CASE REPORT
A 10 year old boy attended the outpatient clinic for evaluation of slimy and bloody stools. His mother had previously been diagnosed with FAP. At the age of 27 years, she underwent prophylactic colectomy. Due to the development of extensive abdominal fibromatosis (desmoid tumour), she died at the age of 32 years. Genetic analysis had revealed a germline de novo frame shift mutation in exon 15 of the APC gene (a 5 bp deletion at codon 1309). The father of the boy is healthy but has a classical family history of HNPCC and carries a germline splice site mutation in the MLH1 gene (codon 226, G to A at position 677).
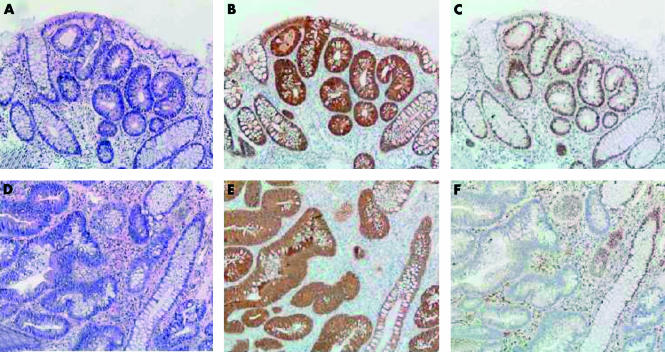
Physical examination was unremarkable. Colonoscopy revealed hundreds of polyps throughout the colon. Some were removed and histopathological examination showed small tubular adenomas with low grade dysplasia. Six months later endoscopic and histopathological examinations were repeated, again showing multiple tubular adenomas, this time with high grade dysplasia (see fig 1▶). Subsequently, the boy underwent proctocolectomy with ileal pouch-anal anastomosis. The surgical specimen showed a large number of adenomas of which many contained areas with high grade dysplasia. Genetic analysis revealed that the patient had inherited both his mother’s APC gene mutation as well as his father’s MLH1 gene defect.
Figure 1 .
Normal colonic mucosa and areas of adenomatous epithelium with low grade dysplasia (A-C) and adenomatous epithelium with high grade dysplasia (D-F). Serial haematoxylin-eosin staining (A, D) and immunohistochemical (brown) staining of β-catenin (B, E) and MLH1 protein (C, F). In the adenomatous areas with low grade dysplasia, β-catenin staining is normal (membranous) in normal mucosa, in contrast with abnormal (cytoplasmic and nuclear) staining in adenomatous areas; MLH1 protein is expressed in both normal mucosa and adenomatous epithelium. In the adenomatous epithelium with high grade dysplasia, β-catenin staining is similar to the adenomatous areas with low grade dysplasia, whereas MLH1 protein expression is seen in normal mucosa but lost in areas with high grade dysplasia (magnification 100×).
DISCUSSION
FAP is characterised by the development of multiple colorectal adenomas at a young age, usually before 20 years. Left untreated, one or more of the adenomas will have progressed to cancer before the age of 45 years. Therefore, most patients have a prophylactic colectomy in the second or third decade of life. The APC protein, dysfunctional in FAP, regulates the intracellular level of β-catenin, and controls diverse physiological processes in the colon epithelium including cell growth, adhesion, and apoptosis.3
HNPCC is caused by germline mutations in one of the DNA MMR genes, including MLH1, MSH2, and MSH6.4 MMR deficient cells rapidly accumulate somatic mutations thus explaining the accelerated tumour progression in HNPCC patients.4 Clinical features of HNPCC include colorectal cancer at an early age (mean 45 years) with a predilection for the proximal colon, an increased frequency of poorly differentiated mucinous tumours, and a high incidence of extracolonic tumours (for example, endometrium, ovary, urinary tract).
To the best of our knowledge, simultaneous inheritance of both types of gene defects in one patient has not been reported previously. The 5 bp deletion at codon 1309 of the APC gene is the most commonly reported change in FAP patients and is typically associated with a severe phenotype with an early onset of disease and the presence of extensive polyposis.5 The MLH1 mutation we found has previously been described and is not associated with a particular phenotype.6
In our patient, the germline APC mutation, followed by somatic loss of the wild-type allele, is likely to have triggered early and multiple adenoma formation. This is illustrated by abnormal expression of β-catenin that was seen in the early adenomas (fig 1▶). Loss of MMR function, due to the germline MLH1 mutation, and loss of the wild-type allele, may well underlie the rapid progression of dysplasia in our patient. The relevance of MMR dysfunction is supported by the finding that progression from low to high grade dysplasia observed in the adenomas was associated with loss of MLH1 expression (fig 1▶).
In addition, evidence has emerged from several animal studies of the important role of MMR genes in accelerated intestinal tumorigenesis, especially in an APC deficient background.7,8 In mice carrying both APC and MLH1 mutations, the incidence of intestinal adenomas was markedly increased compared with mice carrying only one of these mutations.7,8 Alternatively, the severe phenotype in our patient may in fact reflect the marked heterogeneity which is known to exist in both FAP and HNPCC.9,10
Our patient probably has a higher risk of developing cancer at other sites than average FAP or HNPCC mutation carriers. Intensive follow up and surveillance are therefore warranted.
In conclusion, our case represents the first report of a patient with adenomatous polyposis who carried both a pathogenic APC and MLH1 germline mutation. The early adenoma onset and the rapid tumour progression in this patient illustrate that the multistep process of colorectal carcinogenesis in this setting was markedly accelerated.
Abbreviations
FAP, familial adenomatous polyposis
APC, adenomatous polyposis coli
HNPCC, hereditary non-polyposis colorectal cancer
MMR, mismatch repair
REFERENCES
- 1.Potter JD. Colorectal cancer: molecules and populations. J Natl Cancer Inst 1999;91:916–32. [DOI] [PubMed] [Google Scholar]
- 2.Jass JR. Familial colorectal cancer: pathology and molecular characteristics. Lancet Oncol 2000;1:220–6. [DOI] [PubMed] [Google Scholar]
- 3.Fodde R, Smits R, Clevers H. APC, signal transduction and genetic instability in colorectal cancer. Nat Rev Cancer 2001;1:55–67. [DOI] [PubMed] [Google Scholar]
- 4.Lynch HT, Lynch J. Lynch syndrome: genetics, natural history, genetic counseling, and prevention. J Clin Oncol 2000;18:19–31S. [PubMed] [Google Scholar]
- 5.Gayther SA, Wells D, SenGupta SB, et al. Regionally clustered APC mutations are associated with a severe phenotype and occur at a high frequency in new mutation cases of adenomatous polyposis coli. Hum Mol Genet 1994;3:53–6. [DOI] [PubMed] [Google Scholar]
- 6.Wijnen J, Meera Khan P, Vasen H, et al. Majority of hMLH1 mutations responsible for hereditary nonpolyposis colorectal cancer cluster at the exonic region 15–16. Am J Hum Genet 1996;58:300–7. [PMC free article] [PubMed] [Google Scholar]
- 7.Edelmann W, Yang K, Kuraguchi M, et al. Tumorigenesis in Mlh1 and Mlh1/Apc1638N mutant mice. Cancer Res 1999;59:1301–7. [PubMed] [Google Scholar]
- 8.Shoemaker AR, Haigis KM, Baker SM, et al. Mlh1 deficiency enhances several phenotypes of Apc(Min)/+ mice. Oncogene 2000;19:2774–9. [DOI] [PubMed] [Google Scholar]
- 9.Benatti P, Roncucci L, Ganazzi D, et al. Clinical and biologic heterogeneity of hereditary nonpolyposis colorectal cancer. Int J Cancer 2001;95:323–8. [DOI] [PubMed] [Google Scholar]
- 10.Scott RJ, Meldrum C, Crooks R, et al. Familial adenomatous polyposis: more evidence for disease diversity and genetic heterogeneity. Gut 2001;48:508–14. [DOI] [PMC free article] [PubMed] [Google Scholar]